Biofilm Formation Reduction by Eugenol and Thymol on Biodegradable Food Packaging Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Film Preparation
2.3. Antibacterial Activity
2.4. Biofilm Formation
2.4.1. MTT Assay
- Non-biofilm formation (−): OD ≤ ODP;
- Weak biofilm formation (+): ODP < OD ≤ 2ODP;
- Strong biofilm formation (++): 2ODP < OD.
2.4.2. Christensen Method
2.4.3. Fluorescence Microscopy
2.5. Material Properties
2.5.1. FTIR-ATR Analysis
2.5.2. Contact Angle Measurement
2.6. Statistical Analysis
3. Results
3.1. Antibacterial Activity
3.2. Biofilm Formation
3.3. Material Properties
3.3.1. Contact Angle
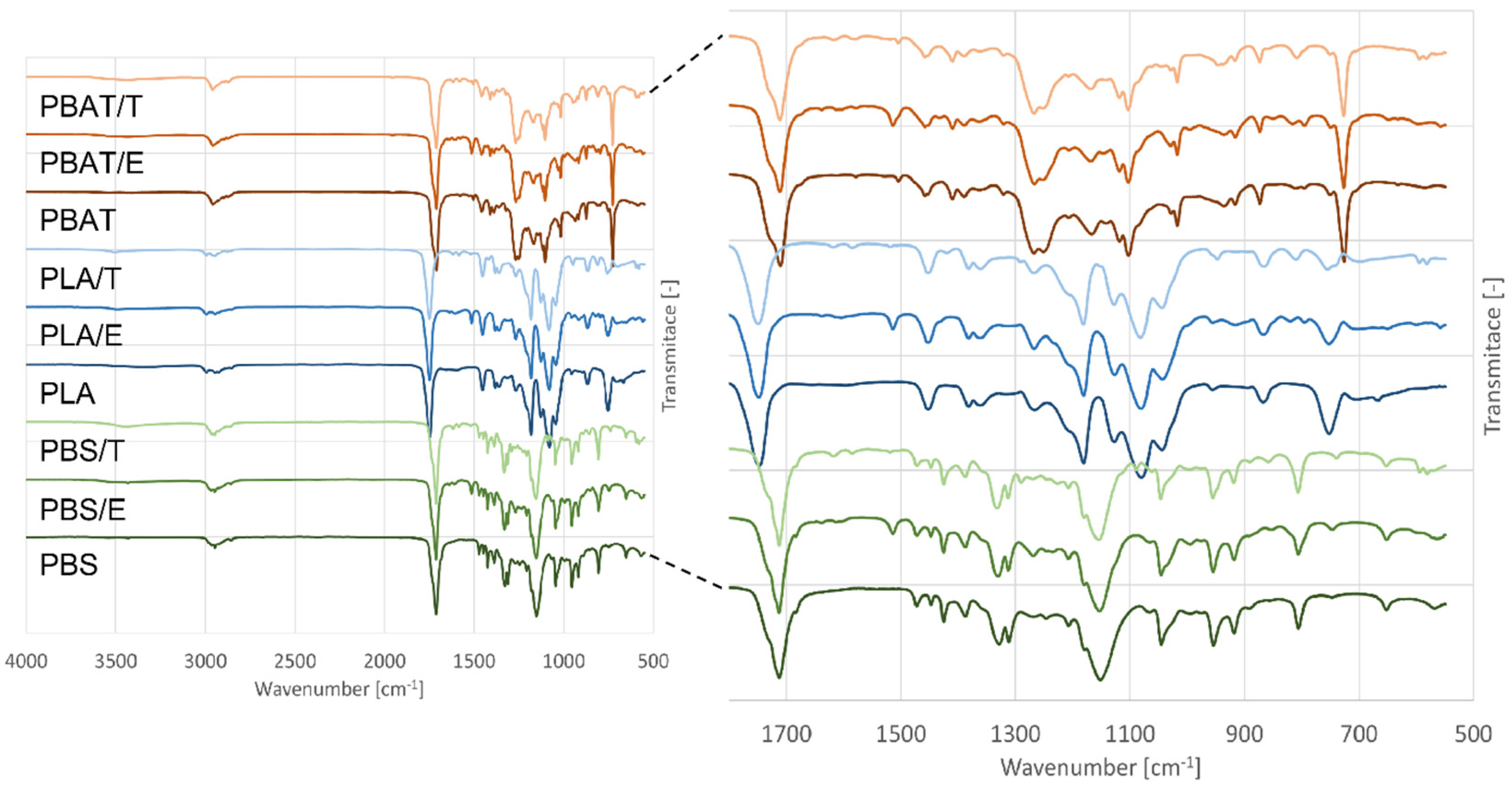
3.3.2. FTIR-ATR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, K.C. (Ed.) Microbial Adhesion and Aggregation, 1st ed.; Springer: Berlin, Heidelberg, 1984; ISBN 9783642701399. [Google Scholar]
- Li, Y.-H.; Tian, X. Quorum Sensing and Bacterial Social Interactions in Biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Cerca, N.; Pier, G.B.; Vilanova, M.; Oliveira, R.; Azeredo, J. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res. Microbiol. 2005, 156, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Wyrwa, J.; Barska, A. Innovations in the food packaging market: Active packaging. Eur. Food Res. Technol. 2017, 243, 1681–1692. [Google Scholar] [CrossRef]
- De Abreu, D.A.P.; Cruz, J.M.; Losada, P.P. Active and Intelligent Packaging for the Food Industry. Food Rev. Int. 2012, 28, 146–187. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EC. Regulation (EC) No. 1935/2004 of the European Parliament and of the Council of 27 October 2004, on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/509/EEC and 89/109/EEC; European Commission: Brussels, Belgium, 2004; Volume 338, pp. 4–17. [Google Scholar]
- EC. Commission Regulation (EC) No. 450/2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food; European Commission: Brussels, Belgium, 2009; Volume 135, pp. 3–11. [Google Scholar]
- Beneventi, E.; Tietz, T.; Merkel, S. Risk Assessment of Food Contact Materials. EFSA J. 2020, 18, e181109. [Google Scholar] [CrossRef]
- Irankhah, S.; Ali, A.A.; Mallavarapu, M.; Soudi, M.R.; Subashchandrabose, S.R.; Gharavi, S.; Ayati, B. Ecological role of Acinetobacter calcoaceticus GSN3 in natural biofilm formation and its advantages in bioremediation. Biofouling 2019, 35, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-D. Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. Int. Biodeterior. Biodegrad. 2003, 52, 69–91. [Google Scholar] [CrossRef]
- Barron, A.; Sparks, T.D. Commercial Marine-Degradable Polymers for Flexible Packaging. iScience 2020, 23, 101353. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Food Contact Surfaces: Challenges, Legislation and Solutions. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Overview of Poly(lactic Acid). In Handbook of Biopolymers and Biodegradable Plastics; Elsevier: Amsterdam, The Netherlands, 2013; pp. 11–54. ISBN 9781455728343. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Hydrolytic degradation of biodegradable polyesters under simulated environmental conditions. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Brocca, D.; Arvin, E.; Mosbæk, H. Identification of organic compounds migrating from polyethylene pipelines into drinking water. Water Res. 2002, 36, 3675–3680. [Google Scholar] [CrossRef]
- Zhang, H.; Dudley, E.G.; Davidson, P.M.; Harte, F. Critical Concentration of Lecithin Enhances the Antimicrobial Activity of Eugenol against Escherichia coli. Appl. Environ. Microbiol. 2017, 83, e03467-16. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Du, Y.; Wang, X.; Feng, T. Self-aggregation of water-soluble chitosan and solubilization of thymol as an antimicrobial agent. J. Biomed. Mater. Res. Part A 2008, 90A, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, S.A.; Roselin, L.S. Recent progress in photochemical reaction on main components of some essential oils. J. Saudi Chem. Soc. 2018, 22, 855–875. [Google Scholar] [CrossRef]
- Khatsee, S.; Daranarong, D.; Punyodom, W.; Worajittiphon, P. Electrospinning polymer blend of PLA and PBAT: Electrospinnability-solubility map and effect of polymer solution parameters toward application as antibiotic-carrier mats. J. Appl. Polym. Sci. 2018, 135, 46486. [Google Scholar] [CrossRef]
- Narayanan, A.; Neera; Mallesha; Ramana, K.V. Synergized Antimicrobial Activity of Eugenol Incorporated Polyhydroxybutyrate Films Against Food Spoilage Microorganisms in Conjunction with Pediocin. Appl. Biochem. Biotechnol. 2013, 170, 1379–1388. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; CLSI Standard M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Zanoaga, M.; Tanasa, F. Photochemical Behavior of Synthetic Polymeric Multicomponent Materials Composites and Nanocomposites. In Photochemical Behavior of Multicomponent Polymeric-Based Materials; Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2016; pp. 109–164. ISBN 978-3-319-25194-3. [Google Scholar]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Molecular Probes. LIVE/DEAD® BacLight Bacterial Viability Kits; Invitrogen: Waltham, MA, USA, 2004; pp. 1–8. [Google Scholar]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Galiè, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riss, T.L. Cell Viability Assays. In Assay Guidance Manual; Moravec, R.A., Niles, A.L., Duellman, S., Benink, H.A., Worzella, T.J., Minor, L., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004; pp. 357–388. [Google Scholar]
- Williams, S.C.; Hong, Y.; Danavall, D.C.A.; Howard-Jones, M.H.; Gibson, D.; Frischer, M.; Verity, P.G. Distinguishing between living and nonliving bacteria: Evaluation of the vital stain propidium iodide and its combined use with molecular probes in aquatic samples. J. Microbiol. Methods 1998, 32, 225–236. [Google Scholar] [CrossRef]
- Williams, G.W.; Schork, M.A.; Brunden, M.N. Basic Statistics for Quality Control in the Clinical Laboratory. CRC Crit. Rev. Clin. Lab. Sci. 1982, 17, 171–199. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Vacheethasanee, K.; Marchant, R.E. Surfactant polymers designed to suppress bacterial (Staphylococcus epidermidis) adhesion on biomaterials. J. Biomed. Mater. Res. 2000, 50, 302–312. [Google Scholar] [CrossRef]
- Chen, S.; Peng, X.; Geng, L.; Wang, H.; Lin, J.; Chen, B.; Huang, A. The effect of polytetrafluoroethylene particle size on the properties of biodegradable poly(butylene succinate)-based composites. Sci. Rep. 2021, 11, 6802. [Google Scholar] [CrossRef] [PubMed]
- Bhole, Y.S.; Karadkar, P.B.; Kharul, U.K. Effect of substituent polarity, bulk, and substitution site toward enhancing gas permeation in dibromohexafluorobisphenol-a based polyarylates. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3156–3168. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of Torrefied Coffee Grounds as Reinforcing Agent To Produce High-Quality Biodegradable PBAT Composites for Food Packaging Applications. ACS Sustain. Chem. Eng. 2016, 5, 1906–1916. [Google Scholar] [CrossRef]
- De Matos Costa, A.R.; Crocitti, A.; de Carvalho, L.H.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(butylene Succinate) (PBS) and Poly(butylene Adipate-co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef]
- Jasim, S.M.; Ali, N.A. Properties characterization of plasticized polylactic acid /Biochar (bio carbon) nano-composites for antistatic packaging. Iraqi J. Phys. 2019, 17, 13–26. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.B.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Kumaraswamy, R.V.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep. 2018, 8, 6650. [Google Scholar] [CrossRef] [Green Version]
- Zamani, Z.; Alipour, D.; Moghimi, H.R.; Mortazavi, S.A.R.; Saffary, M. Development and Evaluation of Thymol Microparticles Using Cellulose Derivatives as Controlled Release Dosage Form. Iran. J. Pharm. Res. 2015, 14, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Achinas, S.; Yska, S.K.; Charalampogiannis, N.; Krooneman, J.; Euverink, G.J.W. A Technological Understanding of Biofilm Detection Techniques: A Review. Materials 2020, 13, 3147. [Google Scholar] [CrossRef]
- Ojima, Y.; Nunogami, S.; Taya, M. Antibiofilm effect of warfarin on biofilm formation of Escherichia coli promoted by antimicrobial treatment. J. Glob. Antimicrob. Resist. 2016, 7, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef]
- Rajamani, S.; Sandy, R.; Kota, K.; Lundh, L.; Gomba, G.; Recabo, K.; Duplantier, A.; Panchal, R.G. Robust biofilm assay for quantification and high throughput screening applications. J. Microbiol. Methods 2019, 159, 179–185. [Google Scholar] [CrossRef]
- Hassan, A.; Usman, J.; Kaleem, F.; Omair, M.; Khalid, A.; Iqbal, M. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz. J. Infect. Dis. 2011, 15, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Morohoshi, T.; Oi, T.; Aiso, H.; Suzuki, T.; Okura, T.; Sato, S. Biofilm Formation and Degradation of Commercially Available Biodegradable Plastic Films by Bacterial Consortiums in Freshwater Environments. Microbes Environ. 2018, 33, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Gzyra-Jagieła, K.; Sulak, K.; Draczyński, Z.; Podzimek, S.; Gałecki, S.; Jagodzińska, S.; Borkowski, D. Modification of Poly(lactic acid) by the Plasticization for Application in the Packaging Industry. Polymers 2021, 13, 3651. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghani, M.M.A.; Azzam, R.A.; Madkour, T.M. Design and Development of Enhanced Antimicrobial Breathable Biodegradable Polymeric Films for Food Packaging Applications. Polymers 2021, 13, 3527. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, H.; Pratiwi, S.U.T.; Hertiani, T. Efficacy of Thymol and Eugenol Against Polymicrobial Biofilm. Indones. J. Pharm. 2018, 29, 214. [Google Scholar] [CrossRef] [Green Version]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.M.; Izadi, M.; Abdollahi, M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, Z.; Jiao, X.; Shang, Y.; Wen, Y. Encapsulation of Thymol in Biodegradable Nanofiber via Coaxial Eletrospinning and Applications in Fruit Preservation. J. Agric. Food Chem. 2019, 67, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Hertiani, T.; Utami, D.T.; Pratiwi, S.U.T.; Haniastuti, T. Eugenol and thymol as potential inhibitors for polymicrobial oral biofilms: An in vitro study. J. Int. Oral Heal. 2021, 13, 45. [Google Scholar] [CrossRef]
- de Morais, S.M.; Vila-Nova, N.S.; Bevilaqua, C.; Rondon, F.C.; Lobo, C.H.; de Alencar Araripe Noronha Moura, A.; Sales, A.D.; Rodrigues, A.P.R.; de Figuereido, J.R.; Campello, C.C.; et al. Thymol and eugenol derivatives as potential antileishmanial agents. Bioorganic Med. Chem. 2014, 22, 6250–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldhuizen, E.J.A.; Tjeerdsma-van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural Requirements for the Antimicrobial Activity of Carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Adil, M.; Singh, K.; Verma, P.K.; Khan, A.U. Eugenol-induced suppression of biofilm-forming genes in Streptococcus mutans: An approach to inhibit biofilms. J. Glob. Antimicrob. Resist. 2014, 2, 286–292. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Jiang, L.; Chuan, Y.; Yuan, M.; Chen, H. Characterization of Active Packaging Films Made from Poly(Lactic Acid)/Poly(Trimethylene Carbonate) Incorporated with Oregano Essential Oil. Molecules 2016, 21, 695. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.; Fortunati, E.; Beltrán, A.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release, Disintegration, Antioxidant, and Antimicrobial Properties of Poly (Lactic Acid)/Thymol/Nanoclay Composites. Polymers 2020, 12, 1878. [Google Scholar] [CrossRef]
- Gazzotti, S.; Todisco, S.A.; Picozzi, C.; Ortenzi, M.A.; Farina, H.; Lesma, G.; Silvani, A. Eugenol-grafted aliphatic polyesters: Towards inherently antimicrobial PLA-based materials exploiting OCAs chemistry. Eur. Polym. J. 2019, 114, 369–379. [Google Scholar] [CrossRef]
- Chen, F.; Shi, Z.; Neoh, K.G.; Kang, E.T. Antioxidant and antibacterial activities of eugenol and carvacrol-grafted chitosan nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Husárová, L.; Pekařová, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of important abiotic and biotic factors in the biodegradation of poly(l-lactic acid). Int. J. Biol. Macromol. 2014, 71, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Šerá, J.; Stloukal, P.; Jančová, P.; Verney, V.; Pekařová, S.; Koutný, M. Accelerated Biodegradation of Agriculture Film Based on Aromatic–Aliphatic Copolyester in Soil under Mesophilic Conditions. J. Agric. Food Chem. 2016, 64, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Šerá, J.; Serbruyns, L.; De Wilde, B.; Koutný, M. Accelerated biodegradation testing of slowly degradable polyesters in soil. Polym. Degrad. Stab. 2019, 171, 109031. [Google Scholar] [CrossRef]

| Samples | B. tequilensis (mm) | B. subtilis (mm) | B. pumilus (mm) | S. maltophilia (mm) | E. coli (mm) | S. aureus (mm) |
|---|---|---|---|---|---|---|
| PLA | * | * | * | * | * | * |
| PLA/T | * | * | 7.8 ± 1.2 | 12.5 ± 0.3 | * | 8.0 ± 0.4 |
| PLA/E | 9.5 ± 0.5 | 9.8 ± 0.3 | 7.3 ± 0.5 | 13.3 ± 0.3 | * | 9.0 ± 0.4 |
| PBS | * | * | * | * | * | * |
| PBS/T | 10.0 ± 0.4 | 9.5 ± 0.3 | 7.0 ± 0.4 | * | 10.5 ± 1.2 | 15.8 ± 0.5 |
| PBS/E | 11.8 ± 0.5 | 13.0 ± 0.7 | 12.3 ± 0.9 | 13.0 ± 0.4 | 15.8 ± 1.1 | 17.8 ± 0.5 |
| PBAT | * | * | * | * | * | * |
| PBAT/T | * | 7.3 ± 0.3 | 7.3 ± 0.3 | 6.3 ± 0.3 | 9.3 ± 0.3 | 10.3 ± 0.3 |
| PBAT/E | 7.8 ± 0.5 | 7.5 ± 0.3 | 9.5 ± 0.3 | 10.8 ± 0.3 | 6.3 ± 0.3 | 8.8 ± 0.5 |
| Materials | Methods | B. tequilensis | B. subtilis | B. pumilus | S. maltophilia | E. coli | S. aureus |
|---|---|---|---|---|---|---|---|
| PLA | MTT assay | − | − | − | − | − | − |
| Christensen method | − | − | − | − | − | − | |
| Fluorescence microscopy (LIVE) | +++ | +++ | +++ | + | +++ | +++ | |
| Fluorescence microscopy (DEAD) | + | − | − | + | ++ | ++ | |
| PBS | MTT assay | + | + | + | + | + | + |
| Christensen method | − | − | + | + | − | − | |
| Fluorescence microscopy (LIVE) | − | ++ | − | ++ | − | + | |
| Fluorescence microscopy (DEAD) | ++ | + | ++ | + | +++ | + | |
| PBAT | MTT assay | − | − | − | − | − | − |
| Christensen method | + | + | + | + | + | + | |
| Fluorescence microscopy (LIVE) | + | + | + | + | +++ | + | |
| Fluorescence microscopy (DEAD) | − | − | − | − | +++ | − |
| Active Compounds | PLA (°) | PBS (°) | PBAT (°) |
|---|---|---|---|
| * | 75 ± 4 aA | 74 ± 2 aA | 56 ± 4 aB |
| 3% w/v thymol | 67 ± 3 aAB | 75 ± 4 aA | 60 ± 4 aB |
| 3% w/v eugenol | 66 ± 2 aA | 74 ± 2 aB | 63 ± 3 aA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleva, P.; Bartošová, L.; Máčalová, D.; Zálešáková, L.; Sedlaříková, J.; Janalíková, M. Biofilm Formation Reduction by Eugenol and Thymol on Biodegradable Food Packaging Material. Foods 2022, 11, 2. https://doi.org/10.3390/foods11010002
Pleva P, Bartošová L, Máčalová D, Zálešáková L, Sedlaříková J, Janalíková M. Biofilm Formation Reduction by Eugenol and Thymol on Biodegradable Food Packaging Material. Foods. 2022; 11(1):2. https://doi.org/10.3390/foods11010002
Chicago/Turabian StylePleva, Pavel, Lucie Bartošová, Daniela Máčalová, Ludmila Zálešáková, Jana Sedlaříková, and Magda Janalíková. 2022. "Biofilm Formation Reduction by Eugenol and Thymol on Biodegradable Food Packaging Material" Foods 11, no. 1: 2. https://doi.org/10.3390/foods11010002
APA StylePleva, P., Bartošová, L., Máčalová, D., Zálešáková, L., Sedlaříková, J., & Janalíková, M. (2022). Biofilm Formation Reduction by Eugenol and Thymol on Biodegradable Food Packaging Material. Foods, 11(1), 2. https://doi.org/10.3390/foods11010002






