Speciation of Arsenic(III) and Arsenic(V) in Plant-Based Drinks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample, Chemicals and Materials
2.2. Instrumentation
2.3. Sample Preparation
3. Results
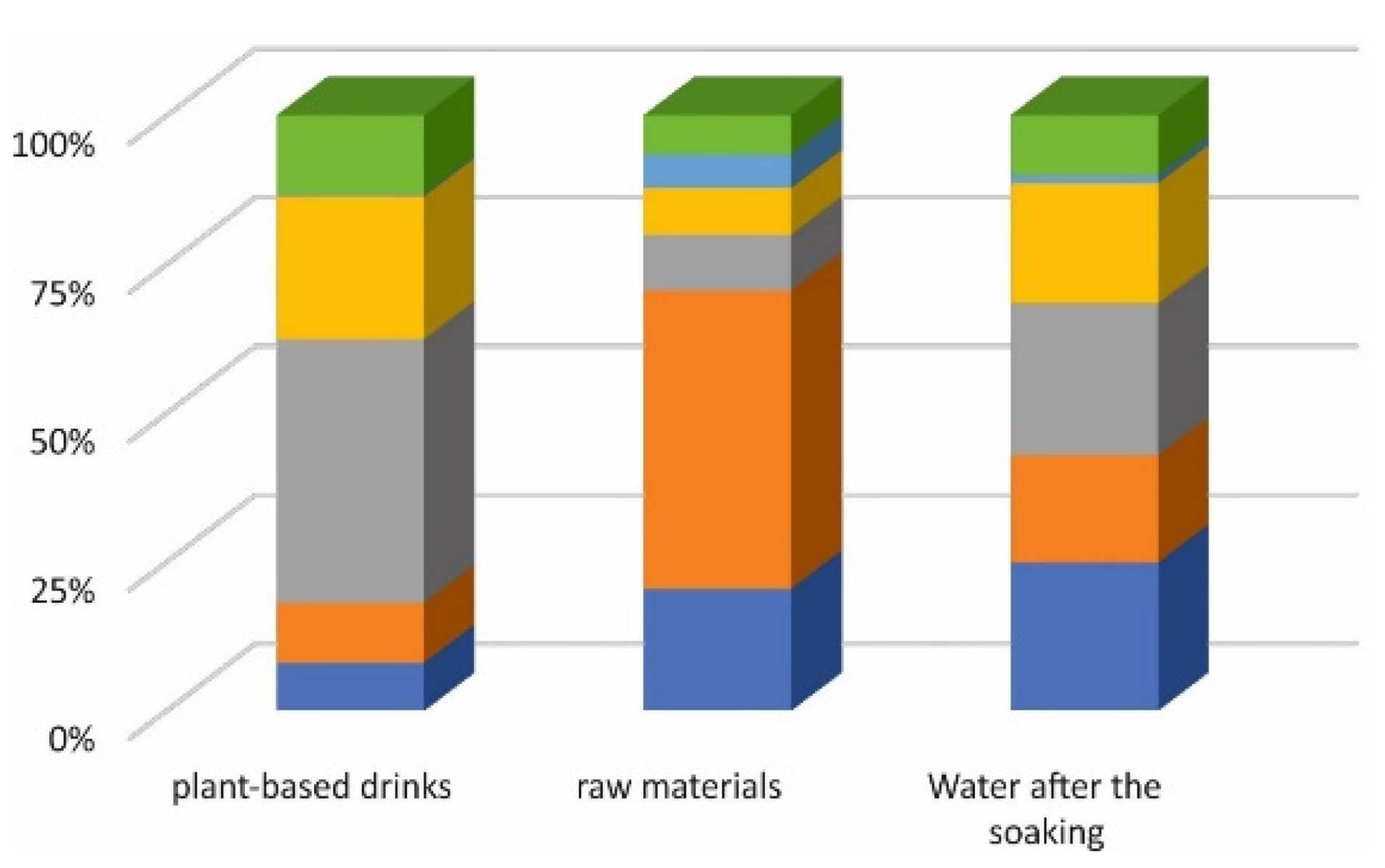
3.1. The Total Content of Arsenic in Plant-Based Drinks
Method Validation
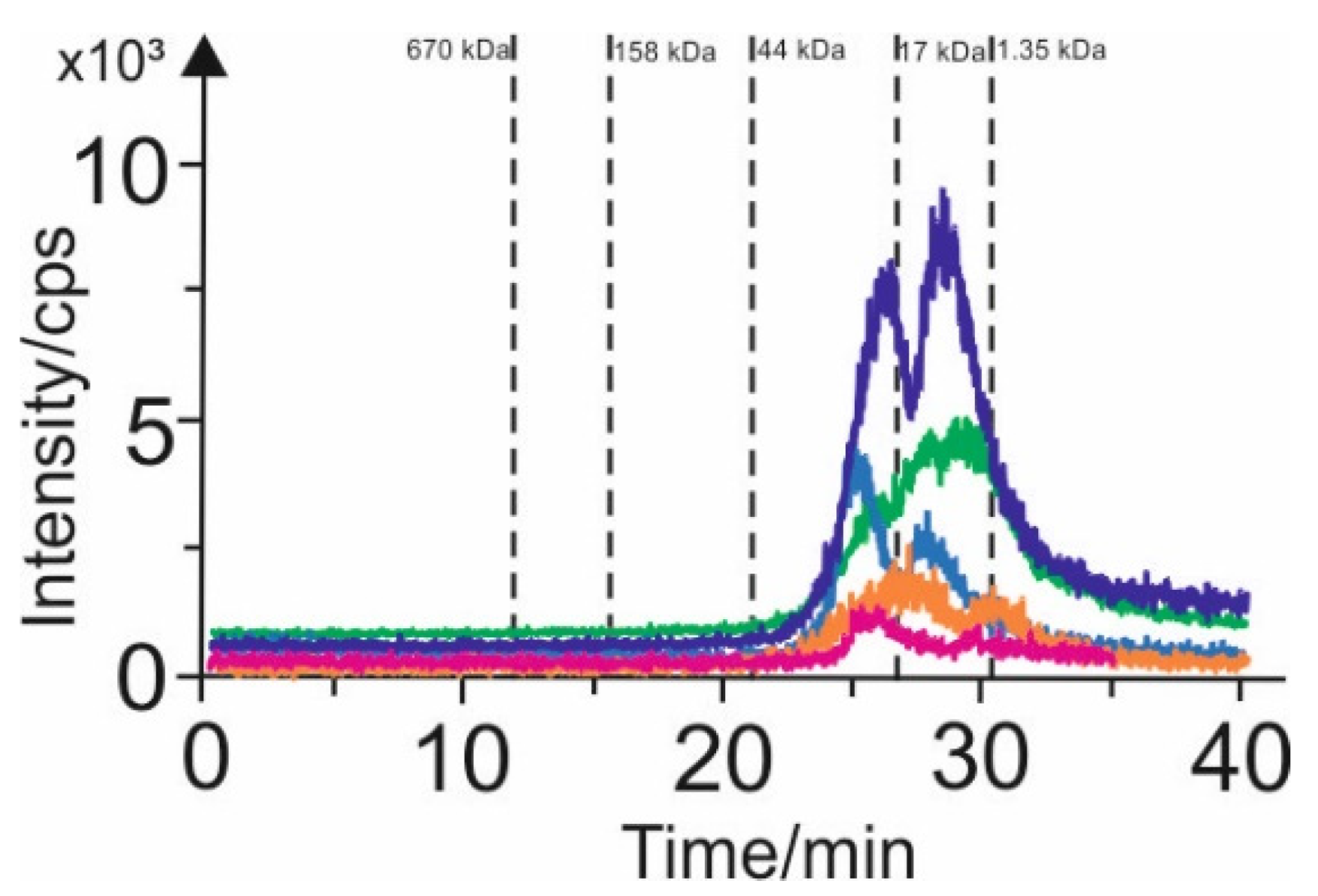
3.2. SEC-ICP-MS Characteristics of Various Extracts Containing Arsenic Species
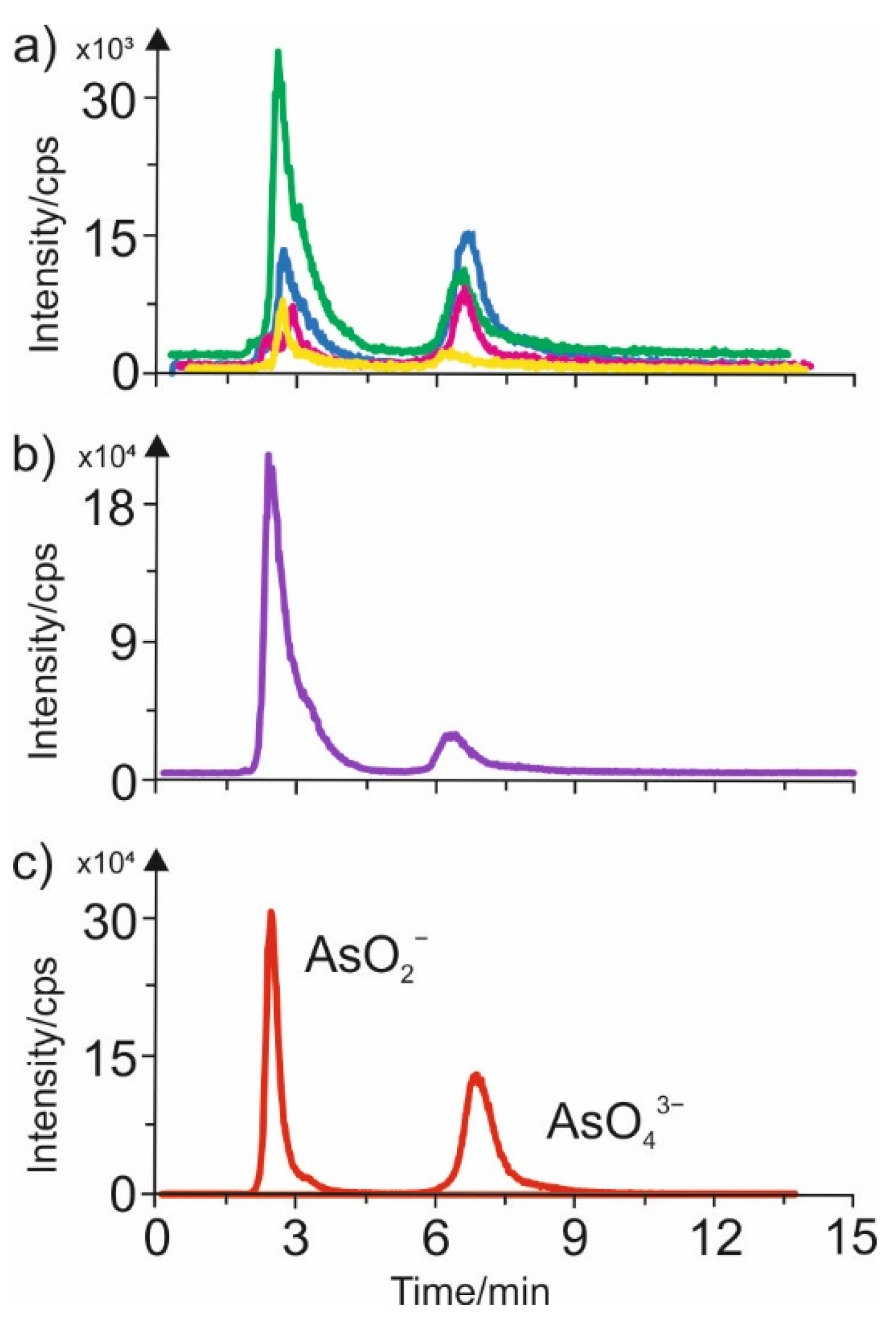
3.3. AEC-ICP-MS Characteristics of Various Extracts Containing Arsenic Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janssen, A.M.; Zunabovic, M.; Domig, K.J. The evolution of a plant-based alternative to meat: From niche markets to widely accepted meat alternatives. Agro FOOD Ind. Hi Tech 2014, 25, 45–49. [Google Scholar]
- Plant-Based Diets and Their Impact on Health, Sustainability and the Environment; WHO/EURO:2021-4007-43766-61591; WHO: Copenhagen, Denmark, 2021.
- FAO. The Future of Food and Agriculture; FAO: Rome, Italy, 2017; pp. 1–80. [Google Scholar]
- Fardet, A. New Concepts and Paradigms for the Protective Effects of Plant-Based Food Components in Relation to Food Complexity. Veg. Plant-Based Diets Health Dis. Prev. 2017, 2017, 293–312. [Google Scholar] [CrossRef]
- Fructuoso, I.; Romão, B.; Han, H.; Raposo, A.; Ariza-Montes, A.; Araya-Castillo, L.; Zandonadi, R.P. An Overview on Nutritional Aspects of Plant-Based Beverages Used as Substitutes for Cow’s Milk. Nutrients 2021, 13, 2650. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Plant-based milk products. Futur. Foods 2022, 12, 233–249. [Google Scholar] [CrossRef]
- Arbach, C.T.; Alves, I.A.; Serafini, M.R.; Stephani, R.; Perrone, Í.T.; de Carvalho da Costa, J. Recent patent applications in beverages enriched with plant proteins. NPJ Sci. Food 2021, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Flores, D.C.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the Effects of Ultra-High-Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical, and Chemical Quality of Almond Beverages. J. Food Sci. 2013, 78, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P. A Review of Plant-based Diets to Prevent and Treat Heart Failure. Card. Fail. Rev. 2018, 4, 54. [Google Scholar] [CrossRef]
- Akin, Z.; Ozcan, T. Functional properties of fermented milk produced with plant proteins. LWT Food Sci. Technol. 2017, 86, 25–30. [Google Scholar] [CrossRef]
- Okon, E.; Ojimelukwe, P. Potentials of Coconut Milk as a Substitute for Cow Milk in Cheese Making. J. Adv. Microbiol. 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Afaneh, I.; Abu-Alruz, K.; Quasem, J.M.; Sundookah, A.; Abbadi, J.; Alloussi, S.; Ayyad, Z. Fundamental Elements to Produce Sesame Yoghurt from Sesame Milk. Am. J. Appl. Sci. 2011, 8, 1086–1092. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Giacalone, D. Barriers to consumption of plant-based beverages: A comparison of product users and non-users on emotional, conceptual, situational, conative and psychographic variables. Food Res. Int. 2021, 144, 110363. [Google Scholar] [CrossRef]
- Haas, R.; Schnepps, A.; Pichler, A.; Meixner, O. Cow Milk versus Plant-Based Milk Substitutes: A Comparison of Product Image and Motivational Structure of Consumption. Sustainability 2019, 11, 5046. [Google Scholar] [CrossRef] [Green Version]
- The environmental impact of dairy production in the EU the practical otions for the improvments of the environmental impact. Eur. Comm. (DGXI) 2000, 1, 1–190.
- Radnitz, C.; Beezhold, B.; DiMatteo, J. Investigation of lifestyle choices of individuals following a vegan diet for health and ethical reasons. Appetite 2015, 90, 31–36. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Bazilian, M.; Griffiths, S.; Kim, J.; Foley, A.; Rooney, D. Decarbonizing the food and beverages industry: A critical and systematic review of developments, sociotechnical systems and policy options. Renew. Sustain. Energy Rev. 2021, 143, 110856. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Kundu, P.; Dhankhar, J.; Sharma, A. Development of non dairy milk alternative using soymilk and almond milk. Curr. Res. Nutr. Food Sci. 2018, 6, 203–210. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. NPJ Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Stanisz, E.; Krawczyk-Coda, M. Speciation Analysis of Food Products. In Analytical Methods in the Determination of Bioactive Compounds and Elements in Food; Springer: Cham, Switzerland, 2021; pp. 309–344. [Google Scholar] [CrossRef]
- Lipiec, E.; Ruzik, L.; Zhou, Y.; Jarosz, M.; Połeć-Pawlak, K. Study of chicken egg protein influence on bioavailability of vitamin B12 by SEC-ICP MS and ESI MS. J. Anal. At. Spectrom. 2011, 26, 608. [Google Scholar] [CrossRef]
- Lipiec, E.; Warowicka, O.; Ruzik, L.; Zhou, Y.; Jarosz, M.; Połeć-Pawlak, K. Investigation of iodine bioavailability from chicken eggs versus iodised kitchen salt with in vitro method. Eur. Food Res. Technol. 2012, 234, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Wojcieszek, J.; Ruzik, L. Operationally defined species characterisation and bioaccessibility evaluation of cobalt, copper and selenium in Cape gooseberry (Physalis peruviana L.) by SEC-ICP MS. J. Trace Elem. Med. Biol. 2016, 34, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ruzik, L.; Pawlak, K.; Jarosz, M. Inorganic and Bioinorganic Speciation Analysis: Problems and Prospects. In Handbook of Trace Analysis; Springer: Cham, Switzerland, 2016; pp. 333–370. [Google Scholar] [CrossRef]
- Ruzik, L. Speciation of challenging elements in food by atomic spectrometry. Talanta 2012, 93, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, K.; Parker, B.; Kannamkumarath, S.S.; Caruso, J.A. Determination of As(III), As(V), monomethylarsonic acid, dimethylarsinic acid and arsenobetaine by HPLC-ICP-MS: Analysis of reference materials, fish tissues and urine. Talanta 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Ackley, K.L.; B’Hymer, C.; Sutton, K.L.; Caruso, J.A. Speciation of arsenic in fish tissue using microwave-assisted extraction followed by HPLC-ICP-MS. J. Anal. At. Spectrom. 1999, 14, 845–850. [Google Scholar] [CrossRef]
- Gallagher, P.; Shoemaker, J.; Wei, X.; Brockhoff-Schwegel, C.; Creed, J.T. Extraction and detection of arsenicals in seaweed via accelerated solvent extraction with ion chromatographic separation and ICP-MS detection. Fresenius. J. Anal. Chem. 2001, 369, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Pizarro, I.; Gómez, M.; Palacios, M.A.; Cámara, C. Evaluation of stability of arsenic species in rice. Anal. Bioanal. Chem. 2003, 376, 102–109. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Reid, M.S.; Hoy, K.S.; Schofield, J.R.M.; Uppal, J.S.; Lin, Y.; Lu, X.; Peng, H.; Le, X.C. Arsenic speciation analysis: A review with an emphasis on chromatographic separations. TrAC Trends Anal. Chem. 2020, 123, 115770. [Google Scholar] [CrossRef]
- Jakubowski, N.; Prohaska, T.; Rottmann, L.; Vanhaecke, F. Inductively coupled plasma- and glow discharge plasma-sector field mass spectrometry. J. Anal. At. Spectrom. 2011, 26, 693–726. [Google Scholar] [CrossRef] [Green Version]
- Ardini, F.; Dan, G.; Grotti, M. Arsenic speciation analysis of environmental samples. J. Anal. At. Spectrom. 2020, 35, 215–237. [Google Scholar] [CrossRef]
- Ruiz-Chancho, M.J.; Sabé, R.; López-Sánchez, J.F.; Rubio, R.; Thomas, P. New Approaches to the Extraction of Arsenic Species from Soils. Microchim. Acta 2005, 151, 241–248. [Google Scholar] [CrossRef]
- Rintala, E.M.; Ekholm, P.; Koivisto, P.; Peltonen, K.; Venäläinen, E.R. The intake of inorganic arsenic from long grain rice and rice-based baby food in Finland—Low safety margin warrants follow up. Food Chem. 2014, 150, 199–205. [Google Scholar] [CrossRef] [Green Version]
| Settings | |
|---|---|
| Pump | Agilent 1260 Series |
| Column | Superdex 200 (10 × 300 mm × 10 µm)—GE Healthcare Life Sciences |
| Mobile phase | 10 mM ammonium acetate buffer (pH 7.4) |
| Elution program | isocratic |
| Flow | 0.75 mL min−1 |
| Injection volume | 100 µL |
| Column temperature | 28 °C |
| Pump | Agilent 1260 Series |
| Column | Agilent io SAX (5 µm, 4.6 × 250 mm) |
| Mobile phase | 5 mM sodium dihydrogen phosphate (pH 6.2) and 0.2 mM EDTA |
| Elution program | isocratic |
| Injection volume | 100 µL |
| Flow rate | 0.7 mL min−1 |
| Column temperature | 22 °C |
| Total Content of Arsenic | ||||||
|---|---|---|---|---|---|---|
| (μg L−1) | RSD (%) | (μg g−1) | RSD (%) | (μg L−1) | RSD (%) | |
| Type of Beverages | Raw Material | Water after the Soaking | ||||
| Soybean | 0.41970 | 1.06 | 0.03412 | 2.25 | 0.33071 | 2.83 |
| Oat | 0.53216 | 2.29 | 0.08410 | 2.23 | 0.24138 | 2.52 |
| Rice | 2.34086 | 2.70 | 0.01566 | 1.47 | 0.34022 | 3.13 |
| Coconut-rice | 1.26839 | 3.62 | 0.01311 | 1.89 | 0.26989 | 2.42 |
| Almond | 0.01616 | 1.95 | 0.00941 | 2.44 | 0.01784 | 0.68 |
| Millet | 0.70325 | 2.23 | 0.01110 | 1.45 | 0.13494 | 1.95 |
| Total Content of Arsenic | |||||
|---|---|---|---|---|---|
| (mg/kg) | After Mineralization (μg kg−1) | Dilution ×5 (μg kg−1) | RSD (%) | Dilution ×30 (μg kg−1) | RSD (%) |
| calculations | |||||
| 0.0490 ± 0.0011 | 0.9801 ± 0.0137 | 0.2023 ± 0.0571 | 2.34 | 0.0301 ± 0.0020 | 3.11 |
| experimentals | |||||
| - | - | 0.1810 ± 0.0350 | 3.05 | 0.0206 ± 0.0012 | 2.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruzik, L.; Jakubowska, M. Speciation of Arsenic(III) and Arsenic(V) in Plant-Based Drinks. Foods 2022, 11, 1441. https://doi.org/10.3390/foods11101441
Ruzik L, Jakubowska M. Speciation of Arsenic(III) and Arsenic(V) in Plant-Based Drinks. Foods. 2022; 11(10):1441. https://doi.org/10.3390/foods11101441
Chicago/Turabian StyleRuzik, Lena, and Małgorzata Jakubowska. 2022. "Speciation of Arsenic(III) and Arsenic(V) in Plant-Based Drinks" Foods 11, no. 10: 1441. https://doi.org/10.3390/foods11101441
APA StyleRuzik, L., & Jakubowska, M. (2022). Speciation of Arsenic(III) and Arsenic(V) in Plant-Based Drinks. Foods, 11(10), 1441. https://doi.org/10.3390/foods11101441






