Antibacterial Mechanism of 3-Carene against the Meat Spoilage Bacterium Pseudomonas lundensis and Its Application in Pork during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Chemicals
2.2. Determination of MIC
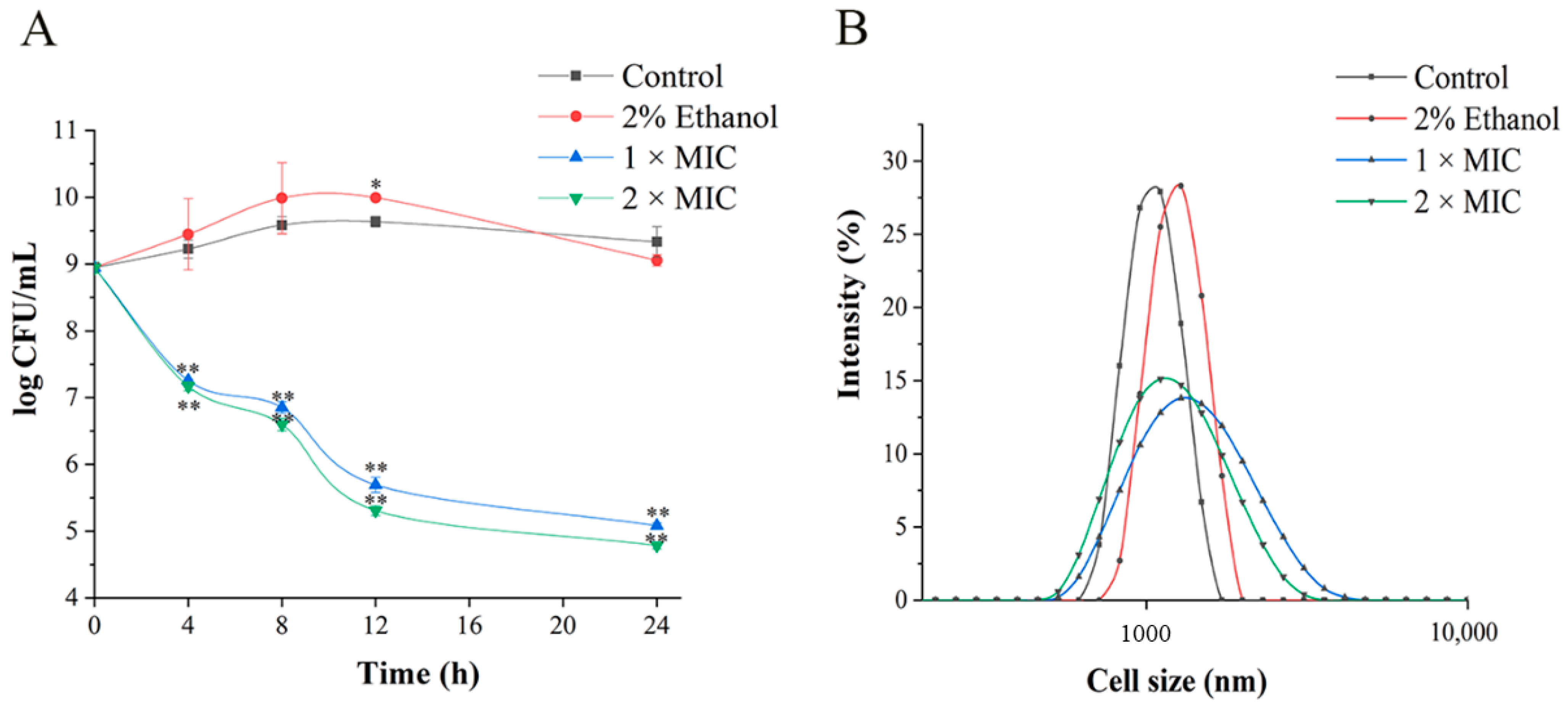
2.3. Time–Kill Assay
2.4. Cell Size Assay
2.5. Morphological Changes
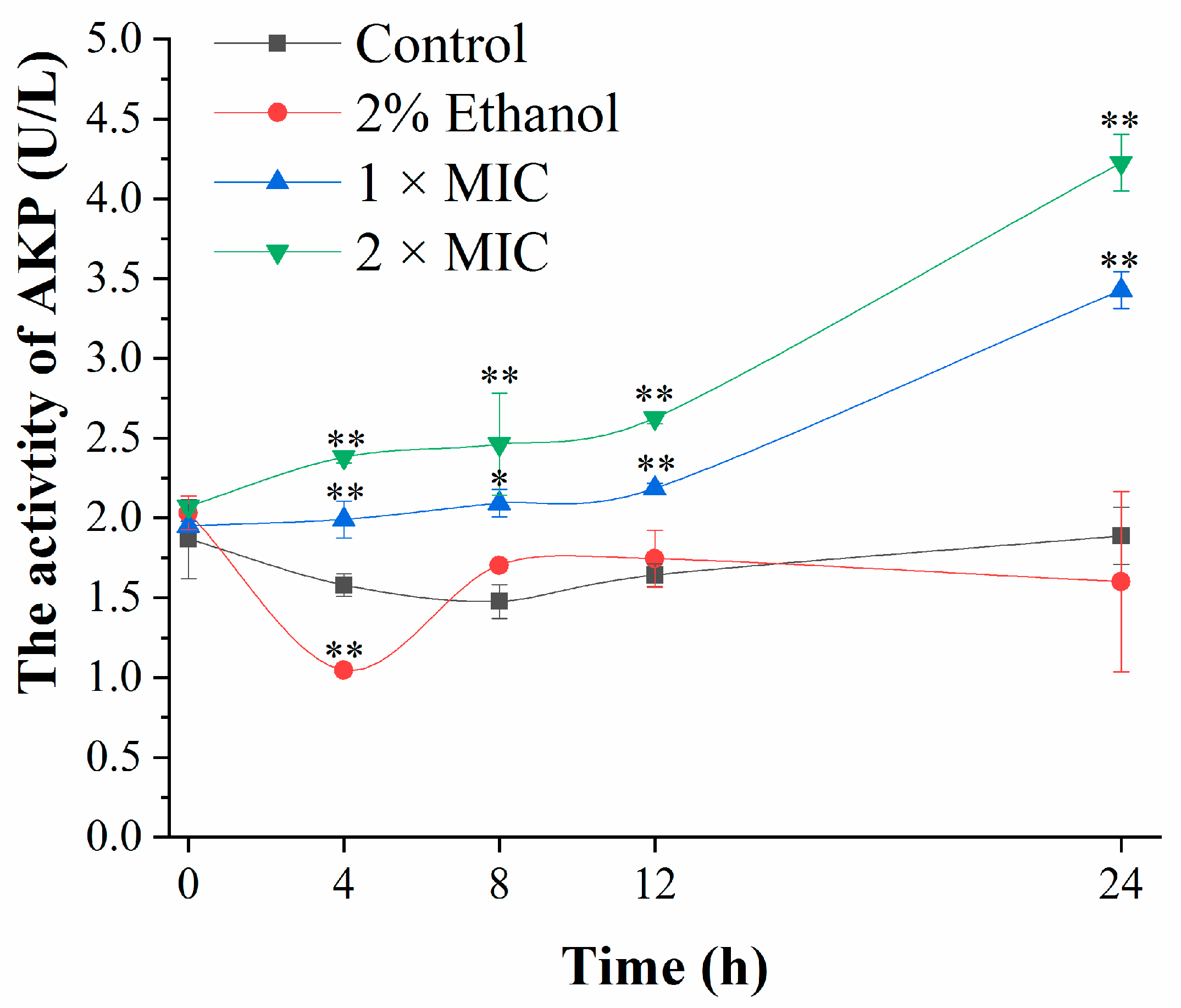
2.6. Determination of Cell Wall Integrity
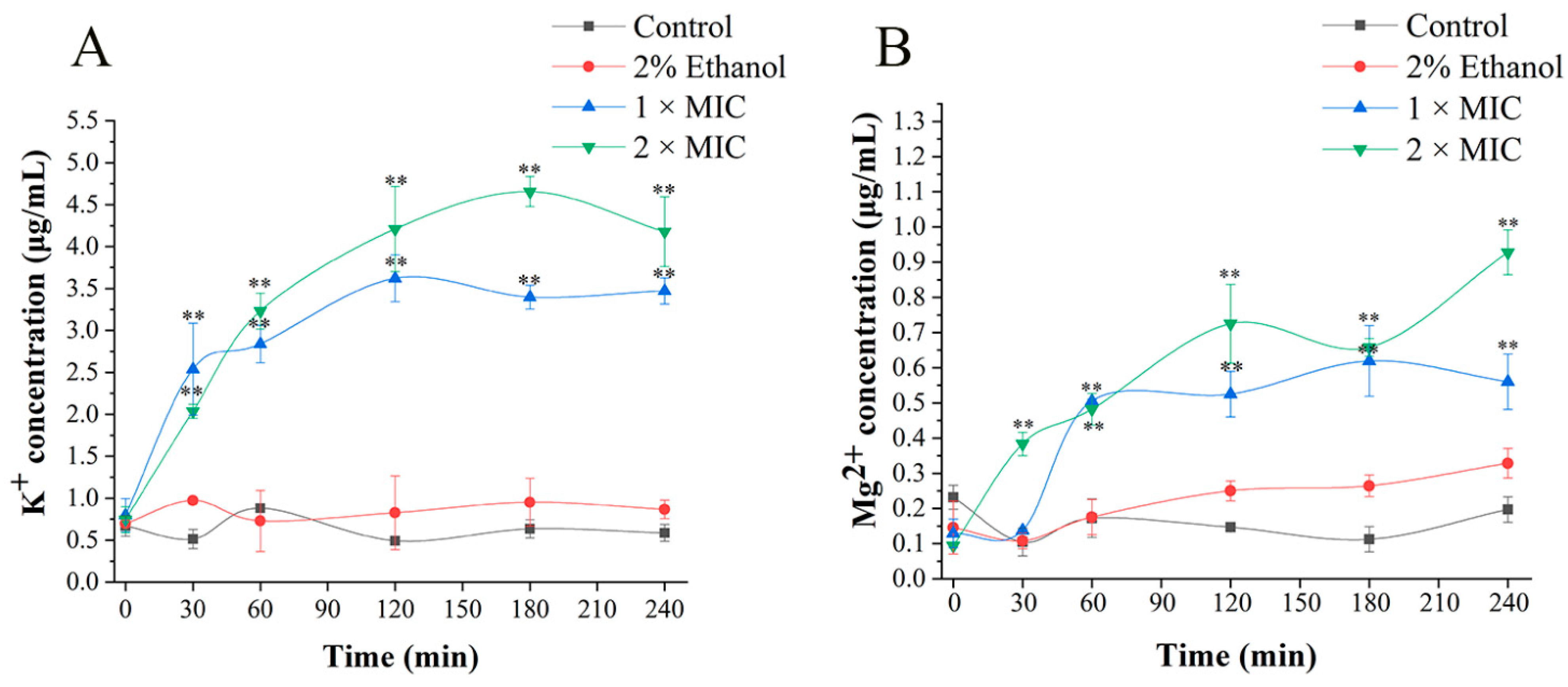
2.7. Ions Release Assay
2.8. Protein and Nucleic Acid Release Assay
2.9. ATPase Activity and ATP Content
2.10. Oxidative Respiratory Metabolism Assay
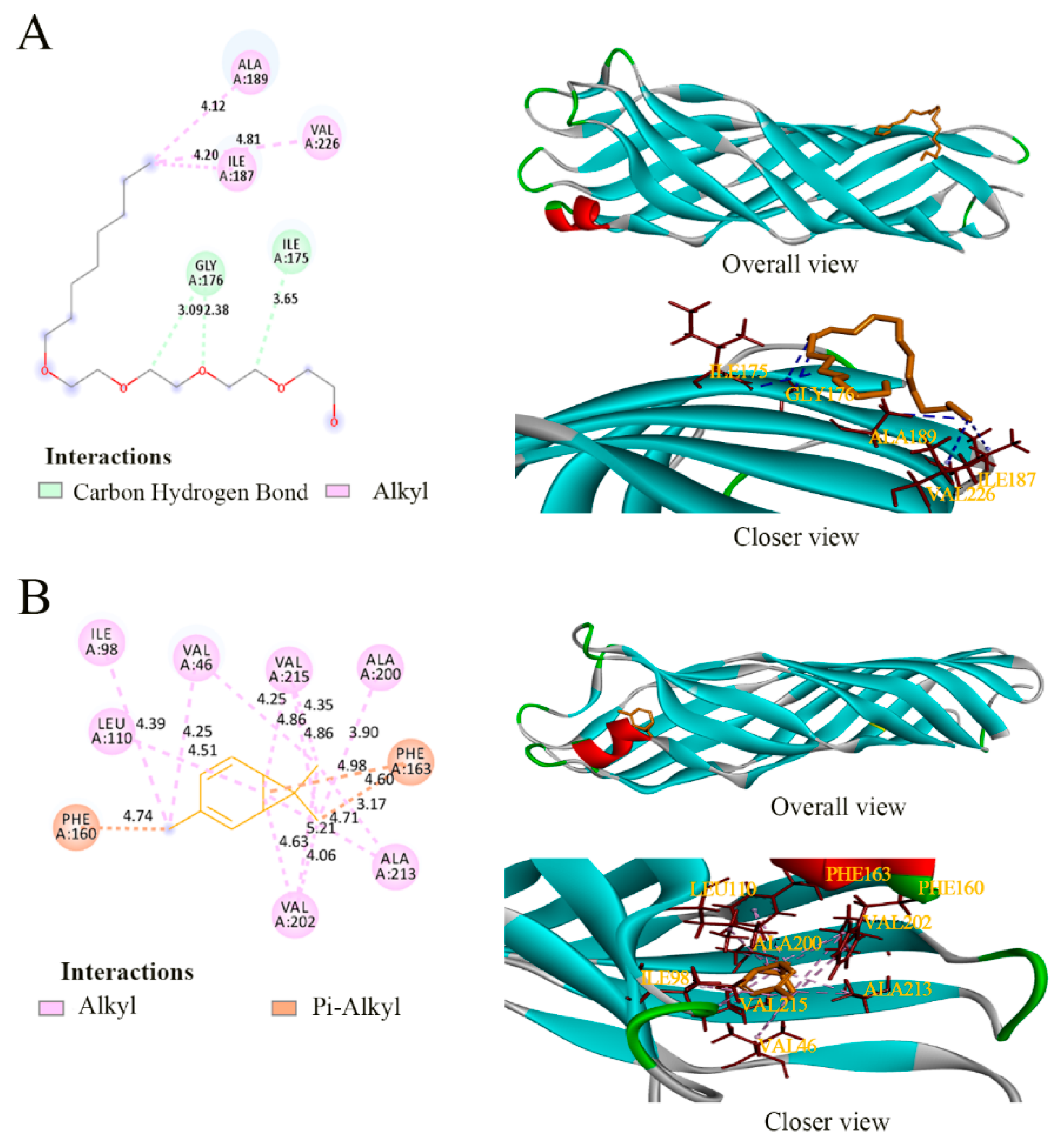
2.11. Molecular Docking of 3-Carene with Three Target Proteins (MurA, OmpW and AtpD)
2.12. Application of 3-Carene as an Antimicrobial Marinade in Pork
2.13. Statistical Analysis
3. Results and Discussion
3.1. Antibacterial Activities Analysis
3.2. Destruction Effect of 3-Carene on the Structure of P. lundensis
3.3. Cell Wall Damage Analysis
3.4. Cell Membrane Damage Caused by 3-Carene
3.5. ATPase (Ca2+-Mg2+-ATPase, Na+-K+-ATPase) Activity and ATP Content
3.6. Oxidative Respiratory Metabolism Characteristics
3.7. Molecular Docking
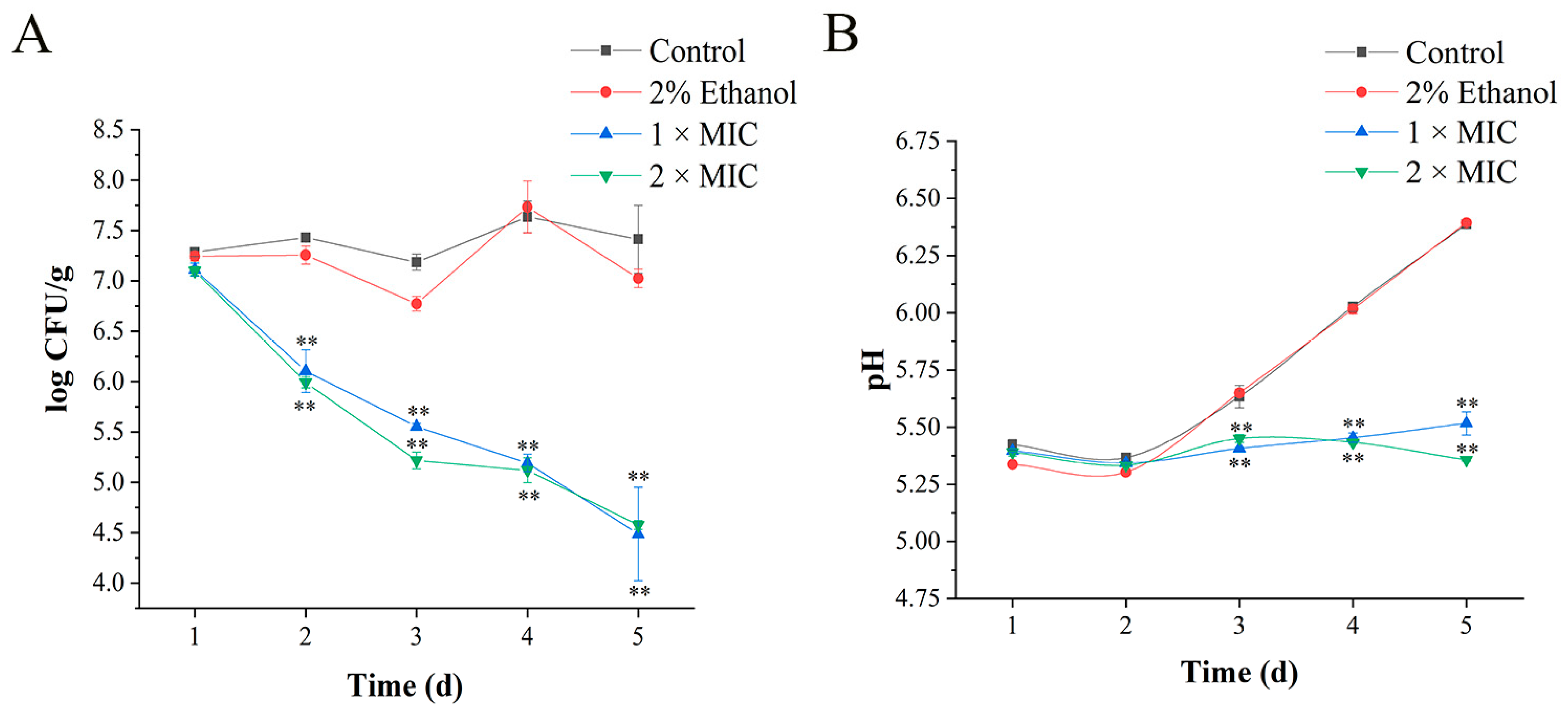
3.8. Application of 3-Carene in Pork
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lahmar, A.; Morcuende, D.; Andrade, M.J.; Chekir-Ghedira, L.; Estévez, M. Prolonging shelf life of lamb cutlets packed under high-oxygen modified atmosphere by spraying essential oils from North-African plants. Meat Sci. 2018, 139, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The antimicrobial effect of thyme essential oil, nisin and their combination against Escherichia coli O157H7 in minced beef during refrigerated storage. Meat Sci. 2008, 80, 159–166. [Google Scholar] [CrossRef]
- Remenant, B.; Jaffrès, E.; Dousset, X.; Pilet, M.F.; Zagorec, M. Bacterial spoilers of food, behavior, fitness and functional properties. Food Microbiol. 2015, 45, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Belák, Á.; Kovács, M.; Hermann, Z.; Holczman, Á.; Márta, D.; Stojakovič, S.; Bajcsi, N.; Maráz, A. Molecular analysis of poultry meat spoiling microbiota and heterogeneity of their proteolytic and lipolytic enzyme activities. Acta Alimet. 2011, 40, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Singh, T.; Tamplin, M.; Chandry, P.S. Genomic and metabolic characterization of spoilage–associated Pseudomonas species. Int. J. Food Microbiol. 2018, 268, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Xie, J.; Zhao, L.J.; Qian, Y.F.; Zhao, Y.; Liu, X. Biofilm Formation Characteristics of Pseudomonas lundensis Isolated from Meat. J. Food Sci. 2015, 80, M2904–M2910. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahoomodally, M.F. A systematic review on black pepper (Piper nigrum L.), from folk uses to pharmacological applications. Crit. Rev. Food Sci. 2019, 59, 210–243. [Google Scholar] [CrossRef]
- Chen, W.X.; Dou, H.G.; Ge, C.; Li, C.F. Comparison of volatile compounds in pepper (Piper Nigrum L.) by simultaneous distillation extraction (SDE) and GC–MS. Adv. Mater. Res. 2011, 1267, 2643–2646. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Pan, S.; Chen, L.; Liu, M.; Yang, K.; Zeng, X.; Tian, T. Analysis of chemical components and biological activities of essential oils from black and white pepper (Piper nigrum L.) in five provinces of southern China. LWT–Food Sci. Technol. 2020, 117, 108644. [Google Scholar] [CrossRef]
- Gil, M.L.; Jimenez, J.; Ocete, M.A.; Zarzuelo, A.; Cabo, M.M. Comparative study of different essential oils of Bupleurum gibraltaricum Lamarck. Pharmazie 1989, 44, 284–287. [Google Scholar] [PubMed]
- Ocete, M.A.; Risco, S.; Zarzuelo, A.; Jimenez, J. Pharmacological activity of the essential oil of Bupleurum gibraltaricum, anti-inflammatory activity and effects on isolated rat uteri. J. Ethnopharmacol. 1989, 25, 305–313. [Google Scholar] [CrossRef]
- Huynh, T.T.T.; Mai, T.C.; Dang, C.H.; Vo, T.T.T.; Nguyen, D.T.; Dang, V.S.; Duy, V.N.K.; Tran, V.T.; Nguyen, T.D. Influence of extractions on physicochemical characterization and bioactivity of Piper nigrum oils, Study on the non-isothermal decomposition kinetic. Arab. J. Chem. 2020, 13, 7289–7301. [Google Scholar] [CrossRef]
- Peng, L.; Xiong, Y.H.; Wang, M.; Han, M.M.; Cai, W.L.; Li, Z.M. Chemical composition of essential oil in Mosla chinensis Maxim cv. Jiangxiangru and its inhibitory effect on Staphylococcus aureus biofilm formation. Open Life Sci. 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W.; Duval, R.E. Antimicrobial activity and proposed action mechanism of 3-carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246. [Google Scholar] [CrossRef] [Green Version]
- Andleeb, S.; Alsalme, A.; Al-Zaqri, N.; Warad, I.; Alkahtani, J.; Bukhari, S.M. In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. J. K. Saud Univ. Sci. 2020, 32, 2053–2058. [Google Scholar] [CrossRef]
- Lai, C.S.; Ponnusamy, Y.; Lim, G.L.; Ramanathan, S. Antibacterial, antibiofilm and antibiotic-potentiating effects of a polyphenol-rich fraction of dicranopteris linearis (Burm.f.) Underw. J. Herb. Med. 2021, 25, 100419. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Wojciuk, B.; Dogowska, B.; Wajs-Bonikowska, A.; Sienkiewicz, M.; Mężyńska, M.; Łopusiewicz, Ł. The influence of essential oil compounds on antibacterial activity of mupirocin-susceptible and induced low-level mupirocin-resistant MRSA strains. Molecules 2019, 24, 3105. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Qian, C.; Xu, H.; Huang, Y. Antibacterial activity of Artemisia asiatica essential oil against some common respiratory infection causing bacterial strains and its mechanism of action in Haemophilus influenzae. Microb. Pathogenesis 2018, 114, 470–475. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Li, C.; Vittayapadung, S.; Cui, H. Antibacterial mechanism of ε-Poly-lysine against Listeria monocytogenes and its application on cheese. Food Control 2018, 91, 76–84. [Google Scholar] [CrossRef]
- Maragkoudakis, P.A.; Mountzouris, K.C.; Psyrras, D.; Cremonese, S.; Fischer, J.; Cantor, M.D.; Tsakalidou, E. Functional properties of novel protective lactic acid bacteria and application in raw chicken meat against Listeria monocytogenes and Salmonella enteritidis. Int. J. Food Microbiol. 2009, 130, 219–226. [Google Scholar] [CrossRef]
- Chen, B.J.; Jamaludin, N.S.; Khoo, C.H.; See, T.H.; Sim, J.H.; Cheah, Y.K.; Halim, S.N.A.; Seng, H.L.; Tiekink, E.R.T. In vitro antibacterial and time kill evaluation of mononuclear phosphanegold(I) dithiocarbamates. J. Inorg. Biochem. 2016, 163, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, Q.; Chen, H.; Li, J. Antibacterial activity and mechanism of the cell-penetrating peptide CF-14 on the gram-negative bacteria, Escherichia coli. Fish Shellfish Immunol. 2020, 100, 489–495. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Cui, H.; Lin, L. Unraveling the anti-bacterial mechanism of Litsea cubeba essential oil against E. coli O157H7 and its application in vegetable juices. Int. J. Food Microbiol. 2021, 338, 108989. [Google Scholar] [CrossRef]
- Diao, M.; Qi, D.; Xu, M.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C.; Zhao, H. Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control 2018, 85, 339–344. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Beshiru, A.; Igbinosa, I.H. Mechanism of action of secondary metabolites from marine-derived Streptoymces on bacterial isolates by membrane permeability. Microb. Pathogenesis 2020, 149, 104532. [Google Scholar] [CrossRef] [PubMed]
- Naghmouchi, K.; Drider, D.; Kheadr, E.; Lacroix, C.; Prévost, H.; Fliss, I. Multiple characterizations of Listeria monocytogenes sensitive and insensitive variants to divergicin M35, a new pediocin-like bacteriocin. J. Appl. Microbiol. 2006, 100, 29–39. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Q.; Wang, X.; Li, R.; Qiao, H.; Ma, P.; Sun, Q.; Zhang, H. Antibacterial activity of xanthan-oligosaccharide against Staphylococcus aureus via targeting biofilm and cell membrane. Int. J. Biol. Macromol. 2020, 153, 539–544. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Baek, K.H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control 2013, 32, 582–590. [Google Scholar] [CrossRef]
- Moghimi, R.; Ghaderi, L.; Rafati, H.; Aliahmadi, A.; McClements, D.J. Superior antibacterial activity of nanoemulsion of Thymus daenensis essential oil against E. coli. Food Chem. 2016, 194, 410–415. [Google Scholar] [CrossRef]
- Abdulkareem, A.O.; Olafimihan, T.F.; Akinbobola, O.O.; Busari, S.A.; Olatunji, L.A. Effect of untreated pharmaceutical plant effluent on cardiac Na+-K+- ATPase and Ca2+-Mg2+-ATPase activities in mice (Mus Musculus). Toxicol. Rep. 2019, 6, 439–443. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, T.; Wei, C.; Lan, W.; Zhao, Y.; Pan, Y.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Zamaraeva, M.V.; Sabirov, R.Z.; Maeno, E.; Ando-Akatsuka, Y.; Bessonova, S.V.; Okada, Y. Cells die with increased cytosolic ATP during apoptosis, a bioluminescence study with intracellular luciferase. Cell Death Differ. 2005, 12, 1390–1397. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Stratford, J.P.; Edwards, C.; Ghanshyam, M.J.; Malyshev, D.; Delise, M.A.; Hayashi, Y.; Asally, M. Electrically induced bacterial membrane-potential dynamics correspond to cellular proliferation capacity. Proc. Natl. Acad. Sci. USA 2019, 116, 9552–9557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.X.; Piao, X.M.; Li, J.; Jin, M.Y.; Jun, J. Extract from Oenothera biennis L. stalks efficiently inhibits Staphylococcus aureus activity by affecting cell permeability and respiratory metabolism. Ind. Crops Prod. 2020, 158, 112961. [Google Scholar] [CrossRef]
- Skarzynski, T.; Mistry, A.; Wonacott, A.; Hutchinson, S.E.; Kelly, V.A.; Duncan, K. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure 1996, 4, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Hrast, M.; Rožman, K.; Jukič, M.; Patin, D.; Gobec, S.; Sova, M. Synthesis and structure-activity relationship study of novel quinazolinone-based inhibitors of MurA. Bioorg. Med. Chem. Lett. 2017, 27, 3529–3533. [Google Scholar] [CrossRef]
- Nandi, B.; Nandy, R.K.; Sarkar, A.; Ghose, A.C. Structural features, properties and regulation of the outer-membrane protein W (OmpW) of Vibrio cholerae. Microbiology 2005, 151, 2975–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soojhawon, I.; Pattabiraman, N.; Tsang, A.; Roth, A.L.; Kang, E.; Noble, S.M. Discovery of novel inhibitors of multidrug-resistant Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2017, 25, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Varsha, P.; Vikas, J. Insights into the physiology and metabolism of a mycobacterial cell in an energy-compromised state. J. Bacteriol. 2019, 201. [Google Scholar]
- Eschenburg, S.; Priestman, M.; Schönbrunn, E. Evidence that the fosfomycin target Cys115 in UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is essential for product release. J. Biol. Chem. 2005, 280, 3757–3763. [Google Scholar] [CrossRef] [Green Version]
- Amit, S.; Harish, S.; Rohit, S.; Jupitara, K.; Tripti, P.; Timir, T. UDP-N-Acetylglucosamine enolpyruvyl transferase (MurA) of Acinetobacter baumannii (AbMurA), Structural and functional properties. Int. J. Biol. Macromol. 2017, 97, 106–114. [Google Scholar]
- Zhou, Z.; Liu, Y.; Liu, Z.; Fan, L.; Dong, T.; Jin, Y.; Saldaña, M.D.A.; Sun, W. Sustained-release antibacterial pads based on nonwovens polyethylene terephthalate modified by β-cyclodextrin embedded with cinnamaldehyde for cold fresh pork preservation. Food Packag. Shelf Life 2020, 26, 100554. [Google Scholar] [CrossRef]





| Bacteria | Control | The Concentration of 3-Carene (mL/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| P. lundensis | Sterile Water | 2% Ethanol | 5 | 10 | 15 | 20 | 25 | 30 |
| +++ | +++ | +++ | +++ | +++ | ++ | − | − | |
| Inhibitors | Respiratory Rate R0 (nmol O2/mL/min) | Respiratory Rate after Inhibitor Treatment R1 (nmol O2/mL/min) | Inhibition Rate IR% |
|---|---|---|---|
| EMP Inhibitor | 2.156 | 0.407 | 81.12 |
| TCA Inhibitor | 2.156 | 1.293 | 40.02 |
| HMP Inhibitor | 2.156 | 1.512 | 29.87 |
| 3-Carene | 2.156 | 1.446 | 32.93 |
| Inhibitors | Respiratory Rate R2 (nmol O2/mL/min) | Superposition Rate SR % |
|---|---|---|
| 3-Carene | 1.446 | |
| 3-Carene and EMP inhibitor | 0.237 | 83.6 |
| 3-Carene and TCA inhibitor | 1.126 | 22.13 |
| 3-Carene and HMP inhibitor | 1.044 | 27.8 |
| Protein | Template Protein Data Bank (PDB) ID | Description | GMQE | QMEAN | Sequence Similarity |
|---|---|---|---|---|---|
| MurA | 6CN1 | UDP-N-acetylglucosamine 1-carboxyvinyl transferase | 0.95 | −0.08 | 0.58 |
| OmpW | 2X27 | Outer membrane protein W | 0.68 | −1.02 | 0.45 |
| AtpD | 2V7Q | ATP synthase subunit beta | 0.86 | −0.63 | 0.52 |
| Protein | Ligands | Binding Energy (Kcal/mol) | Residues Binding at Ligand–Protein Complex |
|---|---|---|---|
| MurA | Fosfomycin | −4.22 | Cys117 Val89 Lys90 Arg93 |
| 3-Carene | −5.19 | Met336 Ala118 | |
| OmpW | (Hydroxyethyloxy) Tri (Ethyloxy) Octane | −0.43 | Gly176 Ile175 Ala189 Ile187 Val226 |
| 3-Carene | −4.22 | Ile98 Val146 Val215 Ala200 Phe163 Ala213 Val202 Leu110 Phe160 | |
| AtpD | adenosine diphosphate (ADP) | −6.75 | Tyr330 Val156 Ala406 Gly151 Lys154 Val152 Thr155 |
| 3-Carene | −4.93 | Ala406 Pro331 Phe409 Tyr330 Met444 Phe403 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Chen, H.; Zhang, M.; Fan, Z.; Zhong, Q.; Chen, W.; Yun, Y.-H.; Chen, W. Antibacterial Mechanism of 3-Carene against the Meat Spoilage Bacterium Pseudomonas lundensis and Its Application in Pork during Refrigerated Storage. Foods 2022, 11, 92. https://doi.org/10.3390/foods11010092
Tang Z, Chen H, Zhang M, Fan Z, Zhong Q, Chen W, Yun Y-H, Chen W. Antibacterial Mechanism of 3-Carene against the Meat Spoilage Bacterium Pseudomonas lundensis and Its Application in Pork during Refrigerated Storage. Foods. 2022; 11(1):92. https://doi.org/10.3390/foods11010092
Chicago/Turabian StyleTang, Zhiling, Haiming Chen, Ming Zhang, Zhuye Fan, Qiuping Zhong, Weijun Chen, Yong-Huan Yun, and Wenxue Chen. 2022. "Antibacterial Mechanism of 3-Carene against the Meat Spoilage Bacterium Pseudomonas lundensis and Its Application in Pork during Refrigerated Storage" Foods 11, no. 1: 92. https://doi.org/10.3390/foods11010092
APA StyleTang, Z., Chen, H., Zhang, M., Fan, Z., Zhong, Q., Chen, W., Yun, Y.-H., & Chen, W. (2022). Antibacterial Mechanism of 3-Carene against the Meat Spoilage Bacterium Pseudomonas lundensis and Its Application in Pork during Refrigerated Storage. Foods, 11(1), 92. https://doi.org/10.3390/foods11010092







