3.1. Terpenoid Fraction
Analysis of the terpenoid fraction isolated from the European species
I. aquifolium revealed the presence of 14 compounds (a table with all compounds is available in the
Supplementary Data), of which the 10 main ones are presented in
Table 3. These include α-amyrin (2.43–4.89 mg/g), lupeol (2.77–1.20 mg/g), betulinic alcohol (2.19–0.99 mg/g), β-amyrin (1.79–0.93 mg/g), ursolic aldehyde (1.05–0.32 mg/g), and ursolic acid (0.84–0.36 mg/g). For comparison, the table also shows the results of triterpenes identified in
I. paraguariensis, showing the similarity of the Argentine
Ilex variety with the European one. In contrast, lower amounts of uvaol, betulinic acid, and oleanic acid, as well as higher contents of lupeol and ursolic aldehyde, were observed in
I. aquifolium, as compared to
I. paraguariensis.
The compounds with biological activity from the triterpene group have also been identified in other
Ilex cultivars. In the leaves of
I. cornuta lupeol, betulonic acid, uvaol, ursolic acid, and α-amyrin were identified. In
I. centrochinensis, the presence of lupeol and oleanic acid was revealed. Equally, lupeol, betulinic alcohol, ursolic acid and uvaol were also identified in the Chinese variety
I. latifolia [
48]. In addition, lupeol and betulin isolated from the leaves of
I. macropoda have been shown to inhibit the activity of a human acyltransferase enzyme involved in cholesterol metabolism [
55]. This evidence suggests the similarity of
Ilex cultivars among plants found in different parts of the world, which may also indicate similar biological properties. With regard to the biological properties of the terpenoid fraction, analogous compounds have already been identified in earlier studies that focused on medicinal plants. Investigations carried out on dandelion leaves have revealed comparable contents of components such as β-sitosterol, α-amyrin, and β-amyrin [
49], where in the leaves of
Ilex species, the amounts of these same components decreased as follows: α-amyrin, β-amyrin, and β-sitosterol. Siddiqui et al. [
50] identified the presence of bioactive terpenoids in the hibiscus plant; however, the authors focused on validating a method to determine oleanolic acid and β-amyrin in different fractions. In sage leaves, the representative triterpenoids are pentacyclic triterpene acids such as oleanic acid, ursolic acid, and betulinic acid [
56]. Niżinski et al. [
57] studied marigold leaves and identified the presence of α-amyrin, β-amyrin, and lupeol, whereas no oleanic acid was identified. Amyrin-rich raw materials include several herbs, i.e.,
Byrsonima crassifolia (0.9 mg/g), Mexican copal (0.5 mg/g), and
Amphipterygium adstringens (2.4 mg/g) [
49]. Amyrine fractions, as well as the pure compounds, possess proven antihyperglycemic and hypolipidemic activity. A dose of 10 mg/kg b.w. α-amyrines and β-amyrines, isolated from
Protium heptaphyllum significantly decreased TC and TG in serum [
58,
59].
3.2. Phenolic Acid Fractions
The phenolic acid profile of European
I. aquifolium showed 31 phenolic acids, of which eight of the major acids are summarized in
Table 4 (a table with all compounds is available in the
Supplementary Data). In
Table 4, caffeic acid (2.88–1.64 mg/g) is the main representative, followed by malic acid (0.55–0.07 mg/g), quinic acid (0.22–0.01 mg/g), and ferulic acid (0.20–0.06 mg/g). For comparison, the phenolic acid profile of
I. paraguariensis was also studied, thus proving the similarity in the phenolic acid profile of the Argentine variety with that of the European variety. Lower amounts of 3-O-Coumaroyl-D-quinic acid, 4-O-Caffeylquinic acid, and chlorogenic acid were observed in the
I. aquifolium cultivars, as compared to
I. paraguariensis, as well as higher amounts of quinic acid and malic acid.
Phenolic acids, known for their antioxidant properties, are also a widely recognized group of active substances. Bojić et al. [
30] identified the following bioactive compounds in
I. paraguariensis, such as chlorogenic acid (2.1 mg/g) and caffeic acid (1.5 mg/g). In the same plant, Vieira et al. [
60] also confirmed the presence of 4,5-di-O-caffeylquinic acid, chlorogenic acid, gallic acid, p-coumaric acid, caffeic acid, and ferulic acid. In other
Ilex species, researchers have identified the presence of phenolic acids. Wang et al. [
61] demonstrated the presence of hydroquinone, protocatechualdehyde, dihydroxyacetophenone, and 4,5-di-O-caffeylquinic acid in
I. pubescens. In
I. asprella, He et al. [
62] identified caffeic acid and coniferyl aldehyde. Phenolic acids possessing antioxidant activity have been characterized in many plants used in medicine and as ingredients to improve food quality. Wojdyło et al. [
63] reported that in
Echinacea purpurea leaves, caffeic acid (6.2 mg/g), neochlorogenic acid (1.15 mg/g), p-coumaric acid (0.20 mg/g), and ferulic acid (0.19 mg/g) were identified, and in
Tanacetum vulgare leaves, the presence of caffeic acid (8.94 mg/g), neochlorogenic acid (3.35 mg/g), and ferulic acid (0.09 mg/g) were confirmed. In other studies, Arceusz and Wesolowski [
64] showed the content of phenolic acids existing in
Melissa officinalis from different manufacturers, in which rosemarinic acid (48.6 mg/g) was found in the highest amount, followed by ferulic acid (1.59 mg/g), caffeic acid (0.71 mg/g), syringic acid (0.53 mg/g), chlorogenic acid (0.33 mg/g), and gallic acid (0.67 mg/g).
3.4. Principal Component Analysis
To summarize and visualize the quantitative differences between the samples, PCA analysis was performed. This method allows for finding the correlated variables in the dataspace (if such variables are indeed present) and combining them into a smaller number of new variables (principal components, PC), which makes it easier for graphical presentation. The analysis was carried out using the quantitative results presented in
Table 2,
Table 3 and
Table 4, which were found to be easily compressible; the first two PCs explained almost 90% of the variance present in the data.
The first PC explained 69.90% of the variance and allowed discrimination between the
Ilex species. The sample of
I. paraguariensis had a negative PC 1 score, whereas all three samples of
I. aquifolium were located on the positive side of PC 1 (
Figure 1A). The loading plot for the variables (
Figure 1B) indicated the constituents, of which the content is responsible for the discrimination. The results were in agreement with the trends noted for particular groups of chemicals. Among the triterpenoids, the higher contents of β-amyrin, α-amyrin, ursolic aldehyde, and lupeol were to the largest extent connected with positive PC 1 scores of the
I. aquifolium samples. On the other hand, oleanolic acid, uvaol, betulinic acid, and betulinic alcohol had strong negative loading on PC 1; thus, higher levels of those constituents were partly responsible for the low PC 1 score of
I. paraguariensis. The PC 1 score was also positively impacted by a higher content of quinic acid but at the same time lower by the content of its derivatives, i.e., chlorogenic acid, 4-caffeoylquinic acid, and coumaroyl quinic acid. This profile was indeed characteristic of all samples of
I. aquifolium. Moreover, higher levels of citric acid and ferulic acid loaded negatively on PC 1. Among sugars, cellobiose and maltose isomers that were present only in
I. aquifolium samples had high positive loading on PC1, whereas glucose isomers, fructose, and mannose isomer 1 influenced that component negatively.
PC 2 explained 19.87% of the variance. The distribution along the PC 2 axis was visible among the samples of different varieties of
I. aquifolium (
Figure 1A) and was connected mainly with differences in the content of ursolic acid, caffeic acid, sucrose, gentiobiose, and mannose isomer 2 (
Figure 1B). The high content of those constituents had a negative loading on PC 2; thus,
I. aquifolium Ferox Argentea, with a high PC 2 score, is characterized by the lowest levels of most of those chemicals.
Overall, the results of the analysis indicated some interesting trends that might be important from a chemotaxonomic point of view; however, further studies on a larger number of samples are required to investigate that topic more thoroughly.
3.5. Caffeine and Theobromine
In the present study, the content of caffeine and theobromine was also analyzed by means of GC-MS and LC-MS in Argentine and European varieties of
Ilex. The presence of caffeine and theobromine was confirmed only in the case of
I. paraguariensis, since in the European varieties of
I. aquifolium the concentration of caffeine, as well as theobromine, was below the detection levels of the equipment used (
Table 6). GC-MS analysis showed that caffeine and theobromine concentrations in
I. paraguariensis leaves were 6.21 mg/g and 1.01 mg/g, respectively, whereas LC-MS analysis showed similar results, with caffeine at 7.77 mg/g and theobromine at 1.38 mg/g.
An important substance from the purine alkaloid family is caffeine, which stimulates the nervous system and supports the body in the process of lipolysis. In earlier work, researchers investigated the presence of caffeine only in some species of
Ilex. The caffeine concentration in
I. aquifolium was not investigated in previous reports. Claudia Anesini et al. [
67] reported that the caffeine concentration in commercial mate tea is 13.5 mg/g. Furthermore, Kaltbach et al. [
68] analyzed 19 different matte teas for caffeine concentrations and obtained results ranging from 5.63–17.57 mg/g. Three species of
Ilex were subjected to tests for caffeine by Negrin et al. [
8]. They obtained results for
Ilex paraguariensis (4.15–11.86 mg/g), Amazonian
Ilex guayusa (19.11–26.94 mg/g), and
Ilex vomitoria (0.004–8.44 mg/g). Despite the lack of caffeine content in the leaves of
Ilex aquifolium, the biological effects of the derived extracts were confirmed, which differs significantly from the findings of Zapata et al., who reported that only the caffeine content of
I. paraguariensis leaves inhibits lipogenesis and fat accumulation and was responsible for the expression of the fatty acid synthase (FASN) and the microsomal TG transfer protein. When Zapata et al. administered the decaffeinated extract of
I. paraguariensis in a Sprague Dawley rat model, no biological effect was observed [
69].
3.6. Hematological and Biochemical Parameters of Blood
Prior to the study, the following groups of rats were selected to monitor blood biochemical parameters: a control group fed a standard diet without any additions (CON), a group fed a high-cholesterol diet (CHOL), a group fed a standard diet with the addition of an aqueous extract of I. aquifolium (ILEX), a group fed a standard diet with the addition of terpenoid fractions (TERP), and a group fed a high-cholesterol diet with the addition of terpenoid fractions (TERPCHOL).
The mean body weight gain of rats fed the cholesterol-supplemented diet was significantly higher (
p < 0.05) than that of the rats fed a standard diet and was 86.25 ± 6.28 g for the CON group and 97.64 ± 8.62 g for the CHOL group. It was lower for the ILEX group at 77.05 ± 4.08 g, the TERP group at 79.63 ± 5.40 g, and the TERPCHOL group at 92.12 ± 4.58 g. A study by Lima et al. [
53] showed that the use of Yerba mate prevented the development of hyperphagia, obesity, visceral obesity, and central leptin resistance in obese rats with early weaning. Caffeine of natural (i.e., mate and coffee) and synthetic origin promoted a reduction in fat accumulation in animals fed a high-fat, sucrose-rich diet [
69]. Considering the results of our own study, the aqueous extract of
I. aquifolium and the terpenoids extracted from it could be considered agents that decrease body weight gain.
Hematological examinations are a basic indicator of metabolism and hematopoiesis. The hematological values found in the control group were in the range of values reported by other authors [
70]. The administration of terpenoids in the TERPCHOL group resulted in a significant increase (
p < 0.05) in WBC values, as compared to the control group. A similar increase (
p < 0.05) was found after treatment with
I. aquifolium (
Table 7 and
Figure 2). In contrast, there was a decrease in WBCs in the CHOL group. The supplementation, both in the ILEX and the TERPCHOL groups, caused a significant increase (
p < 0.05) in RBCs; however, such changes did not occur after the application of terpenoids. In addition, the hemoglobin concentration was the highest in the ILEX and TERPCHOL groups. The HCT value was highest (
p < 0.05) in the TERPCHOL group.
I. aquifolium supplementation increased PLT, but these changes were not statistically confirmed.
The hypercholesterolemic diet resulted in an increase in cholesterol, LDL fraction, TG, glucose, and insulin in the CHOL and TERPCHOL groups, as compared to the other groups (
Table 8 and
Figure 3). Changes in lipid parameters after treatment with terpenoids extracted from
I. aquifolium were small, and the levels found were similar to those that were found in the control group. Supplementation with
I. aquifolium resulted in a reduction in cholesterol and LDL fraction in the ILEX group, as compared to the CHOL and CON groups. The use of terpenoids reduced the serum LDL fraction, whereas a marked (
p < 0.05) increase in TG and enhanced lipolysis was observed in the TERPCHOL group. From the perspective of the functional properties of the
Ilex species, caffeoylquinone ester and triterpenoid saponin fractions may play a role in lowering low-density lipoprotein (LDL) levels in the blood [
56,
57]. An interesting relationship was observed in a study conducted by Zapata et al. [
69] regarding the efficacy of phenolic compounds, specifically caffeine and caffeoylquinic acids contained in Yerba mate. The experiment involved a comparison between the isolated caffeine from mate, the whole extract containing a fraction of phenolic compounds, and the same extract devoid of caffeine. The results showed reduced in vitro lipid accumulation in rat tissues. This effect was assigned to the modulation of the FASN fatty acid synthase in 3T3-L1 adipocytes, leading to weight loss and liver fat accumulation in rats fed a high-fat, high-sucrose diet (HFSD). Preliminary in silico studies (molecular docking tests) of these substances were also performed in the context of finding potential ligands that could interact with enzymes involved in lipogenesis, as well as in lipid metabolism [
71]. The administered extracts or active compounds caused an increase in blood insulin levels. There was marked hyperinsulinemia in the CHOL group, as compared to the control group (201.33 pg/mL vs. 56.67 pg/mL, respectively).
I. aquifolium terpenoids caused a smaller increase in serum insulin concentration in the ILEX group, as compared to the CON group. The use of terpenoids with a hypercholesterolemic diet reduced the increase in blood insulin concentrations in the TERPCHOL group, indicating the effective prevention of hyperinsulinemia even when the dietary cholesterol content was high.
Feeding cholesterol to the rats resulted in an increase (p < 0.05) in AST and ALT activity, as compared to the control group. In addition, a similar increase occurred after supplementation with I. aquifolium and terpenoids in the ILEX and TERP groups. However, AST activity decreased (p < 0.05) with the hypercholesterolemic diet. The TERPCHOL group also had the lowest creatinine concentrations. The concentrations of TP and Alb were lower in the experimental groups, as compared to the control group.
The daily dose of cholesterol used in our study resulted in moderate hypercholesterolemia. This is supported by [
72], where similar levels of total blood cholesterol were reported with a hypercholesterolemic diet. There are a number of methods to lower cholesterol, one of which is to adjust the proportion of protein and fiber in the diet, as well as the use of probiotics or phytobiotics [
73,
74,
75]. Hypercholesterolemic rats are characterized by low LDLR gene expression and high SREBP-1c protein expression in the liver [
76]. An increase in plasma FFA is one of the strongest signals stimulating the liver to synthesize triglycerides and other lipids [
77]. These processes are associated with a decrease in fat mass. In our study, these relationships were also present in the CHOL group pups. The use of terpenoids with the hypercholesterolemic diet reduced the intensity of the lipolysis. In a previous study, a high-fat diet and supplementation with Yerba mate extracts from
I. paraguariensis St. Hil. in rats prevented endothelial dysfunction by increasing nitric oxide production and regulating the expression of genes responsible for lipid metabolism [
78]. Other studies have found no reduction in serum lipids with the consumption of Yerba mate (
I. paraguariensis), polyphenols, or saponins from mate [
79]. Zapata et al. [
69] indicated that caffeine had been responsible for the effects of Yerba mate tea on adipogenesis and lipogenesis. However, no differences were found between total cholesterol and triglyceride levels among the groups used in that study. However, the literature indicates that differences in the preparations, doses, or timing of
Ilex spp. aqueous extracts and their administration may be responsible for various effects on the metabolic response [
78,
79]. In our study, the use of the
I. aquifolium extract lowered blood cholesterol levels, reduced lipolysis, and had a beneficial effect on glucose and insulin levels. Moreover, in other studies in which mate tea was used, it prevented an increase in insulin and decreased HDL cholesterol [
80].
In recent years, a number of plants and plant extracts have been used in the prevention or treatment of diseases of civilization. Plant extracts contain large amounts of antioxidants that can play an important role in adsorbing and neutralizing free radicals, quenching oxygen, or decomposing peroxides [
81,
82]. Plants rich in flavonoids (e.g., ginger, turmeric, anise, coriander, and green tea), as well as anthocyanins, have antioxidant activity [
83]. The composition of the active compounds depends on the type of plant. In an extract from
Rubia tinctorum L., the antioxidant activity may be due to vanillin, rosmarinic acid, quercetin, catechin, syringic acid, or cinnamic acid [
82]. In studies conducted in animal models involving obesity-inducing diets and associated insulin resistance, supplementation with Yerba extracts was shown to alleviate hyperglycemia and improve insulin sensitivity and plasma lipids [
84].
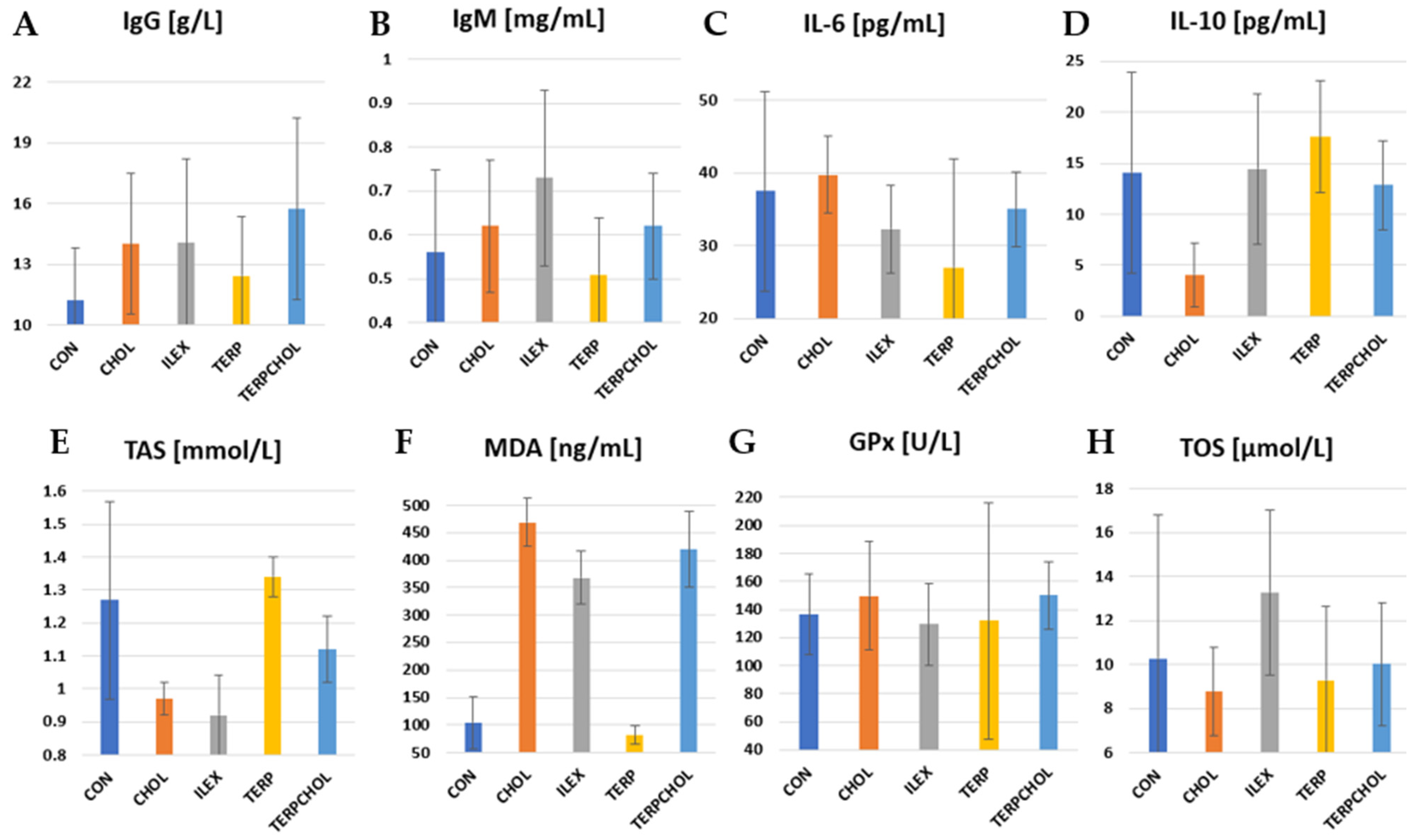
The mean values of immunological parameters and selected parameters of antioxidant status are summarized in
Table 9 and in
Figure 4. IL-10 is recognized as an anti-inflammatory cytokine. It inhibits the production of pro-inflammatory cytokines such as IL-2, IL-3, and TNF-α. There was a significant (
p < 0.05) reduction in IL-10 levels in the CHOL-treated group compared to the other groups. Terpenoids caused an increase in the value of this parameter, whereas in the ILEX group, the concentration of IL-10 was similar to that found in the CON group. The lowest TOS value was recorded in the CHOL group, whereas the highest value was recorded in the ILEX group. The application of terpenoids with a hypercholesterolemic diet resulted in an increase in TOS values in the TERPCHOL group, as compared to the CHOL group.
I. aquifolium had no effect on the blood concentrations of GPx, whereas the administration of cholesterol only or cholesterol together with the terpenoid fraction increased GPx concentrations in the CHOL and TERPCHOL groups. However, these changes were not statistically confirmed. The hypercholesterolemic diet had no significant effect on the blood concentration of IL-6. The greatest reduction in IL-6 was found after treatment with the terpenoid fraction, as well as a similar but slightly weaker effect from supplementation with
I. aquifolium. The increase (
p < 0.05) in MDA levels in the CHOL group indicated that oxidative stress occurred sufficiently to cause free radical-mediated lipid peroxidation in the cell membrane. A marked reduction (
p < 0.05) in MDA was caused by terpenoid supplementation; however, the terpenoid fraction with the hypercholesterolemic diet did not reduce the increase in the MDA concentration. The use of
I. aquifolium had no beneficial effect on MDA concentrations. The greatest increase in IgG was found in the TERPCHOL group. The IgM concentration was statistically equal (
p < 0.05) between the ILEX and TERP groups.
Hypercholesterolemia is a risk factor of cardiovascular disease (CVD), type 2 diabetes mellitus, and metabolic syndrome [
85]. Furthermore, some oxidative stress markers such as MDA increase in degenerative diseases such as diabetes mellitus [
86]. In our study, TOS, GPx, and MDA levels were reduced after supplementation with terpenoids alone. Surprisingly,
Ilex extract increased the level of serum MDA. This could be caused by other compounds, which have not been investigated in our experiment. An increasing liver MDA concentration during the administration of
I. paraguariensis water extract was also observed by Bravo [
87].
However, long-term hypercholesterolemia cannot be alleviated by the effects of terpenoids. The efficacy of these compounds has only been demonstrated in animals without metabolic disorders. In studies using hypercholesterolemic rats consuming a high-cholesterol diet, mate consumption had no effect on HDL-cholesterol or protein carbonyls, yet it showed a marked hypolipidemic action by decreasing TG, total and LDL-cholesterol, and serum MDA levels [
87]. Furthermore,
I. paraguariensis has been shown to be capable of minimizing oxidative stress during perimenopause by modulating antioxidant defense [
75]. Bassalat et al. [
88] showed that
T. leucocladum manifested antihyperglycemic and antihyperlipidemic effects and also increased the antioxidative defense system and reduced the lipid peroxidation process in experimental diabetic rats. Recent research indicates that mate from
I. paraguariensis has a direct action on ROS accumulation and activates various molecules involved in antioxidant responses such as p-CREB, NRF2, SIRT1, and SOD2 [
89]. The current study showed that high concentrations of polyphenols from mate may be useful for the prevention of retinal damage in AMD and other retinal degenerations in which oxidative stress has been a pathogenic mechanism.
3.8. Histopathological Examination of Tissue
The liver is the most important organ, and it predominantly controls the metabolism of endogenous and exogenous agents, as well as glucose and lipid metabolisms. As a result, many substances can react with the basic cellular components and, consequently, induce many types of liver damage and dysfunction [
99,
100]. In our research, the histological investigation of the rat livers (
Figure 5) in the control group showed a normal liver architecture, with hepatocytes containing a single, centrally located nucleus. The hepatocytes were characterized by individual cytoplasmic fat vacuoles with no sign of inflammation, necrosis, or cholestasis. In the groups of rats fed a high-cholesterol diet, the histological examinations of the liver sections demonstrated steatosis and minor lymphocytic infiltration around the liver vessels and enlarged portal-bile spaces. Some of the hepatocytes showed changes in cell nuclei, including pyknotic nuclei, a reduced form of the nucleolus, and the presence of more heterochromatin. The presence of numerous binuclear hepatocytes may indicate an intensifying process of cell proliferation in response to damage. The liver structures of the ILEX rats demonstrated an enlargement of the blood vessels, mainly the central veins, with no signs of increased cellular blood components within the sinusoids or the normal portal bile spaces or of steatosis changes in hepatocytes due to increased lipid storage. In rats supplemented with terpenoid fractions, the hepatocytes featured swelling and blood stagnation in both arterial and venous vessels, with no perivascular infiltration visible. The architecture of the lobuli was disturbed. The sinusoids were poorly visible in contrast to the portal-bile spaces. Numerous hepatocyte changes within the cytoplasm were observed, including an increased volume of cells and/or pycnotic nuclei and the cytoplasm itself was translucent, without any granules or solid material. In the livers of the TERPCHOL group, small perivascular lymphocytic infiltrates were present. The sinusoids were clearly visible, whereas the biliary canaliculi were poorly visible. The structures of the cell nuclei and the cytoplasm were similar to the TERP group, except for the increase in the content of fat and water vacuoles. The level of liver damage and the number of bi-nucleated or multinucleated hepatocytes decreased, suggesting that the overall damage had been lessened.
Ilex fractions show the potential for liver protection [
101,
102], and the consumption of the
Ilex extract may be used as a preventative step for hypercholesterolemia [
97,
103]. Similarly, terpenoids have been shown to have a protective effect in the liver and may have potential in the treatment of liver diseases [
104]. Terpenoids have been applied as activators of peroxisome proliferator-activated receptors (PPAR) and may decrease the risk of obesity-associated disorders [
97,
105,
106]. In our study, histopathological assessments of the liver sections from the control group showed normal hepatic tissues, whereas steatosis was observed in animals fed a high-cholesterol diet. Cholesterol and LDL levels, as well as AST and ALT levels, are important indicators of liver function, and we found that AST and ALT levels were elevated in rats fed a high-cholesterol diet. Histologically, we observed liver steatosis and small leukocyte infiltrates in these animals, which indicated metabolic disruption, as compared to the control group. The inflammatory infiltrates were visible in every group receiving the high-cholesterol diet. In this study,
Ilex extracts reduced the fatty liver changes in animals fed a high-cholesterol diet. This affirmed the results of previous studies, in which
Ilex extracts were shown to decrease the differentiation of pre-adipocytes and to reduce the accumulation of lipids in adipocytes and overall inflammation, as well as suppressing adipocyte differentiation and triglyceride accumulation [
102]. As compared to other studies [
103,
104], the animals treated with terpenoids showed prominent changes that are typical of liver damage, including higher rates of hepatocyte swelling and hepatocyte vacuolization, as well as the shifting of nuclei to the cellular periphery and cytoplasmic vacuolization. In addition, our research did not show that proliferative processes were intensified, which may indicate that terpenoids have an inhibitory effect on the proliferation of hepatocytes.