Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Defatting, Processing, and Protein Extraction
2.3. Amino Acid and Essential Amino Acid Index
2.4. Surface Hydrophobicity
2.5. Protein Solubility
2.6. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. pH Measurements
2.8. Color Measurements
2.9. Foam Capacity and Stability
2.10. Emulsion Capacity and Emulsion Stability
2.11. Statistical Analysis
3. Results and Discussion
3.1. Amino Acid Composition and Essential Amino Acid Index
3.2. Surface Hydrophobicity, Protein Solubility, pH, and Color
3.3. SDS-PAGE
3.4. Foaming Capacity and Foam Stability
3.5. Emulsifying Capacity and Emulsion Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Jung, S.; Kim, H.W.; Choi, Y.S. Effects of organic solvent on functional properties of defatted proteins extracted from Protaetia brevitarsis larvae. Food Chem. 2021, 336, 127679. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, Y.-K.; Kim, S.-W.; Song, D.-H.; Kim, H.-W.; Kim, I.-S. Nutritional composition of white-spotted flower chafer (Protaetia brevitarsis) larvae produced from commercial insect farms in Korea. Food Sci. Anim. Resour. 2021, 41, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, T.-K.; Jeong, C.H.; Yong, H.I.; Cha, J.Y.; Kim, B.-K.; Choi, Y.-S. Biological activity and processing technologies of edible insects: A review. Food Sci. Biotechnol. 2021, 30, 1003–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Cha, J.Y.; Yong, H.I.; Jang, H.W.; Jung, S.; Choi, Y.-S. Application of edible insects as novel protein sources and strategies for improving their processing. Food Sci. Anim. Resour. 2022, 42, 372–388. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. What Governs Selection and Acceptance of Edible Insect Species? In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 331–351. [Google Scholar]
- Purschke, B.; Bruggen, H.; Scheibelberger, R.; Jager, H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.). Eur. Food Res. Technol. 2018, 244, 269–280. [Google Scholar] [CrossRef] [Green Version]
- da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Barsics, F.; Megido, R.C.; Brostaux, Y.; Barsics, C.; Blecker, C.; Haubruge, E.; Francis, F. Could new information influence attitudes to foods supplemented with edible insects? Br. Food J. 2017, 119, 2027–2039. [Google Scholar] [CrossRef]
- Castro, M.; Chambers, E. Willingness to eat an insect based product and impact on brand equity: A global perspective. J. Sens. Stud. 2019, 34, e12486. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.-S.; Lee, S.-D.; Cho, S.-J. Effect of three defatting solvents on the techno-functional properties of an edible insect (Gryllus bimaculatus) protein concentrate. Molecules 2021, 26, 5307. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes-Extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russin, T.A.; Boye, J.I.; Arcand, Y.; Rajamohamed, S.H. Alternative techniques for defatting soy: A practical review. Food Bioprocess Technol. 2011, 4, 200–223. [Google Scholar] [CrossRef]
- Melo, D.; Álvarez-Ortí, M.; Nunes, M.A.; Costa, A.S.; Machado, S.; Alves, R.C.; Pardo, J.E.; Oliveira, M.B.P. Whole or defatted sesame seeds (Sesamum indicum L.)? The effect of cold pressing on oil and cake quality. Foods 2021, 10, 2108. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Yong, H.I.; Kang, M.-C.; Jung, S.; Jang, H.W.; Choi, Y.-S. Effects of high hydrostatic pressure on technical functional properties of edible insect protein. Food Sci. Anim. Resour. 2021, 41, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Yong, H.I.; Chun, H.H.; Lee, M.A.; Kim, Y.B.; Choi, Y.S. Changes of amino acid composition and protein technical functionality of edible insects by extracting steps. J. Asia-Pac. Entomol. 2020, 23, 298–305. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Sante-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Laemmli, U. SDS-page Laemmli method. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- FAO; WHO; UNU. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; Food and Agriculture Organization; World Health Organization; The United Nations University: Geneva, Switzerland, 1985; p. 206. [Google Scholar]
- Zayas, J.F. Functionality of Proteins in Food; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Queiroz, L.S.; Regnard, M.; Jessen, F.; Mohammadifar, M.A.; Sloth, J.J.; Petersen, H.O.; Ajalloueian, F.; Brouzes, C.M.C.; Fraihi, W.; Fallquist, H.; et al. Physico-chemical and colloidal properties of protein extracted from black soldier fly (Hermetia illucens) larvae. Int. J. Biol. Macromol. 2021, 186, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Z.; Li, Y.; Meng, X.; Sui, X.; Qi, B.; Zhou, L. Relationship between surface hydrophobicity and structure of soy protein isolate subjected to different ionic strength. Int. J. Food Prop. 2015, 18, 1059–1074. [Google Scholar] [CrossRef]
- L’Hocine, L.; Boye, J.I.; Arcand, Y. Composition and functional properties of soy protein isolates prepared using alternative defatting and extraction procedures. J. Food Sci. 2006, 71, C137–C145. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering capacity of dairy products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour difference∆ E-A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Williams, J.; Williams, J.; Kirabo, A.; Chester, D.; Peterson, M. Nutrient Content and Health Benefits of Insects. In Insects as Sustainable Food Ingredients; Academic press: San Diego, CA, USA, 2016; pp. 61–84. [Google Scholar]
- De Graaf, L.A. Denaturation of proteins from a non-food perspective. J. Biotechnol. 2000, 79, 299–306. [Google Scholar] [CrossRef]
- Damodaran, S. Protein stabilization of emulsions and foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Influence of low molecular weight surfactants on the stability of model infant formula emulsions based on hydrolyzed rice protein. LWT-Food Sci. Technol. 2022, 154, 112544. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, Y.; Liang, X.; Piatko, M.; Campbell, S.; Lo, S.K.; Liu, Y. Synergetic interfacial adsorption of protein and low-molecular-weight emulsifiers in aerated emulsions. Food Hydrocoll. 2018, 81, 15–22. [Google Scholar] [CrossRef]
- O’sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]

 ,
,  ,
,  ,
,  ,
,  ).
).
 ,
,  ,
,  ,
,  ,
,  ).
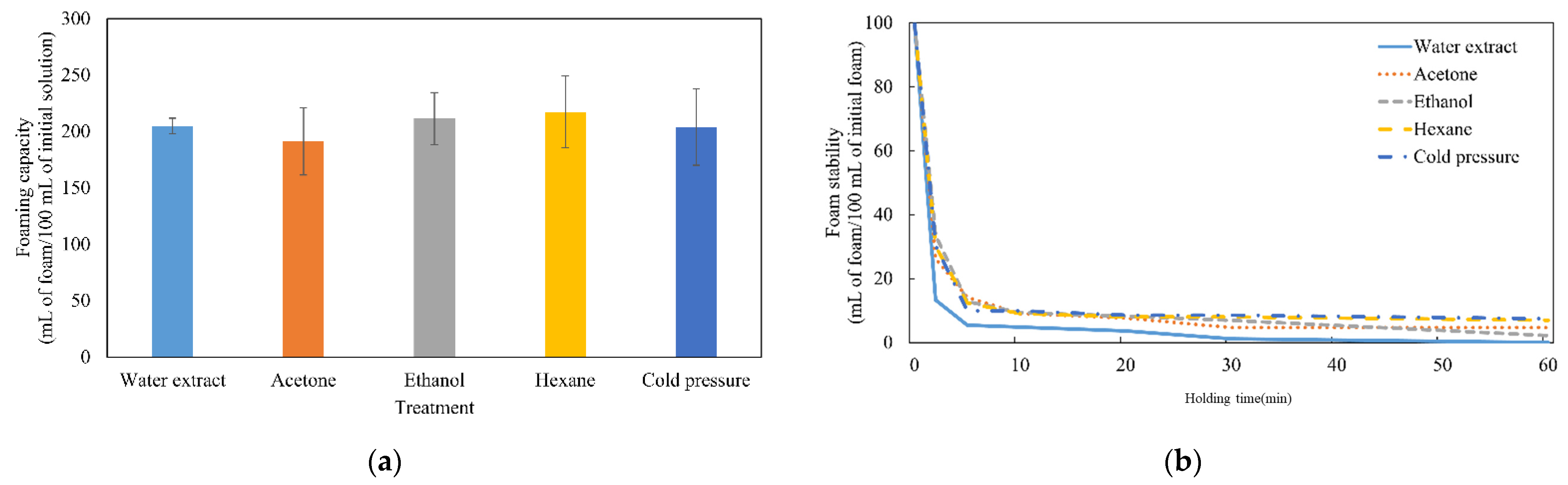
).
 ,
,  ,
,  ,
,  ,
,  ). Different letters on top of columns indicated significant differences among treatments (p < 0.05).
). Different letters on top of columns indicated significant differences among treatments (p < 0.05).
 ,
,  ,
,  ,
,  ,
,  ). Different letters on top of columns indicated significant differences among treatments (p < 0.05).
). Different letters on top of columns indicated significant differences among treatments (p < 0.05).
| Water Extract | Acetone | Ethanol | Hexane | Cold Pressure | ReferenceValue 1 | |
|---|---|---|---|---|---|---|
| Essential amino acids | ||||||
| His | 44.87 ± 1.16 bc | 44.06 ± 0.01 c | 45.78 ± 0.23 b | 44.56 ± 0.47 bc | 56.02 ± 0.44 a | 15 |
| Ile | 27.17 ± 0.01 b | 26.89 ± 0.10 b | 26.63 ± 0.56 bc | 25.86 ± 0.12 c | 28.82 ± 0.64 a | 30 |
| Leu | 38.77 ± 0.57 b | 38.05 ± 0.45 bc | 36.80 ± 0.79 c | 36.84 ± 0.10 c | 41.16 ± 0.53 a | 59 |
| Lys | 95.09 ± 0.31 a | 87.93 ± 0.02 d | 88.65 ± 0.28 c | 91.86 ± 0.26 b | 86.48 ± 0.27 e | 45 |
| Met + Cys | 11.69 ± 0.06 c | 12.09 ± 0.02 b | 12.10 ± 0.01 b | 12.57 ± 0.03 a | 10.21 ± 0.06 d | 22 |
| Phe + Tyr | 99.50 ± 0.35 b | 98.27 ± 0.40 bc | 97.00 ± 0.40 c | 93.68 ± 1.40 d | 108.30 ± 0.13 a | 38 |
| Thr | 35.31 ± 0.18 c | 35.68 ± 0.17 c | 38.13 ± 0.58 a | 36.61 ± 0.03 b | 36.61 ± 0.11 b | 23 |
| Val | 37.02 ± 0.47 bc | 37.57 ± 0.34 b | 36.67 ± 0.20 c | 36.85 ± 0.04 bc | 42.08 ± 0.26 a | 39 |
| Sum of EAA | 389.4 ± 0.34 b | 380.51 ± 0.26 cd | 381.74 ± 1.29 c | 378.8 ± 0.69 d | 409.64 ± 1.95 a | 271 |
| Nonessential amino acids | ||||||
| Ala | 123.81 ± 0.23 b | 123.98 ± 0.69 b | 117.3 ± 0.17 c | 123.17 ± 0.18 b | 135.78 ± 0.47 a | |
| Arg | 47.12 ± 0.09 d | 47.67 ± 0.21 cd | 49.15 ± 0.51 b | 47.94 ± 0.17 c | 52.58 ± 0.09 a | |
| Asp | 85.55 ± 0.36 c | 88.64 ± 0.71 b | 92.76 ± 0.11 a | 89.36 ± 0.07 b | 91.95 ± 0.09 a | |
| Glu | 185.79 ± 0.83 c | 191.50 ± 1.20 b | 196.44 ± 0.81 a | 193.12 ± 0.10 b | 155.88 ± 0.60 d | |
| Pro | 73.94 ± 1.52 a | 73.16 ± 3.38 a | 64.25 ± 0.67 b | 73.53 ± 0.60 a | 62.94 ± 3.09 b | |
| Gly | 48.86 ± 0.05 b | 49.34 ± 0.42 b | 50.47 ± 0.03 a | 49.04 ± 0.14 b | 52.11 ± 0.09 a | |
| Ser | 45.56 ± 0.36 b | 45.24 ± 0.84 b | 47.94 ± 0.8 a | 45.07 ± 0.37 b | 39.16 ± 0.02 c | |
| Sum of nonessential amino acids | 610.61 ± 0.34 c | 619.5 ± 0.26 ab | 618.27 ± 1.29 b | 621.21 ± 0.69 a | 590.37 ± 1.95 d | |
| Essential amino acid index | 1.26 ± 0.01 b | 1.25 ± 0.01 bc | 1.25 ± 0.01 bc | 1.24 ± 0.01 c | 1.31 ± 0.01 a | 1 |
| Water Extract | Acetone | Ethanol | Hexane | Cold Pressure | |
|---|---|---|---|---|---|
| Surface hydrophobicity (Bromophenol blue bound, μg) | 35.69 ± 3.79 a | 33.90 ± 6.84 a | 30.58 ± 6.47 a | 37.45 ± 2.80 a | 11.65 ± 1.75 b |
| Protein solubility (mg/mL) | 41.60 ± 0.17 d | 52.42 ± 0.74 a | 52.80 ± 0.78 a | 50.40 ± 0.29 b | 44.19 ± 0.02 c |
| pH | 7.14 ± 0.01 ab | 7.13 ± 0.01 b | 7.14 ± 0.01 ab | 7.14 ± 0.00 a | 7.12 ± 0.01 c |
| CIE L* | 16.67 ± 0.01 c | 16.37 ± 0.01 d | 16.83 ± 0.02 c | 18.01 ± 0.01 a | 17.24 ± 0.31 b |
| CIE a* | 1.86 ± 0.06 d | 1.82 ± 0.06 d | 1.95 ± 0.08 c | 2.22 ± 0.02 a | 2.09 ± 0.09 b |
| CIE b* | 4.07 ± 0.04 b | 3.98 ± 0.03 c | 3.85 ± 0.06 d | 4.14 ± 0.03 a | 3.87 ± 0.03 d |
| Color difference (∆E) | - 1 | 0.32 ± 0.03 c | 0.31 ± 0.06 c | 1.39 ± 0.01 a | 0.65 ± 0.31 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-K.; Lee, J.-H.; Yong, H.I.; Kang, M.-C.; Cha, J.Y.; Chun, J.Y.; Choi, Y.-S. Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae. Foods 2022, 11, 1400. https://doi.org/10.3390/foods11101400
Kim T-K, Lee J-H, Yong HI, Kang M-C, Cha JY, Chun JY, Choi Y-S. Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae. Foods. 2022; 11(10):1400. https://doi.org/10.3390/foods11101400
Chicago/Turabian StyleKim, Tae-Kyung, Jae-Hoon Lee, Hae In Yong, Min-Cheoul Kang, Ji Yoon Cha, Ji Yeon Chun, and Yun-Sang Choi. 2022. "Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae" Foods 11, no. 10: 1400. https://doi.org/10.3390/foods11101400
APA StyleKim, T.-K., Lee, J.-H., Yong, H. I., Kang, M.-C., Cha, J. Y., Chun, J. Y., & Choi, Y.-S. (2022). Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae. Foods, 11(10), 1400. https://doi.org/10.3390/foods11101400







