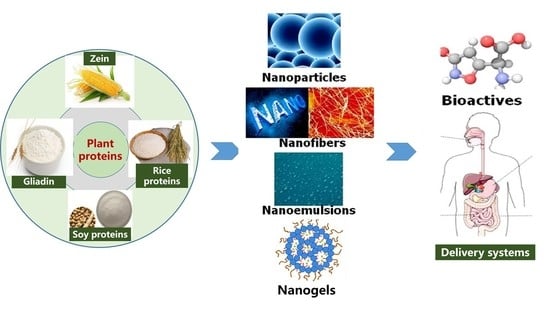
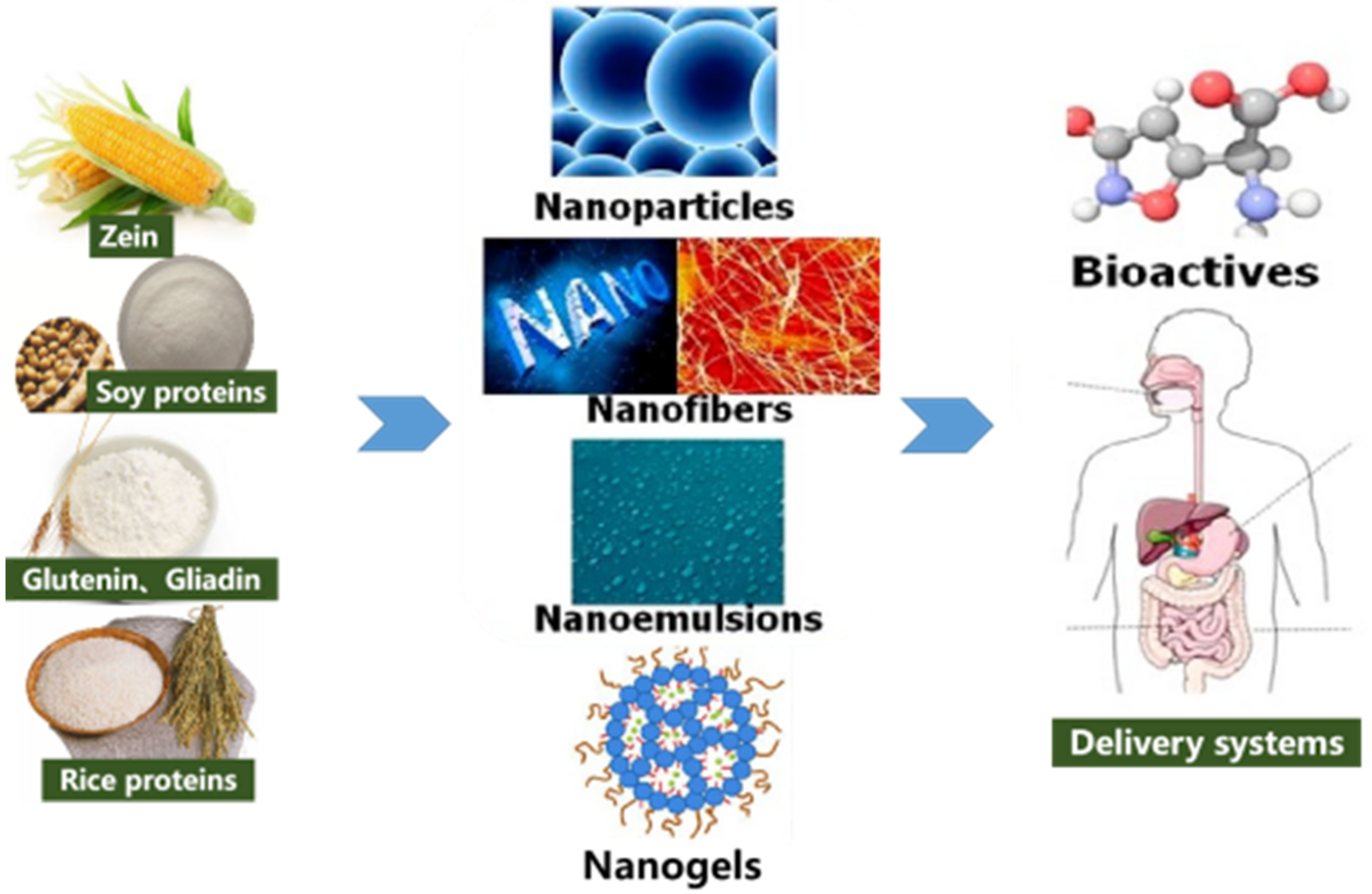
Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems
Abstract
:1. Introduction
2. Plant-Based Protein Properties
2.1. Zein
2.2. Soy Proteins
2.3. Glutenin and Gliadin
2.4. Rice Proteins and Other Plant Proteins
3. Application of Different Types of Plant Protein-Based Nano-Enabled Carriers in the Encapsulation, Protection, and Delivery of Bioactive Components
3.1. Plant Protein-Based Nanoparticles
3.2. Plant Protein-Based Emulsions and Gels
3.3. Plant Protein-Based Films and Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Bard, J.M.; Fumeron, F.; Lecerf, J.M. Functional foods containing phytosterols or phytostanols: Benefits and risks. Prat. Nutr. 2016, 12, 32–35. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S.; Santosh, O.; Bajwa, H.K.; Indira, A. Mineral elements in bamboo shoots and potential role in food fortification. J. Food Compos. Anal. 2021, 95, 103662. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; McClements, D.J.; Li, X.; Chen, L.; Long, J.; Jiao, A.; Xie, F.; Wang, J.; Jin, Z.; Qiu, C. Improved art bioactivity by encapsulation within cyclodextrin carboxylate. Food Chem. 2022, 384, 132429. [Google Scholar] [CrossRef]
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef]
- Qiu, C.; McClements, D.J.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation. Food Chem. 2020, 17, 126328. [Google Scholar] [CrossRef]
- Wang, C.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Resistant starch and its nanoparticles: Recent advances in their green synthesis and application as functional food ingredients and bioactive delivery systems. Trends Food Sci. Technol. 2022, 119, 90–100. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ramezanzade, L.; McClements, D.J. Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends Food Sci. Technol. 2021, 109, 322–339. [Google Scholar] [CrossRef]
- Yang, M.; Liang, Z.; Wang, L.; Qi, M.; Luo, Z.; Li, L. Microencapsulation Delivery System in Food Industry-Challenge and the Way forward. Adv. Polym. Technol. 2020, 2020, 7531810. [Google Scholar] [CrossRef]
- Qiu, C.; Hu, Y.; Jin, Z.; McClements, D.J.; Qin, Y.; Xu, X.; Wang, J. A review of green techniques for the synthesis of size-controlled starch-based nanoparticles and their applications as nanodelivery systems. Trends Food Sci. Technol. 2019, 92, 138–151. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, J.; Zhang, H.; Qin, Y.; Xu, X.; Jin, Z. Novel approach with controlled nucleation and growth for green synthesis of size-controlled cyclodextrin-based metal-organic frameworks based on short-chain starch nanoparticles. J. Agr. Food Chem. 2018, 66, 9785–9793. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; Gao, Y.; McClements, D.J. Food-Grade covalent complexes and their application as nutraceutical delivery systems: A review. Compr. Rev. Food Sci. Saf. 2017, 16, 76–95. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade Pickering emulsions for encapsulation and delivery of bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Qiu, C.; Wang, J.; Qin, Y.; Xu, X.; Jin, Z. Characterization and Mechanisms of Novel Emulsions and Nanoemulsion Gels Stabilized by Edible Cyclodextrin-Based Metal-Organic Frameworks and Glycyrrhizic Acid. J. Agr. Food Chem. 2019, 67, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Akman, P.K.; Bozkurt, F.; Balubaid, M.; Yilmaz, M.T. Fabrication of curcumin-loaded gliadin electrospun nanofibrous structures and bioactive properties. Fiber Polym. 2019, 20, 187–1199. [Google Scholar] [CrossRef]
- Alinaqi, Z.; Khezri, A.; Rezaeinia, H. Sustained release modeling of clove essential oil from the structure of starch-based bio-nanocomposite film reinforced by electrosprayed zein nanoparticles. Int. J. Biol. Macromol. 2021, 173, 193–202. [Google Scholar] [CrossRef]
- Inchaurraga, L.; Martínez-López, A.L.; Martin-Arbella, N.; Irache, J.M. Zein-based nanoparticles for the oral delivery of insulin. Drug Deliv. Transl. Res. 2020, 10, 1601–1611. [Google Scholar] [CrossRef]
- Ning, F.; Wang, X.; Zheng, H.; Zhang, K.; Bai, C.; Peng, H.; Huang, Q.; Xiong, H. Improving the bioaccessibility and in vitro absorption of 5-demethylnobiletin from chenpi by se-enriched peanut protein nanoparticles-stabilized pickering emulsion. J. Funct. Foods 2019, 55, 76–85. [Google Scholar] [CrossRef]
- Niva, M.; Vainio, A. Towards more environmentally sustainable diets? Changes in the consumption of beef and plant- and insect-based protein products in consumer groups in Finland. Meat Sci. 2021, 182, 108635. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.P.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein demand: Review of plant and animal proteins used in alternative protein product development and production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, Z.; Zhang, H.; Chen, G.; Jin, B.; Ba, C.; Zhang, C.; Li, Z. Advance in assembly and stability of zein composite nanoparticles. J. Chinese Cereal Oil Assoc. 2020, 35, 188–195, 202. [Google Scholar]
- Gomes, A.; Sobral, P.J.D.A. Plant Protein-Based Delivery Systems: An Emerging Approach for Increasing the Efficacy of Lipophilic Bioactive Compounds. Molecules 2022, 27, 60. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein- vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2021, 118, 111538. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Kong, X.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Fabrication and characterization of resveratrol-loaded gliadin nanoparticles stabilized by gum Arabic and chitosan hydrochloride. LWT-Food Sci. Technol. 2020, 129, 109532. [Google Scholar] [CrossRef]
- Bonke, A.; Sieuwerts, S.; Petersen, I.L. Amino Acid Composition of Novel Plant Drinks from Oat, Lentil and Pea. Foods 2020, 9, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Evans, N.M.; Liu, H.; Shao, S. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Saf. 2020, 19, 2639–2656. [Google Scholar] [CrossRef]
- Tang, C. Assembly of food proteins for nano-encapsulation and delivery of nutraceuticals. Food Hydrocoll. 2021, 117, 106710. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, H.; McClements, D.J.; Zhang, Z.; Zhang, R.; Jin, Z.; Tian, Y. Effect of dietary fibers on the structure and digestibility of fried potato starch: A comparison of pullulan and pectin. Carbohydr. Polym. 2019, 215, 47–57. [Google Scholar] [CrossRef]
- Tang, C. Nano-architectural assembly of soy proteins: A promising strategy to fabricate nutraceutical nanovehicles. Adv. Colloid Interface Sci. 2021, 291, 102402. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ganesh, S.; Wang, W.; Amiji, M. Protein Corona-Enabled Systemic Delivery and Targeting of Nanoparticles. AAPS J. 2020, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Weissmueller, N.T.; Lu, H.; Hurley, A.; Prud‘homme, R.K. Nanocarriers from GRAS zein proteins to encapsulate hydrophobic actives. Biomacromolecules 2016, 17, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.; Yang, Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011, 29, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. Zein and zein-based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in research on interactions between polyphenols and biology-based nano-delivery systems and their applications in improving the bioavailability of polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Bila, M.; Iqbal, H.M.; Wang, J. Zein-based micro- and nano-constructs and biologically therapeutic cues with multi-functionalities for oral drug delivery systems. J. Drug Deliv. Sci. Technol. 2020, 58, 101818. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kugimiya, W.; Maeda, H.; Yano, H.; Kusumoto, K.; Nabetani, H. Trends in plant-based substitutes for animal proteins. J. Jpn. Soc. Food Sci. 2021, 67, 459–473. [Google Scholar] [CrossRef]
- Mariotti, F. Animal and plant protein sources and cardiometabolic health. Adv. Nutr. 2019, 10, S351–S366. [Google Scholar] [CrossRef]
- Tang, C. Nanostructured soy proteins: Fabrication and applications as delivery systems for bioactives. Food Hydrocoll. 2019, 91, 2–116. [Google Scholar] [CrossRef]
- Baranes-Zeevi, M.; Goder, D.; Zilberman, M. Novel drug-eluting soy-protein structures for wound healing applications. Polym. Adv. Technol. 2019, 30, 2523–2538. [Google Scholar] [CrossRef]
- Matsliah, L.; Goder, D.; Giladi, S.; Zilberman, M. In vitro characterization of novel multidrug-eluting soy protein wound dressings. J. Biomater. Appl. 2020, 35, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, N.W.; Lopez, E.C.; Gaur, S.; Feng, H.; Engeseth, N.J.; Andrade, J. Soy protein nanoemulsions improve stability and bioavailability of vitamin D. FASEB J. 2017, 31, lb388. [Google Scholar]
- Cheng, C.; Gao, Y.; Wu, Z.; Miao, J.; Gao, H.; Ma, L.; Zou, L.; Peng, S.; Liu, C.; Liu, W. Gliadin nanoparticles pickering emulgels for β-Carotene delivery: Effect of particle concentration on the stability and bioaccessibility. Molecules 2020, 25, 4188. [Google Scholar] [CrossRef] [PubMed]
- Voci, S.; Fresta, M.; Cosco, D. Gliadins as versatile biomaterials for drug delivery applications. J. Control Release 2021, 329, 385–400. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L. The composition, extraction, functionality and applications of rice proteins: A review. Trends Food Sci. Technol. 2017, 64, 1–12. [Google Scholar] [CrossRef]
- Phongthai, S.; Homthawornchoo, W.; Rawdkuen, S. Preparation, properties and application of rice bran protein: A review. Int. Food Res. J. 2017, 24, 25–34. [Google Scholar]
- Xu, Y.; Ma, X.; Gong, W.; Li, X.; Huang, H.; Zhu, X. Nanoparticles based on carboxymethylcellulose-modified rice protein for efficient delivery of lutein. Food Funct. 2020, 11, 2380–2394. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y. Nanoparticles derived from plant proteins for controlled release and targeted delivery of therapeutics. Nanomedicine 2015, 10, 2001–2004. [Google Scholar] [CrossRef]
- Kumagai, S.; Daikai, T.; Onodera, T. Bovine Spongiform Encephalopathy—A Review from the Perspective of Food Safety. Food Saf. 2019, 7, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Peterson, S.C. Optimal conditions for the encapsulation of menthol into zein nanoparticles. LWT-Food Sci. Technol. 2021, 144, 111213. [Google Scholar] [CrossRef]
- Calliari, C.M.; Campardelli, R.; Pettinato, M.; Perego, P. Encapsulation of hibiscus sabdariffa extract into zein nanoparticles. Chem. Eng. Technol. 2020, 43, 2062–2072. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Ruiz-Cruz, S.; Juárez, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; López-Ahumada, G.A.; Rodríguez-félix, F. Gallic acid-loaded zein nanoparticles by electrospraying process. J. Food Sci. 2019, 84, 818–831. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, M.; Zhang, S.; Liu, C.; Chen, P.; Li, H.; Wang, D.; Xu, Y. Effect of sophorolipid on the curcumin-loaded ternary composite nanoparticles self-assembled from zein and chondroitin sulfate. Food Hydrocoll. 2021, 113, 106493. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, S.; Zhang, L.; Dai, L.; Tai, K.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y.; Mackie, A. Fabrication, characterization and in vitro digestion of food grade complex nanoparticles for co-delivery of resveratrol and coenzyme Q10. Food Hydrocoll. 2020, 105, 105791. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Salvatici, M.C.; Fresta, M.; Cosco, D. Brij-stabilized zein nanoparticles as potential drug carriers. Colloid Surface B 2021, 201, 111647. [Google Scholar] [CrossRef]
- Khan, M.A.; Yue, C.; Fang, Z.; Hu, S.; Cheng, H.; Bakry, A.M.; Liang, L. Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol. J. Food Eng. 2019, 258, 45–53. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.; Cheng, J.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Ju, M.; Zhu, G.; Huang, G.; Shen, X.; Zhang, Y.; Jiang, L.; Sui, X. A novel pickering emulsion produced using soy protein-anthocyanin complex nanoparticles. Food Hydrocoll. 2020, 99, 105329. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, L.; Feng, S.; Liu, F.; Luo, Y. Mechanistic study on the nanocomplexation between curcumin and protein hydrolysates from Great Northern bean (Phaseolus vulgaris L.) for delivery applications in functional foods. LWT-Food Sci. Technol. 2020, 139, 110572. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Ouyang, X.; Ling, J. Fabrication of soy protein isolate/cellulose nanocrystal composite nanoparticles for curcumin delivery. Int. J. Biol. Macromol. 2020, 165, 1468–1474. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Ouyang, X.; Ling, J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zeng, X.; Yi, J.; Zhang, Y. Fabrication of pea protein nanoparticles with calcium-induced cross-linking for the stabilization and delivery of antioxidative resveratrol. Int. J. Biol. Macromol. 2021, 152, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, M.; Koocheki, A.; Varidi, M.J.; Varidi, M. Encapsulation of curcumin using Grass pea (Lathyrus sativus) protein isolate/Alyssum homolocarpum seed gum complex nanoparticles. Innov. Food Sci. Emerg. Technol. 2021, 72, 102728. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Wang, P.; Li, R.; Ma, A.; Zhang, H. Core-shell pea protein-carboxymethylated corn fiber gum composite nanoparticles as delivery vehicles for curcumin. Carbohydr. Polym. 2020, 240, 116273. [Google Scholar] [CrossRef]
- Shi, A.; Wang, Q.; Chen, X.; Liu, H.; Liu, L.; Hu, H.; Yang, Y. Calcium-induced peanut protein nanoparticles for resveratrol delivery. J. Control Release 2017, 259, e6–e7. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Hu, Y.; Xu, N.; Li, D.; Wang, C.; Li, D. Study on condition of ultrasound-assisted thermo-alkali-modified peanut protein embedding curcumin for nanoparticles. J. Food Sci. Technol. 2020, 57, 1049–1060. [Google Scholar] [CrossRef]
- Asadi, M.; Salami, M.; Hajikhani, M.; Emam-Djomeh, Z.; Aghakhani, A.; Ghasemi, A. Electrospray production of curcumin-walnut protein nanoparticles. Food Biophys. 2020, 16, 15–26. [Google Scholar] [CrossRef]
- Yang, S.; Liu, L.; Chen, H.; Wei, Y.; Dai, L.; Liu, J.; Yuan, F.; Mao, L.; Li, Z.; Chen, F.; et al. Impact of different crosslinking agents on functional properties of curcumin-loaded gliadin-chitosan composite nanoparticles. Food Hydrocoll. 2021, 112, 106258. [Google Scholar] [CrossRef]
- Yang, S.; Dai, L.; Sun, C.; Gao, Y. Characterization of curcumin loaded gliadin-lecithin composite nanoparticles fabricated by antisolvent precipitation in different blending sequences. Food Hydrocoll. 2018, 85, 185–194. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, W.; Li, R.; Jia, X.; Cheng, Y. Impact of deamidation on gliadin-based nanoparticle formation and curcumin encapsulation. J. Food Eng. 2019, 260, 30–39. [Google Scholar] [CrossRef]
- Chen, S.; Ma, Y.; Dai, L.; Liao, W.; Zhang, L.; Liu, J.; Gao, Y. Fabrication, characterization, stability and re-dispersibility of curcumin-loaded gliadin-rhamnolipid composite nanoparticles using pH-driven method. Food Hydrocoll. 2021, 118, 106758. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, Y.; Chen, L. Intestinal uptake of barley protein-based nanoparticles for β-carotene delivery. Acta Pharm. Sin. B 2019, 9, 87–96. [Google Scholar] [CrossRef]
- Liu, C.; Yang, X.; Wu, W.; Long, Z.; Xiao, H.; Luo, F.; Shen, Y.; Lin, Q. Elaboration of curcumin-loaded rice bran albumin nanoparticles formulation with increased in vitro bioactivity and in vivo bioavailability. Food Hydrocoll. 2018, 77, 834–842. [Google Scholar] [CrossRef]
- Peng, H.; Gan, Z.; Xiong, H.; Luo, M.; Yu, N.; Wen, T.; Wang, R.; Li, Y. Self-assembly of protein nanoparticles from rice bran waste and their use as delivery system for curcumin. ACS Sustain. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar] [CrossRef]
- Ma, X.; Chen, X.; Ma, M.; Xu, Y.; Wu, X.; Mu, G.; Zhu, M. Lutein transport systems loaded with rice protein-based self-assembled nanoparticles. Food Biosci. 2021, 42, 101061. [Google Scholar] [CrossRef]
- Almajano, M.P.; Gordon, M.H. Synergistic effect of BSA on antioxidant activities in model food emulsions. J. Am. Oil Chem. Soc. 2004, 81, 275–280. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Yazdani, N. Reinforcement of hydrogel scaffold using oxidized-guar gum incorporated with curcumin-loaded zein nanoparticles to improve biological performance. Int. J. Biol. Macromol. 2020, 167, 59–65. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Theivendran, P.; Baskararaj, S.; Sankaranarayanan, B.; Palanisamy, P.; Saravanan, G.; Arunachalam, S.; Sankaranarayanan, M.; Natarajan, J.; Somasundaram, B.; et al. Modeling a pH-sensitive Zein-co-acrylic acid hybrid hydrogels loaded 5-fluorouracil and rutin for enhanced anticancer efficacy by oral delivery. 3 Biotech 2019, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Bodoki, E.; Vostinaru, O.; Samoila, O.; Dinte, E.; Bodoki, A.E.; Swetledge, S.; Astete, C.E.; Sabliov, C.M. Topical nanodelivery system of lutein for the prevention of selenite-induced cataract. Nanomedicine 2019, 15, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, S.; Hou, J.; Guo, J.; Wang, J.; Yang, X. Zein based oil-in-glycerol emulgels enriched with β-carotene as margarine alternatives. Food Chem. 2016, 211, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, R.; Mao, L.; Gao, Y. Ethanol-induced composite hydrogel based on propylene glycol alginate and zein: Formation, characterization and application. Food Chem. 2018, 255, 390–398. [Google Scholar] [CrossRef]
- Hu, B.; Han, L.; Ma, R.; Phillips, G.O.; Nishinari, K.; Fang, Y. All-natural food-grade hydrophilic-hydrophobic core-shell microparticles: Facile fabrication based on gel-network-restricted antisolvent method. ACS Appl. Mater. Interfaces 2019, 11, 11936–11946. [Google Scholar] [CrossRef]
- Yan, J.; Liang, X.; Ma, C.; McClements, D.J.; Liu, X.; Liu, F. Design and characterization of double-cross-linked emulsion gels using mixed biopolymers: Zein and sodium alginate. Food Hydrocoll. 2021, 113, 106473. [Google Scholar] [CrossRef]
- Liu, M.; Wang, F.; Pu, C.; Tang, W.; Sun, Q. Nanoencapsulation of lutein within lipid-based delivery systems: Characterization and comparison of zein peptide stabilized nano-emulsion, solid lipid nanoparticle, and nano-structured lipid carrier. Food Chem. 2021, 358, 129840. [Google Scholar] [CrossRef]
- Yan, W.; Jia, X.; Zhang, Q.; Chen, H.; Zhu, Q.; Yin, L. Interpenetrating polymer network hydrogels of soy protein isolate and sugar beet pectin as a potential carrier for probiotics. Food Hydrocoll. 2020, 113, 106453. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C. Soy glycinin as food-grade Pickering stabilizers: Part. III. Fabrication of gel-like emulsions and their potential as sustained-release delivery systems for β-carotene. Food Hydrocoll. 2016, 56, 434–444. [Google Scholar] [CrossRef]
- Brito-Oliveira, T.C.; Bispo, M.; Moraes, I.C.; Campanella, O.H.; Pinho, S.C. Stability of curcumin encapsulated in solid lipid microparticles incorporated in cold-set emulsion filled gels of soy protein isolate and xanthan gum. Food Res. Int. 2017, 102, 759–767. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, L.; Su, Y.; Chang, C.; Yang, Y.; Li, J. Development of soy protein isolate/κ-carrageenan composite hydrogels as a delivery system for hydrophilic compounds: Monascus yellow. Int. J. Biol. Macromol. 2021, 172, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Wu, Y.; Zhong, L.; Ma, N.; Zhao, L.; Ma, G.; Cheng, N.; Nakata, P.; Xu, J. In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll. 2021, 112, 106340. [Google Scholar] [CrossRef]
- Yi, F.; Wu, K.; Yu, G.; Su, C. Preparation of Pickering emulsion based on soy protein isolate-gallic acid with outstanding antioxidation and antimicrobial. Colloid Surface B 2021, 206, 111954. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wang, B.; Wang, Y.; Teng, Y. Effects of colloidal complexes formation between resveratrol and deamidated gliadin on the bioaccessibility and lipid oxidative stability. Food Hydrocoll. 2017, 69, 466–472. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (-)-Epigallocatechin-3-gallate and Quercetin in Particle-Stabilized W/O/W Emulsion Gels: Controlled Release and Bioaccessibility. J. Agr. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef]
- Fereydouni, N.; Movaffagh, J.; Amiri, N.; Darroudi, S.; Gholoobi, A.; Goodarzi, A.; Hashemzadeh, A.; Darroudi, M. Synthesis of nano-fibers containing nano-curcumin in zein corn protein and its physicochemical and biological characteristics. Sci. Rep. 2021, 11, 1902. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, L.; Niu, B.; Jiang, S.; Cheng, J.; Jiang, S. Kinetics and antioxidant capacity of proanthocyanidins encapsulated in zein electrospun fibers by cyclic voltammetry. J. Agr. Food Chem. 2016, 64, 3083–3090. [Google Scholar] [CrossRef]
- Li, Y.; Lim, L.T.; Kakuda, Y. Electrospun zein fibers as carriers to stabilize (-)-epigallocatechin gallate. J. Food Sci. 2009, 74, C233–C240. [Google Scholar] [CrossRef]
- Wang, H.; Hao, L.; Wang, P.; Chen, M.; Jiang, S.; Jiang, S. Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocoll. 2017, 63, 437–446. [Google Scholar] [CrossRef]
- Horuz, T.I.; Belibağlı, K.B. Nanoencapsulation of carotenoids extracted from tomato peels into zein fibers by electrospinning. J. Sci. Food Agr. 2019, 99, 759–766. [Google Scholar] [CrossRef]
- Krumreich, F.D.; Prietsch, L.P.; Antunes, M.D.; Jansen-Alves, C.; Mendonça, C.R.; Borges, C.D.; Zavareze, E.R.; Zambiazi, R.C. Avocado oil incorporated in ultrafine zein fibers by electrospinning. Food Biophys. 2019, 14, 383–392. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Pathumban, S.; Yoovidhya, T. Effect of entrapped α-tocopherol on mucoadhesivity and evaluation of the release, degradation, and swelling characteristics of zein-chitosan composite electrospun fibers. J. Food Eng. 2014, 120, 110–117. [Google Scholar] [CrossRef]
- Evangelho, J.A.; Crizel, R.L.; Chaves, F.C.; Prietto, L.; Pinto, V.Z.; Miranda, M.Z.; Dias, A.G.; Zavareze, E.R. Thermal and irradiation resistance of folic acid encapsulated in zein ultrafine fibers or nanocapsules produced by electrospinning and electrospraying. Food Res. Int. 2019, 124, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, S.M.; Jayaramudu, J.; Sadiku, E.R.; Ray, S.S.; Varaprasad, K.; Aderibigbe, B.A. Application of cross-linked soy protein isolate with resorcinol films for release studies of naturally occurring bioactive agent with antiproliferative activity. J. Drug Deliv. Sci. Technol. 2014, 24, 86–91. [Google Scholar] [CrossRef]
- Bisharat, L.; Barker, S.A.; Narbad, A.; Craig, D.Q. In vitro drug release from acetylated high amylose starch-zein films for oral colon-specific drug delivery. Int. J. Pharmaceut. 2019, 556, 311–319. [Google Scholar] [CrossRef] [PubMed]

| Type of Nanoparticles | Preparation Method | Encapsulated Bioactives | Encapsulation Efficiency | Major Outcomes | Refs |
|---|---|---|---|---|---|
| Zein nanoparticles | Liquid–liquid dispersion method | Menthol | >90% | A feasible encapsulation carrier was designed for bioactive substances soluble in 90% ethanol. | [52] |
| Zein nanoparticles | Liquid antisolvent precipitation | Hibiscus sabdariffa extract | 89% | At the same time, high encapsulation efficiency and good particle size control in the nanometer range were obtained. | [53] |
| Zein nanoparticles | Desolvation procedure | Insulin | 8% (payload) | The pharmacological activity and relative availability of insulin were significantly improved after insulin was loaded with zein nanoparticles. | [19] |
| Zein nanoparticles | Electrospraying | Gallic acid | - | The preparation of zein gallic acid nanoparticles by the electrospray method was a feasible technology, which had a potential protective effect on gallic acid. | [54] |
| Zein nanoparticles | Nanoprecipitation method | Rutin | ~88% | Zein nanosystem improved the stability and controlled release of rutin. | [25] |
| Zein–chondroitin sulfate–sophorolipid composite nanoparticles | Self-assembly technology | Curcumin | 63.4% to 98.21% | The ternary nanocrystalline delivery system had good biocompatibility and provided a new idea for the delivery of bioactive substances. | [55] |
| Zein–propylene glycol alginate–rhamnolipid complex nanoparticles | Emulsification–evaporation method | Resveratrol; Coenzyme Q10 | 49.54 ± 4.37% to 91.80 ± 4.62%; 84.06 ± 1.49% to 95.51 ± 0.61% | The co-transfer of resveratrol and coenzyme Q10 was achieved, and the chemical stability and synergistic sustained release of resveratrol and coenzyme Q10 were improved. | [56] |
| Brij-stabilized zein nanoparticles | Nanoprecipitation technique | Rhodamine B; Bromophenol blue | ~40%; ~80% | Brij-stabilized zein nanosystem prolonged the release time of the active compound and was a promising and innovative nanomaterial. | [57] |
| Alginate/chitosan-coated zein nanoparticles | Electrostatic deposition technique | Resveratrol | >70% | The alginate–chitosan layer significantly promoted the release and bioavailability of resveratrol in zein nanoparticles. | [58] |
| Zein/carboxymethyl dextrin nanoparticles | Antisolvent precipitation | Curcumin | 85.5% | The nanoparticles significantly enhanced the photochemical stability, thermal stability, antioxidant activity, and gastrointestinal slow-release effect of curcumin. | [59] |
| Zein/soluble soybean polysaccharide composite nanoparticles | Antisolvent precipitation method | Lutein | >80% | The complex system was a promising lutein delivery system that could be added as an ingredient to beverages or functional foods. | [60] |
| Soy protein nanoparticles | Alkali soluble acid precipitation | Anthocyanin | 90.02 ± 0.04% to 94.18 ± 0.04% | It provided a valuable reference for the preparation of a new type of Pickering emulsion and improved the stability of bioactive substances. | [61] |
| Soy protein nanoparticles | Self-assembled nanocomplexation | Curcumin | - | The 5% hydrolyzed soybean protein had the highest loading capacity for curcumin, relatively small particle size, and the best storage stability. | [62] |
| Soy protein isolate/cellulose nanocrystal composite nanoparticles | Self-assembly technology | Curcumin | 88.3% | Composite nanoparticles had high encapsulation efficiency and slow release effect, and were a promising delivery carrier for hydrophobic bioactive substances. | [63] |
| Soybean protein isolate and fucoidan nanoparticles | Electrostatic interaction | Curcumin | >95% | The composite nanoparticles had a spherical core–shell structure, the embedding rate of curcumin could reach 95%, and the system had long-term dispersion stability. | [64] |
| Pea protein nanoparticles | Calcium-induced cross-linking | Resveratrol | 74.08% | The nanoparticles could be efficient, powerful nanocarriers for the delivery of hydrophobic polyphenols, with great potential in functional beverages. | [65] |
| Grass pea protein isolate/Alyssum homolocarpum seed gum complex nanoparticles | Antisolvent precipitation | Curcumin | 88.22% | The particles could delay the release of Cur under in vitro gastrointestinal conditions. | [66] |
| Core–shell pea protein–carboxymethylated corn fiber gum composite nanoparticles | Liquid–liquid dispersion method | Curcumin | 99.2 ± 0.8% (pH = 3.5) | The core–shell structure afforded curcumin higher antioxidant activity, which provided a new strategy for the delivery of unstable hydrophobic active substances. | [67] |
| Peanut protein nanoparticles | Calcium-induced | Resveratrol | 82.7% | This resveratrol-loaded PPN could serve as a promising delivery system for long-term anti-cancer. | [68] |
| Peanut protein nanoparticles | Ultrasound-assisted thermo–alkali modification | Curcumin | 83.27 ± 1.06% | Compared with pure curcumin, the antioxidant activity was increased with the presence of peanut protein nanoparticles. | [69] |
| Peanut protein nanoparticles | Alkali extraction and acid precipitation methods | 5-demethylnobiletin | - | It provided a new delivery strategy for 5-demethylnobiletin in functional food and beverages. | [20] |
| Walnut protein nanoparticles | Electrospray technique | Curcumin | 61.45 ± 1.61% | The nanosystem could be used as a unique food-grade carrier to improve the water solubility and sustained release of curcumin. | [70] |
| Gliadin nanoparticles | Antisolvent precipitation | Resveratrol | 68.2% | The stability, solubility, and antioxidant capacity of resveratrol were improved by the combination of gliadin nanoparticles and gum Arabic. | [26] |
| Gliadin–chitosan composite nanoparticles | Antisolvent precipitation | Curcumin | 86.1% | The chitosan-modified gliadin nanoparticles showed higher encapsulation efficiency, better stability, and stronger antioxidant capacity for curcumin. | [71] |
| Gliadin–lecithin composite nanoparticles | Antisolvent precipitation | Curcumin | 90.7 ± 0.3% | Gliadin–lecithin composite nanoparticles possessed higher encapsulation efficiency, better stability, and higher antioxidant activity. | [72] |
| Gliadin nanoparticles | Antisolvent precipitation | Curcumin | 91% | Deaminated gliadin nanoparticles had a good encapsulation and protection effect on curcumin and had a good application prospect in the field of nutrition transmission. | [73] |
| Gliadin–rhamnolipid composite nanoparticles | pH-driven method | Curcumin | 98.70% | Composite nanoparticles prepared by pH-driven phytic acid had the potential to be a good nanoparticle delivery system for curcumin in functional foods. | [74] |
| Barley protein nanoparticles | High-pressure homogenizing method | β-carotene | - | Barley protein nanoparticles could improve the adsorption performance and may be used as a carrier of hydrophobic compounds. | [75] |
| Rice bran albumin nanoparticles | Antisolvent precipitation approach | Curcumin | 95.94% | Nanoparticulate curcumin formulation showed improved in vitro antioxidant activity, anti-inflammatory activity, and in vitro antiproliferative activity on tumor cells of curcumin in aqueous solution as compared with free curcumin. | [76] |
| Rice bran albumin–chitosan nanoparticles | Self-assembly technology | Curcumin | 93.56% | Composite nanoparticles had good biodegradability and had great potential as green and renewable materials in the transport of hydrophobic active substances. | [77] |
| Rice protein | Antisolvent method | Lutein | 89.8% to 94.1% | It provided a reference strategy for the stabilization of lutein and nutrient delivery. | [78] |
| Carboxymethylcellulose-modified rice protein nanoparticles | Antisolvent method | Lutein | - | This nano-system enhanced the absorption of lutein, which is helpful for the further development and application of new nano-delivery systems of lutein. | [48] |
| Type of Gels | Encapsulated Bioactives | Encapsulation Efficiency | Major Outcomes | Refs |
|---|---|---|---|---|
| Zein hydrogels | Curcumin | - | The hydrogel network structure supported by curcumin had good biocompatibility, degradability, low cytotoxicity, and antibacterial properties. | [80] |
| Zein–co-acrylic acid hybrid hydrogels | Rutin | 81.47% | The hydrogel structure had good stability, high encapsulation rate, and drug loading capacity for rutin, and was a good carrier of bioactive substances. | [81] |
| Zein thermosensitive gel | Lutein | 95.9 ± 3.2% | The thermal gel had a good loading effect on lutein and improved the bioavailability of lutein. | [82] |
| Zein-based oil-in-glycerol emulgels | β-carotene | - | Zein-based oil-in-glycerol emulgels enhanced the stability and bioavailability of beta carotene. | [83] |
| Zein ethanol-induced composite hydrogel | Curcumin | - | The composite hydrogels had a good ability to maintain curcumin release under simulated gastrointestinal conditions. | [84] |
| Zein hydrophilic–hydrophobic core shell hydrogel | Bioactives | - | A general method for hydrophilic and hydrophobic core–shell hydrogels was designed to provide a new carrier for the delivery and controlled release of bioactive substances. | [85] |
| Zein and sodium alginate double cross-linked emulsion gels | Curcumin; Resveratrol | - - | The double cross-linked emulsion gels afforded higher light stability and bioaccessibility than the single-cross-linked ones. | [86] |
| Zein nano-emulsion | Lutein | - | Zein stable nanoemulsions had good protective and release effects on lutein, providing valuable insights for improving the bioavailability of fat-soluble bioactive substances. | [87] |
| Soy protein isolate and sugar beet pectin interpenetrating polymer network hydrogels | Probiotics | 88.9% | The probiotics achieved better storage stability and higher vitality after loading the gel structure. | [88] |
| Soy glycinin gel-like emulsions | β-carotene | - | Gelatinous network slowed down the release of beta carotene and improved its bioavailability. | [89] |
| Soy protein cold-set emulsion filled gels | Curcumin | - | A new method was proposed to improve the stability of curcumin in the soybean protein gel system. | [90] |
| Soy protein isolate/κ-carrageenan composite hydrogels | Monascus yellow | - | The monaskoid yellow pigment in hydrogel system had higher stability and a better sustained release effect. | [91] |
| Soy protein isolate -Pleurotus eryngii polysaccharide conjugate-stabilized emulsion | β-carotene | - | Conjugated emulsion system was helpful to enhance the bioavailability of β -carotene and had broad application prospects in the transportation of fat-soluble nutrients. | [92] |
| Soy protein isolate emulsion | Lipophilic bioactive substance | 86.97% to 91.68% | A new method for preparing functional Pickering emulsion as a transfer medium for functional lipophilic raw materials was provided. | [93] |
| Gliadin nanoparticles Pickering emulgels | β-carotene | - | Gliadin nanoparticle Pickering emulgels could improve the stability and bioavailability of β-carotene. | [44] |
| Gliadin emulsion system | Resveratrol | - | The emulsion system based on gliadin had a good encapsulation and delivery effect on resveratrol, which provided a valuable reference for the application of bioactive substances in the food system. | [94] |
| Gliadin emulsion gels | EGCG; Quercetin | 65.5%; 97.2% | The emulsion gel increased the chemical stability and solubility of quercetin in simulated gastrointestinal tract conditions, and the effective bioaccessibility of quercetin was increased by four times. | [95] |
| Type of Material | Preparation Method | Encapsulated Bioactives | Encapsulation Efficiency | Major Outcomes | Refs |
|---|---|---|---|---|---|
| Zein fibers | Electrospinning technique | Curcumin | 94% ± 3.04% | Curcumin was successfully woven into zein fibers and maintained its functional properties. | [96] |
| Zein fibers | Electrospinning technique | Proanthocyanidins | close to 100% | Zein–proanthocyanidin fiber had good controlled release effect on proanthocyanidin. | [97] |
| Zein fibers | Continuous electrospinning | EGCG | - | EGCG was encapsulated in zein fiber, which provided a new strategy for biological delivery of EGCG. | [98] |
| Zein fibers | Electrospinning technique | Curcumin | close to 100% | The encapsulation rate of curcumin was close to 100%, and the good antioxidant activity and bacteriostasis were retained after encapsulation. | [99] |
| Zein fibers | Electrospinning technique | Carotenoids | >90% | Zein fiber had a good stabilizing effect on carotenoids and had a broad application prospect in the production of functional food. | [100] |
| Zein fibers | Electrospinning technique | Carotenoids | >77% | Good encapsulation of carotenoids and an ideal release effect of carotenoids in gastrointestinal tract were achieved. | [101] |
| Zein–chitosan composite electrospun fibers | Electrospinning | α-tocopherol | - | Composite electrostatic fibers had good adhesion and release properties in gastric mucosa, and had potential applicability in the transport of hydrophobic compounds to the gastrointestinal tract. | [102] |
| Zein ultrafine fibers | Electrospinning | Folic acid | >80% | The folic acid stability of the zein packaging was improved, which was beneficial to expand its application in functional foods. | [103] |
| Gliadin nanofiber | Electrospinning | Curcumin | 80% to 85% | The inclusion of curcumin in nanofibers significantly enhanced their antioxidant and antibacterial activities, and gliadin nanofibers had potential applications in the food industry and other biological activity delivery systems. | [17] |
| Pea protein core–shell composite fiber | - | Curcumin | 99.2 ± 0.8% | The core–shell composite nanomaterial showed excellent encapsulation performance and stability, and showed higher antioxidant activity and free radical-scavenging ability than free curcumin. | [67] |
| Soy protein isolate films | Casting method | Curcumin | 42% to 68% | Soybean protein isolate membrane had higher curcumin loading capacity and improved the bioavailability of curcumin. | [104] |
| Zein–high-amylose starch films | - | Paracetamol | - | Zein membrane could achieve colon-targeted drug delivery, which provided a feasible strategy for improving the bioavailability of bioactive substances. | [105] |
| Zein–starch composite film | - | Clove essential oil | - | The compound membrane had a higher loading rate and better release effect on clove essential oil. | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C.; et al. Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods 2022, 11, 1562. https://doi.org/10.3390/foods11111562
Guan T, Zhang Z, Li X, Cui S, McClements DJ, Wu X, Chen L, Long J, Jiao A, Qiu C, et al. Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods. 2022; 11(11):1562. https://doi.org/10.3390/foods11111562
Chicago/Turabian StyleGuan, Tongwei, Zhiheng Zhang, Xiaojing Li, Shaoning Cui, David Julian McClements, Xiaotian Wu, Long Chen, Jie Long, Aiquan Jiao, Chao Qiu, and et al. 2022. "Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems" Foods 11, no. 11: 1562. https://doi.org/10.3390/foods11111562
APA StyleGuan, T., Zhang, Z., Li, X., Cui, S., McClements, D. J., Wu, X., Chen, L., Long, J., Jiao, A., Qiu, C., & Jin, Z. (2022). Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods, 11(11), 1562. https://doi.org/10.3390/foods11111562