Differential Nutrition-Health Properties of Ocimum basilicum Leaf and Stem Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Crude Extracts
2.3. Fingerprint of Polyphenols Extracts
2.4. Antioxidant Activity
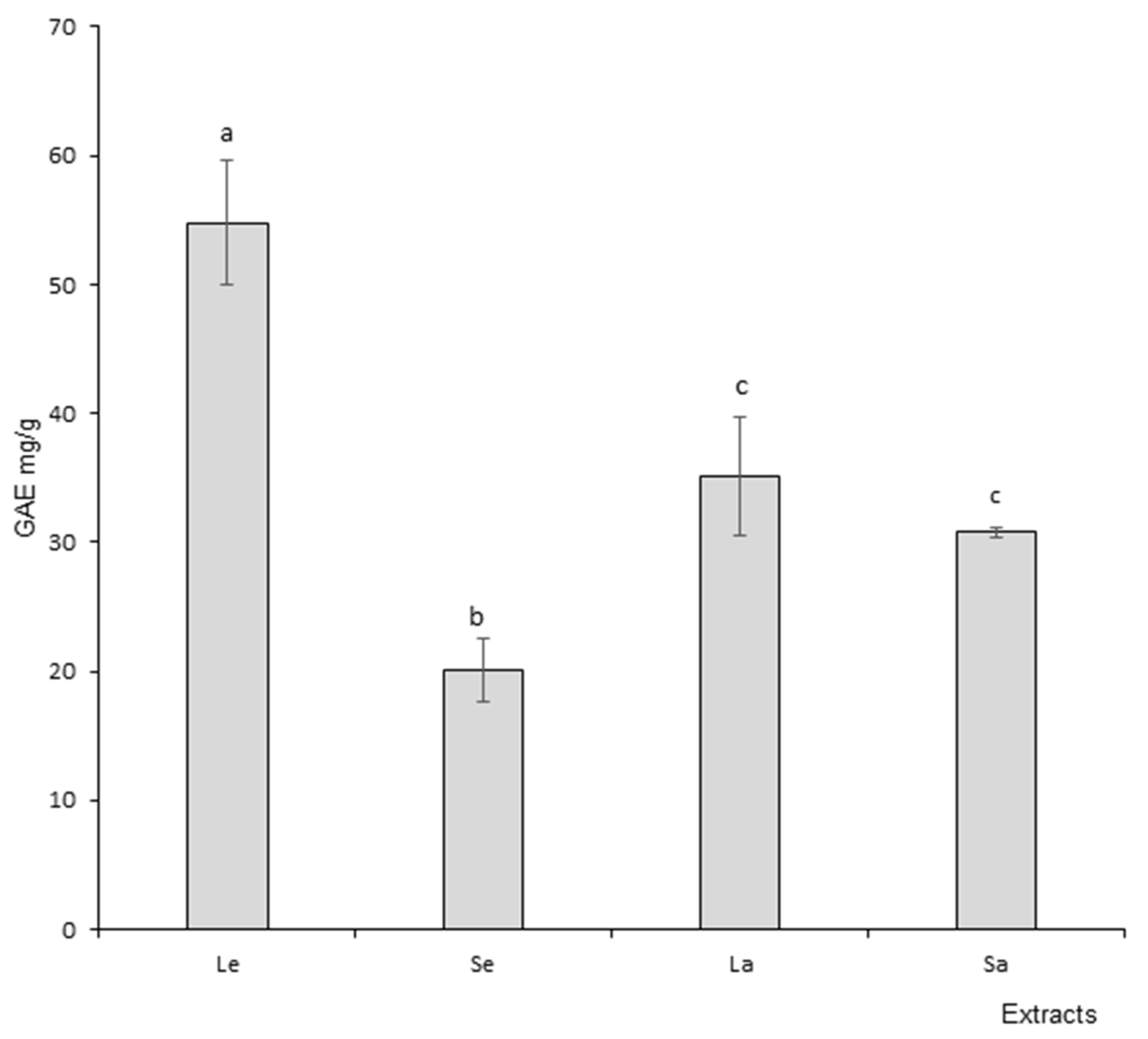
2.4.1. TPC (Total Phenolic Content) Test
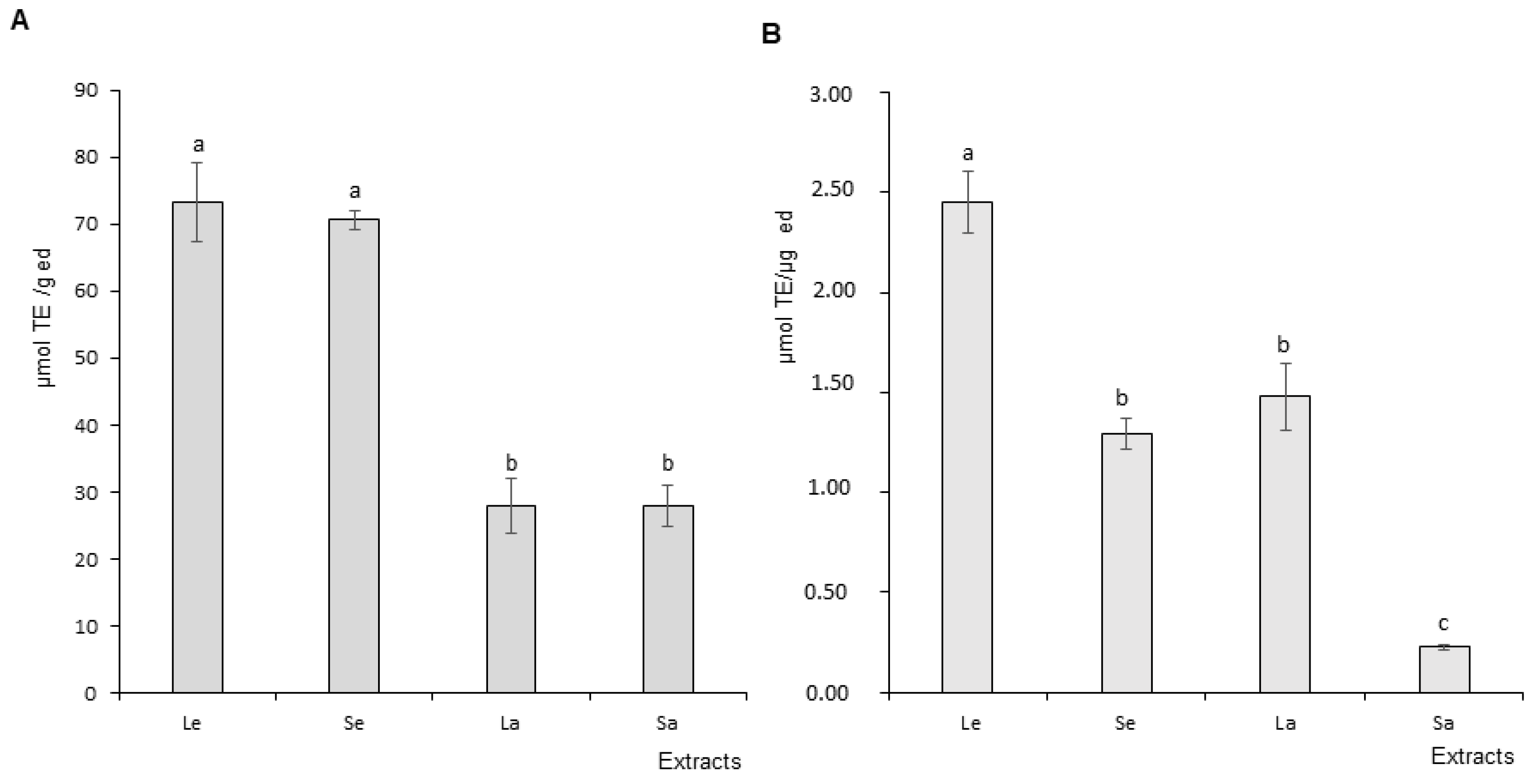
2.4.2. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Scavenging Activity
2.4.3. ORAC (Oxygen Radical Absorbance Capacity) Assay
2.5. Anti-Inflammatory Activity
2.5.1. Macrophage Culture
2.5.2. Cell Viability Assay
2.5.3. Determination of Nitrites (NO)
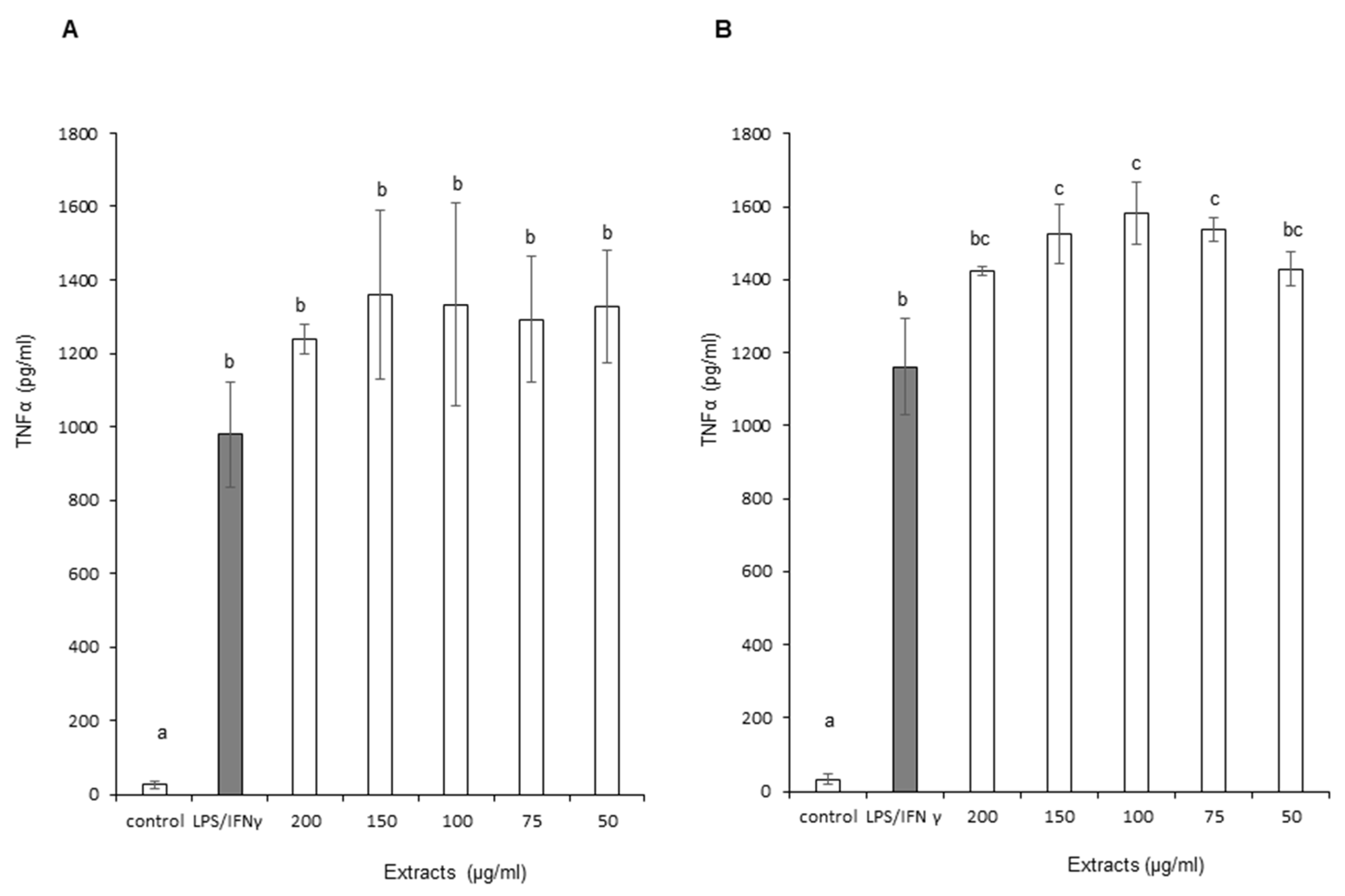
2.5.4. TNF-α (Tumor Necrosis Factor Alpha) Assay
2.5.5. IL-6 (Interleukin 6) Assay
2.5.6. Prostaglandin Assay
2.5.7. MCP-1 (Monocyte Chemoattractant Protein-1) Assay
2.6. Antispasmodic Activity
2.6.1. Antispasmodic Model
Animal Model and Isolated Jejunum Preparation
Spasmolytic Activity
2.7. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Polyphenols
DPPH and ORAC Assay Results
3.3. Anti-Inflammatory Activity
3.3.1. Cell Viability
3.3.2. Nitric Oxide (NO) Production and Scavenging
Nitric Oxide (NO) Production
Nitric Oxide (NO) Scavenging
3.3.3. Tumor Necrosis Factor Alpha (TNF-α)
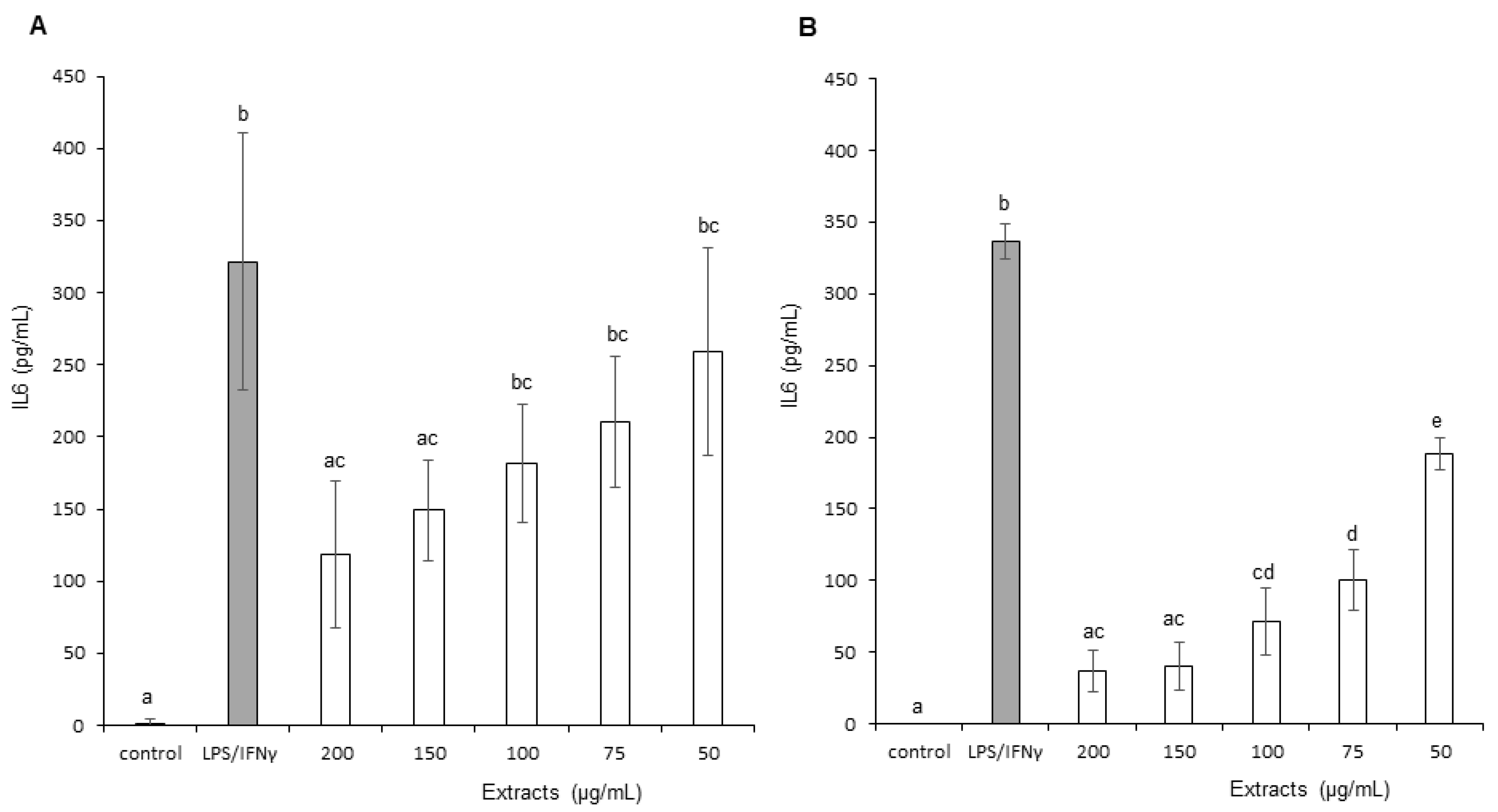
3.3.4. Interleukin 6 (IL-6)
3.3.5. Monocyte Chemoattractant Protein-1 (MCP-1)
3.3.6. Prostaglandin E2 (PGE-2)
3.4. Antispasmodic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilal, A.; Jahan, N.; Ahmed, A.; Bilal, S.N.; Habib, S.; Hajra, S. Phytochemical and pharmacological studies on Ocimum basilicum Linn-A review. Int. J. Curr. Res. Rev. 2012, 4, 73–83. [Google Scholar]
- Putievsky, E.; Galambosi, B. Production Systems of Sweet Basil. In Basil: The Genus Ocimum; Hiltunen, R., Holm, Y., Eds.; Medicinal and Aromatic Plants—Industrial Profiles; Taylor and Francis: Amsterdam, The Netherlands, 2005; Volume 10, pp. 37–63. [Google Scholar]
- Nadeem, F.; Hanif, M.A.; Bhatti, I.A.; Jilani, M.I.; Al-Yahyai, R. Chapter 4: Basil. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 47–62. [Google Scholar]
- Pushpangadan, P.; George, V. Basil. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 55–72. [Google Scholar]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil Seeds as a Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- Naji-Tabasi, S.; Razavi, S.M.A. Functional properties and applications of basil seed gum: An overview. Food Hydrocoll. 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Kisa, D.; İmamoğlu, R.; Genç, N.; Şahin, S.; Qayyum, M.A.; Elmastaş, M. The interactive effect of aromatic amino acid composition on the accumulation of phenolic compounds and the expression of biosynthesis-related genes in Ocimum basilicum. Physiol. Mol. Biol. Plants 2021, 27, 2057–2069. [Google Scholar] [CrossRef]
- Irondi, E.A.; Agboola, S.O.; Oboh, G.; Boligon, A.A. Inhibitory effect of leaves extracts of Ocimum basilicum and Ocimum gratissimum on two key enzymes involved in obesity and hypertension in vitro. J. Intercult. Ethnopharmacol. 2016, 5, 396–402. [Google Scholar] [CrossRef]
- Al Abbasy, D.W.; Pathare, N.; Al-Sabahi, J.N.; Khan, S.A. Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum Linn.). Asian Pac. J. Trop. Dis. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Vieira, R.F.; Simon, J.E. Chemical Characterization of basil (Ocimum spp.) found in the markets and used in traditional medicine in Brazil. Econ. Bot. 2000, 4, 207–216. [Google Scholar] [CrossRef]
- Abirami, S.G.; Nirmala, P.A. Comparative in vitro study of anticancer effect of Mentha piperita, Ocimum basilicum and Coleus aromaticus against human laryngeal epidermoid carcinoma (HEP-2) cell lines. J. Med. Plants Stud. 2014, 2, 6–9. [Google Scholar]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical Characterization of Basil (Ocimum basilicuml L.) Found in Local Accessions and Used in Traditional Medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Eftekhar, N.; Moghimi, A.; Mohammadian Roshan, N.; Saadat, S.; Boskabady, M.H. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement. Altern. Med. 2019, 19, 349. [Google Scholar] [CrossRef]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.; Marquez, S.; De Mejia, E.G. The Health Benefits of Selected Culinary Herbs and Spices Found in the Traditional Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2016, 56, 2728–2746. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.S.; de Arrigoni-Blank, M.F.; De Carvalho Filho, J.L.; De Santana, A.D.; de Santos, D.A.; Alves, P.B.; Blank, A.F. Chemical diversity in basil (Ocimum sp.) germplasm. Sci. World J. 2015, 2015, 352638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soro, L.C.; Munier, S.; Pelissier, Y.; Grosmaire, L.; Yada, R.; Kitts, D.; Ocho-Anin Atchibri, A.L.; Guzman, C.; Boudard, F.; Menut, C.; et al. Influence of geography, seasons and pedology on chemical composition and anti-inflammatory activities of essential oils from Lippia multiflora Mold leaves. J. Ethnopharmacol. 2016, 194, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus L.) for an antidiabetic effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgari Lajayer, B.; Najafi, N.; Moghiseh, E.; Mosaferi, M.; Hadian, J. Effects of Gamma Irradiated and Non-Irradiated Sewage Sludge on Essential Oil Content and Constituents of Ocimum basilicum L. J. Med. Plants 2019, 18, 99–117. [Google Scholar] [CrossRef]
- Rui, W.; Xia, W.; Zhao, W.; Li, B.; Li, J.; Feng, Y.; Chen, H.; Zhao, S. Differential Constituents in Roots, Stems and Leaves of Polygonum multiflorum Thunb. Screened by UPLC/ESI-Q-TOF-MS and Multivariate Statistical Analysis. J. Chromatogr. Sci. 2020, 58, 136–143. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhang, C.; Sun, X.; Liao, X.; Zheng, W.; Yin, Q.; Yang, J.; Mao, D.; Wang, B.; et al. The qualitative and quantitative analyses of Gelsemium elegans. J. Pharm. Biomed. Anal. 2019, 172, 329–338. [Google Scholar] [CrossRef]
- Genfi, A.K.A.; Larbie, C.; Emikpe, B.O.; Oyagbemi, A.A.; Firempong, C.K.; Adjei, C.O. Modulation of Oxidative Stress and Inflammatory Cytokines as Therapeutic Mechanisms of Ocimum americanum L. Extract in Carbon Tetrachloride and Acetaminophen-Induced Toxicity in Rats. J. Evid. Based Integr. Med. 2020, 25. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Shankar, C.G.; Girija, S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [CrossRef]
- Kelm, M.A.; Nair, M.G.; Strasburg, G.M.; DeWitt, D.L. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine 2000, 7, 7–13. [Google Scholar] [CrossRef]
- Madeira, S.V.; Matos, F.J.; Leal-Cardoso, J.H.; Criddle, D.N. Relaxant effects of the essential oil of Ocimum gratissimum on isolated ileum of the guinea pig. J. Ethnopharmacol. 2002, 81, 1–4. [Google Scholar] [CrossRef]
- Madeira, S.V.; Rabelo, M.; Soares, P.M.; Souza, E.P.; Meireles, A.V.; Montenegro, C.; Lima, R.F.; Assreuy, A.M.; Criddle, D.N. Temporal variation of chemical composition and relaxant action of the essential oil of Ocimum gratissimum L. (Labiatae) on guinea-pig ileum. Phytomedicine 2005, 12, 506–509. [Google Scholar] [CrossRef]
- Franca, C.S.; Menezes, F.S.; Costa, L.C.; Niculau, E.S.; Alves, P.B.; Pinto, J.E.; Marçal, R.M. Analgesic and antidiarrheal properties of Ocimum selloi essential oil in mice. Fitoterapia 2008, 79, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.D.; Franca, C.S.; Niculau, E.S.; Costa, L.C.; Pinto, J.E.; Alves, P.B.; Marçal, R.M. Antispasmodic effect of Ocimum selloi essential oil on the guinea-pig ileum. Nat. Prod. Res. 2015, 29, 2125–2128. [Google Scholar] [CrossRef]
- Morel, S.; Arnould, S.; Vitou, M.; Boudard, F.; Guzman, C.; Poucheret, P.; Fons, F.; Rapior, S. Antiproliferative and Antioxidant Activities of Wild Boletales Mushrooms from France. Int. J. Med. Mushrooms 2018, 20, 13–29. [Google Scholar] [CrossRef]
- Bony, E.; Boudard, F.; Dussossoy, E.; Portet, K.; Brat, P.; Giaimis, J.; Michel, A. Chemical composition and anti-inflammatory properties of the unsaponifiable fraction from awara (Astrocaryum vulgare M.) pulp oil in activated J774 macrophages and in a mice model of endotoxic shock. Plant Foods Hum. Nutr. 2012, 67, 384–392. [Google Scholar] [CrossRef]
- Deme, P.; Aluganti Narasimhulu, C.; Parthasarathy, S. Evaluation of Anti-Inflammatory Properties of Herbal Aqueous Extracts and Their Chemical Characterization. J. Med. Food. 2019, 22, 861–873. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Basile, M.J.; Kennelly, E.J. Analysis of Polyphenolic Antioxidants from the Fruits of Three Pouteria Species by Selected Ion Monitoring Liquid Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 5873–5878. [Google Scholar] [CrossRef]
- Kumar, S.; Bouic, P.J.; Rosenkranz, B. In Vitro Assessment of the Interaction Potential of Ocimum Basilicum (L.) Extracts on CYP2B6, 3A4, and Rifampicin Metabolism. Front. Pharmacol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics Composition and Antioxidant Activity of Sweet Basil (Ocimum Basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.Y.M.; Mansour, S.M.; Elkady, W.M. Phytochemical Profile and Protective Effect of Ocimum Basilicum Aqueous Extract in Doxorubicin/Irradiation-Induced Testicular Injury. J. Pharm. Pharmacol. 2020, 72, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, B.; Chen, P.; Charles, D.; Yu, L. Characterisation of Organic and Conventional Sweet Basil Leaves Using Chromatographic and Flow-Injection Mass Spectrometric (FIMS) Fingerprints Combined with Principal Component Analysis. Food Chem. 2014, 154, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Yan, Y.; Guo, D. Characterization of Phenolic Compounds in the Chinese Herbal Drug Tu-Si-Zi by Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1469–1484. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G. Anti-Acetylcholinesterase Potential and Metabolome Classification of 4 Ocimum Species as Determined via UPLC/QTOF/MS and Chemometric Tools. J. Pharm. Biomed. Anal. 2016, 125, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Burducea, M.; Zheljazkov, V.D.; Lobiuc, A.; Pintilie, C.A.; Virgolici, M.; Silion, M.; Asandulesa, M.; Burducea, I.; Zamfirache, M.-M. Biosolids Application Improves Mineral Composition and Phenolic Profile of Basil Cultivated on Eroded Soil. Sci. Hortic. 2019, 249, 407–418. [Google Scholar] [CrossRef]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Scagel, C.F. Chicoric Acid Found in Basil (Ocimum Basilicum L.) Leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef]
- Selvakkumar, C.; Gayathri, B.; Vinaykumar, K.S.; Lakshmi, B.S.; Balakrishnan, A. Potential anti-inflammatory properties of crude alcoholic extract of Ocimum basilicum L. in human peripheral blood mononuclear cells. J. Health Sci. 2007, 53, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Singh, S. Mechanism of action of anti-inflammatory effect of fixed oil of Ocimum basilicum Linn. Indian J. Exp. Biol. 1999, 37, 248–252. [Google Scholar] [PubMed]
- Noor, Z.I.; Ahmed, D.; Rehman, H.M.; Qamar, M.T.; Froeyen, M.; Ahmad, S.; Mirza, M.U. In Vitro Antidiabetic, Anti-Obesity and Antioxidant Analysis of Ocimum basilicum Aerial Biomass and in Silico Molecular Docking Simulations with Alpha-Amylase and Lipase Enzymes. Biology 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Ninfali, P. Polyphenols and antioxidant capacity of vegetables under fresh and frozen conditions. J. Agric. Food Chem. 2003, 51, 2222–2226. [Google Scholar] [CrossRef]
- Oalđe Pavlović, M.; Kolarević, S.; Đorđević, J.; Jovanović Marić, J.; Lunić, T.; Mandić, M.; Kračun Kolarević, M.; Živković, J.; Alimpić Aradski, A.; Marin, P.D.; et al. A Study of Phytochemistry, Genoprotective Activity, and Antitumor Effects of Extracts of the Selected Lamiaceae Species. Plants 2021, 10, 2306. [Google Scholar] [CrossRef]
- Sevgi, E.; Dag, A.; Kızılarslan-Hançer, Ç.; Atasoy, S.; Kurt, B.Z.; Aksakal, Ö. Evaluation of cytotoxic and antioxidant potential of Dittrichia viscosa (L.) Greuter used in traditional medicine. J. Ethnopharmacol. 2021, 276, 114211. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Meneses, C.C.B.; Pizzatto, L.N.; Andrade, F.F.; Sipert, C.R. Prostaglandin E2 Affects Interleukin 6 and Monocyte Chemoattractant Protein 1/CCL2 Production by Cultured Stem Cells of Apical Papilla. J. Endod. 2020, 46, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L.; Mira, L.; Corvo, M.L. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr. Med. Chem. 2008, 15, 1586–1605. [Google Scholar] [CrossRef]
- Aziba, P.I.; Bass, D.; Elegbe, Y. Pharmacological investigation of Ocimum gratissimum in rodents. Phytother. Res. 1999, 13, 427–429. [Google Scholar] [CrossRef]
- Pires, A.F.; Madeira, S.V.; Soares, P.M.; Montenegro, C.M.; Souza, E.P.; Resende, A.C.; de Soares Moura, R.; Assreuy, A.M.; Criddle, D.N. The role of endothelium in the vasorelaxant effects of the essential oil of Ocimum gratissimum in aorta and mesenteric vascular bed of rats. Can. J. Physiol. Pharmacol. 2012, 90, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Interaminense, L.F.; Jucá, D.M.; Magalhães, P.J.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Pharmacological evidence of calcium-channel blockade by essential oil of Ocimum gratissimum and its main constituent, eugenol, in isolated aortic rings from DOCA-salt hypertensive rats. Fundam. Clin. Pharmacol. 2007, 21, 497–506. [Google Scholar] [CrossRef]
- Eftekhar, N.; Moghimi, A.; Hossein Boskabady, M.; Kaveh, M.; Shakeri, F. Ocimum basilicum affects tracheal responsiveness, lung inflammatory cells and oxidant-antioxidant biomarkers in sensitized rats. Drug Chem. Toxicol. 2019, 42, 286–294. [Google Scholar] [CrossRef]
- Makharia, G.K. Understanding and treating abdominal pain and spasms in organic gastrointestinal diseases: Inflammatory bowel disease and biliary diseases. J. Clin. Gastroenterol. 2011, 45, S89–S93. [Google Scholar] [CrossRef]
- Lahlou, S.; Interaminense, L.F.; Leal-Cardoso, J.H.; Morais, S.M.; Duarte, G.P. Cardiovascular effects of the essential oil of Ocimum gratissimum leaves in rats: Role of the autonomic nervous system. Clin. Exp. Pharmacol. Physiol. 2004, 31, 219–225. [Google Scholar] [CrossRef]
- Rashidian, A.; Roohi, P.; Mehrzadi, S.; Ghannadi, A.R.; Minaiyan, M. Protective Effect of Ocimum basilicum Essential Oil Against Acetic Acid-Induced Colitis in Rats. J. Evid. Based Complement. Altern. Med. 2016, 21, NP36–NP42. [Google Scholar] [CrossRef]
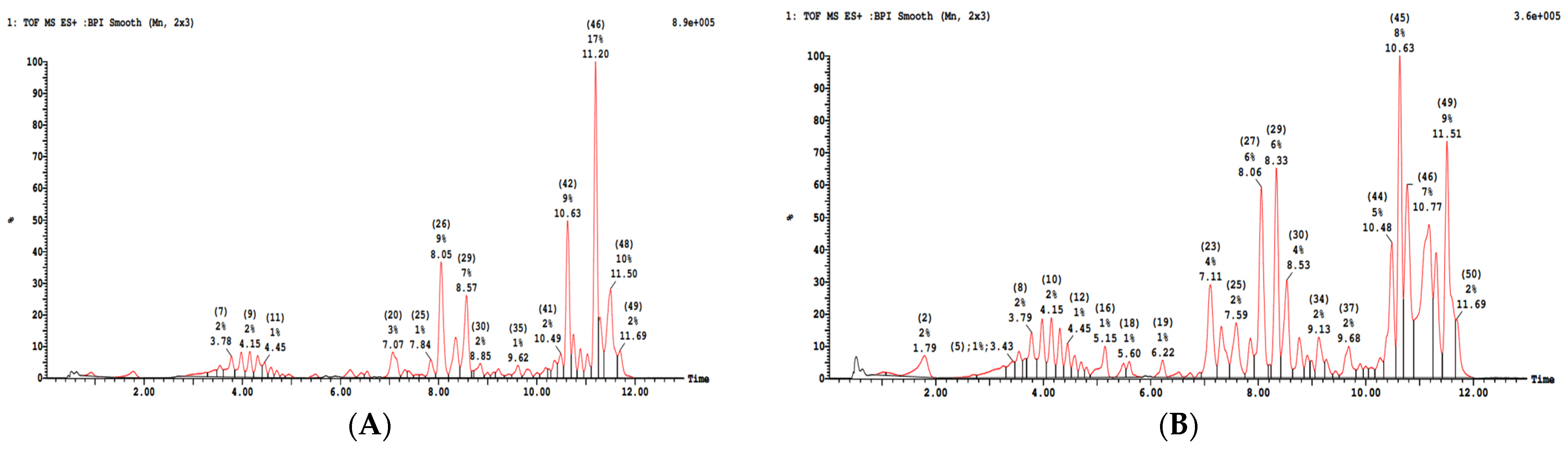





| RT (min) | M/Z | Water | Ethanol | Identification Based on Bibliography | Ref |
|---|---|---|---|---|---|
| 3.25 | 305.2 | x | x | (+)-Gallocatechin | [32,33] |
| 3.78 | 371.2 | x | x | Medioresinole (Furanoide lignine) | [34,35] |
| 4.31 | 503.3 | x | x | luteolin acetyl-glucuronide | [35,36,37] |
| 4.45 | 547.3 | x | x | 5.50-Dihydroxy-d-sesamin-5-O-glucoside | [38] |
| 5.31 | 329.2 | x | Trihydroxy octadecenoic acid (fatty Acid) | [34] | |
| 6.21 | 351.2 | x | Salvigenin(5-Hydroxy-6,7,4′-trimethoxyflavone) (Flavon) | [34] | |
| 6.51 | 329.2 | x | x | Trihydroxy octadecenoic acid (fatty Acid) | [34] |
| 6.54 | 359.1 | x | rosmarinic Acid | [34,35] | |
| 6.93 | 517.3 | x | luteolin acetyl-glucuronide | [35,36,37] | |
| 7.07 | 345.1 | x | Eupatorin (3′,5-Dihydroxy-4′,6,7-trimethoxyflavone) Flavon | [34] | |
| 8.05 | 329.1 | x | Trihydroxy Acid octadecenoic ((Fatty Acid) | [34] | |
| 8.57 | 325.1 | x | x | feruloyltartaric acid | [39,40,41] |
| 9.62 | 325.1 | x | feruloyltartaric acid | [39,41] | |
| 10.49 | 609.3 | x | Rutin (flavonoid) | [34,36,37] |
| RT (min) | M/Z | Water | Ethanol | Identification Based on Bibliography | Ref |
|---|---|---|---|---|---|
| 1 | 191.1 | x | Isocitric/citric acid | [34] | |
| 3.57 | 327.2 | x | Salvigenin(5-Hydroxy-6,7,4′-trimethoxyflavone) Flavones | [34] | |
| 3.78 | 387.2 | x | x | Medioresinole (Furanoide lignine) | [34,36] |
| 4.21 | 507.2 | x | Quercitin-acetyl-O-glucoside | [40] | |
| 4.32 | 503.3 | x | luteolin acetyl-glucuronide | [35,36,37] | |
| 4.45 | 547.3 | x | x | 5.50-Dihydroxy-d-sesamin-5-O-glucoside | [38] |
| 4.98 | 359.1 | x | Rosmarinic acid | [34,35] | |
| 5.14 | 359.1 | x | Rosmarinic acid | [34,35] | |
| 5.51 | 435.1 | x | Naringenin glucoside | [41] | |
| 6.22 | 327.2 | x | x | Salvigenin(5-Hydroxy-6,7,4′-trimethoxyflavone) Flavone | [34] |
| 6.52 | 329.2 | x | x | Trihydroxy octadecenoic acid (Fatty Acid) | [34] |
| 7.85 | 311.2 | x | Caftaric acid | [34,35,42] | |
| 8.06 | 329.1 | x | Trihydroxy octadecenoic acid (fatty Acid) | [34] | |
| 8.33 | 359.1 | x | Rosmarinic Acid | [34,35] | |
| 8.53 | 299.1 | x | hydroxybenzoic acid-O-glucoside(salicylic acid-O-glucoside) | [39] | |
| 8.91 | 295.2 | x | Cinnamyl malic acid | [42] | |
| 9.13 | 311.2 | x | x | caftaric acid | [34,35] |
| 9.74 | 353.2 | x | x | Chlorogenic acid | [36,38] |
| 10.48 | 609.3 | x | Rutin (flavonoid) | [34,36,37] | |
| 10.63 | 593.3 | x | Vicenin II (flavonoid) | [39] | |
| 10.77 | 593.3 | x | x | feruloyltartaric acid | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensaid, A.; Boudard, F.; Servent, A.; Morel, S.; Portet, K.; Guzman, C.; Vitou, M.; Bichon, F.; Poucheret, P. Differential Nutrition-Health Properties of Ocimum basilicum Leaf and Stem Extracts. Foods 2022, 11, 1699. https://doi.org/10.3390/foods11121699
Bensaid A, Boudard F, Servent A, Morel S, Portet K, Guzman C, Vitou M, Bichon F, Poucheret P. Differential Nutrition-Health Properties of Ocimum basilicum Leaf and Stem Extracts. Foods. 2022; 11(12):1699. https://doi.org/10.3390/foods11121699
Chicago/Turabian StyleBensaid, Aicha, Frederic Boudard, Adrien Servent, Sylvie Morel, Karine Portet, Caroline Guzman, Manon Vitou, Florence Bichon, and Patrick Poucheret. 2022. "Differential Nutrition-Health Properties of Ocimum basilicum Leaf and Stem Extracts" Foods 11, no. 12: 1699. https://doi.org/10.3390/foods11121699






