Amino Acid Profiles in Peach (Prunus persica L.) Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Collection
2.3. Amino Acids Analysis
2.4. Flesh Parameters
2.5. Protein Quality Calculations
2.6. Statistics
3. Results
3.1. Flesh Parameters
3.2. Amino Acids
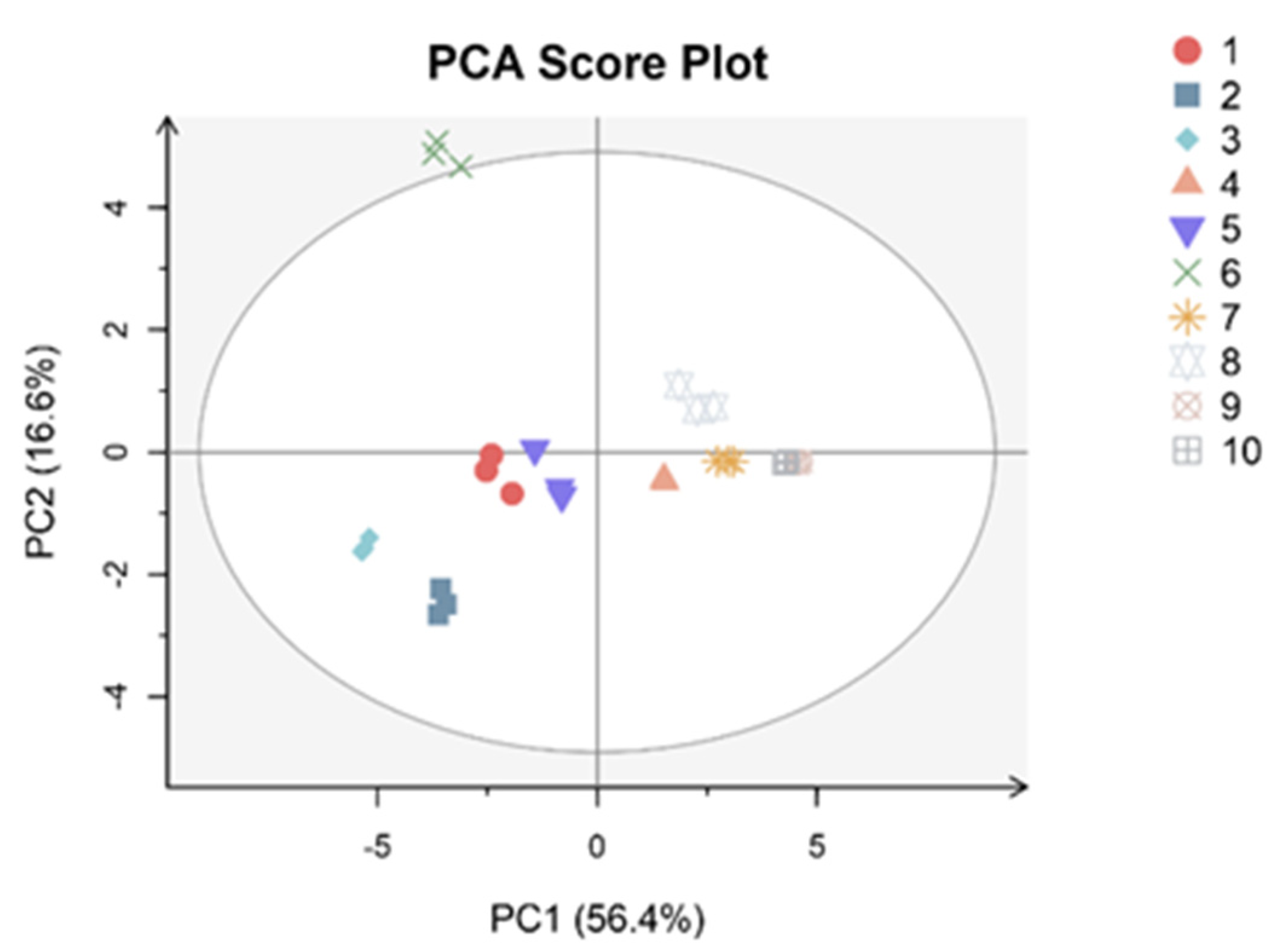
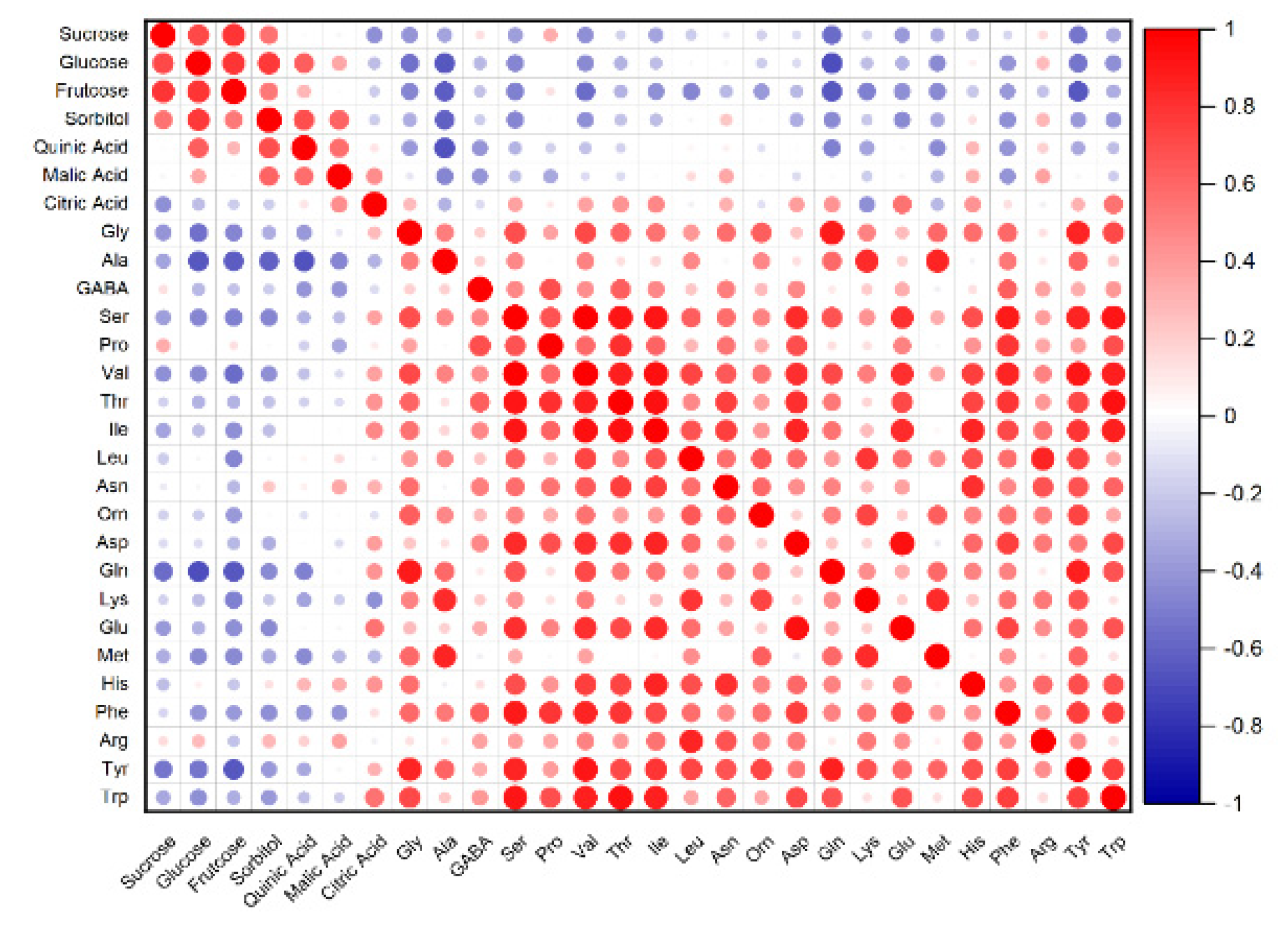
3.3. PCA and Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Weisskopf, A.; Fuller, D.Q. Peach: Origins and Development; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Bender, D.A. Amino Acids Synthesized from Glutamate: Glutamine, Proline, Ornithine, Citrulline and Arginine. In Amino Acid Metabolism, 3rd ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 157–223. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Oxidative phosphorylation & photophosphorylation. InLehninger Principles of Biochemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 2008; pp. 947–948. [Google Scholar]
- Covarrubias, M.P.; Lillo-Carmona, V.; Melet, L.; Benedetto, G.; Andrade, D.; Maucourt, M.; Deborde, C.; Fuentealba, C.; Moing, A.; Valenzuela, M.L.; et al. Metabolite Fruit Profile Is Altered in Response to Source–Sink Imbalance and Can Be Used as an Early Predictor of Fruit Quality in Nectarine. Front. Plant Sci. 2021, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative Study of Early- and Mid-Ripening Peach (Prunus persica L.) Varieties: Biological Activity, Macro-, and Micro- Nutrient Profile. Foods 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Famiani, F.; Bonghi, C.; Chen, Z.-H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone Fruits: Growth and Nitrogen and Organic Acid Metabolism in the Fruits and Seeds—A Review. Front. Plant Sci. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Dabbou, S.; Maatallah, S.; Antonelli, A.; Montevecchi, G. Variation of amino acids in Prunus persica cultivars leaves with regard to leaf age. Sci. Hortic. 2021, 281, 110001. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines: Anatomy and Physiology; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar] [CrossRef]
- Davidović, S.M.; Veljović, M.S.; Pantelić, M.M.; Baošić, R.M.; Natić, M.M.; Dabić, D.; Pecić, S.P.; Vukosavljević, P.V. Physicochemical, Antioxidant and Sensory Properties of Peach Wine Made from Redhaven Cultivar. J. Agric. Food Chem. 2013, 61, 1357–1363. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Ren, T.; Wang, J.; Niu, C.; Zheng, F.; Li, Q. Effect of Saccharomyces cerevisiae and non-Saccharomyces strains on alcoholic fermentation behavior and aroma profile of yellow-fleshed peach wine. LWT Food Sci. Technol. 2022, 155, 112993. [Google Scholar] [CrossRef]
- Gregan, S.; Wargent, J.; Liu, L.; Shinkle, J.; Hofmann, R.; Winefield, C.; Trought, M.; Jordan, B. Effects of solar ultraviolet radiation and canopy manipulation on the biochemical composition of Sauvignon Blanc grapes. Aust. J. Grape Wine Res. 2012, 18, 227–238. [Google Scholar] [CrossRef]
- Shen, Z.J.; Ma, R.J.; Yu, M.L.; Cai, Z.X.; Song, H.F.; Li, X. Regularity Analysis of Main Sugar and Acid in Fruit Development of Peach. Agric. Boreali-Sin. 2007, 22, 130–135. [Google Scholar]
- Nosworthy, M.G.; Franczyk, A.J.; Medina, G.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of Processing on the in Vitro and in Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum). J. Agric. Food Chem. 2017, 65, 7790–7796. [Google Scholar] [CrossRef]
- Botoran, O.R.; Ionete, R.E.; Miricioiu, M.G.; Costinel, D.; Radu, G.L.; Popescu, R. Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin. Molecules 2019, 24, 4500. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.; Vercambre, G.; Jordan, M.-O. Spatial-temporal management of nitrogen and carbon on the peach tree (Prunus persicae L. Batsch.). Sci. Hortic. 2020, 273, 109613. [Google Scholar] [CrossRef]
- Maatallah, S.; Dabbou, S.; Castagna, A.; Guizani, M.; Hajlaoui, H.; Ranieri, A.M.; Flamini, G. Prunus persica by-products: A source of minerals, phenols and volatile compounds. Sci. Hortic. 2019, 261. [Google Scholar] [CrossRef]
- Farina, V.; Bianco, R.L.; Mazzaglia, A. Evaluation of Late-Maturing Peach and Nectarine Fruit Quality by Chemical, Physical, and Sensory Determinations. Agriculture 2019, 9, 189. [Google Scholar] [CrossRef]
- Rodriguez, C.E.; Bustamante, C.A.; Budde, C.O.; Müller, G.L.; Drincovich, M.F.; Lara, M.V. Peach Fruit Development: A Comparative Proteomic Study Between Endocarp and Mesocarp at Very Early Stages Underpins the Main Differential Biochemical Processes Between These Tissues. Front. Plant Sci. 2019, 10, 715. [Google Scholar] [CrossRef]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic Profiling during Peach Fruit Development and Ripening Reveals the Metabolic Networks That Underpin Each Developmental Stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Fedeli, C.; Morgutti, S.; Negrini, N.; Cocucci, M.; Espen, L. Peach fruit ripening: A proteomic comparative analysis of the mesocarp of two cultivars with different flesh firmness at two ripening stages. Phytochemistry 2011, 72, 1251–1262. [Google Scholar] [CrossRef]
- Guan, L.; Li, Y.; Wang, L. Detection and application of genome-wide variations in peach for association and genetic relationship analysis current status: Accepted subject areas plant molecular biology and genetics. BMC Genet. 2019, 20, 101. [Google Scholar] [CrossRef]
- Milne, R.; Grof, C.P.; Patrick, J.W. Mechanisms of phloem unloading: Shaped by cellular pathways, their conductances and sink function. Curr. Opin. Plant Biol. 2018, 43, 8–15. [Google Scholar] [CrossRef]
- Moing, A.; Carbonne, F.; Zipperlin, B.; Svanella, L.; Gaudillere, J.-P. Phloem loading in peach: Symplastic or apoplastic? Physiol. Plant 1997, 101, 489–496. [Google Scholar] [CrossRef]
- Millard, P.; Wendler, R.; Grassi, G.; Grelet, G.-A.; Tagliavini, M. Translocation of nitrogen in the xylem of field-grown cherry and poplar trees during remobilization. Tree Physiol. 2006, 26, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Sonia, E.V.; Maldonado, A.M.; Francisco, A.R.; Berta, S.; Fernando, R.; Fernando, P.A.; Juan, M.B.; Caballero, J.L. Evidence for a positive regulatory role of strawberry (Fragaria × ananassa) Fa WRKY1 and Arabidopsis At WRKY75 proteins in resistance. J. Exp. Bot. 2009, 60, 3043–3065. [Google Scholar]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hu, X.; Yu, J.; Yang, A.; Liu, Y. Caffeoyl Shikimate Esterase has a Role in Endocarp Lignification in Peach (Prunus persica L.) Fruit. Korean J. Hortic. Sci. 2017, 35, 59–68. [Google Scholar] [CrossRef]
- Arias-Gil, M.; Garde-Cerdán, T.; Ancín-Azpilicueta, C. Influence of addition of ammonium and different amino acid concentrations on nitrogen metabolism in spontaneous must fermentation. Food Chem. 2007, 103, 1312–1318. [Google Scholar] [CrossRef]
- Henschke, P.A.; Jiranek, V. Yeasts-metabolism of nitrogen compounds. In Wine Microbiology and Biotechnology; Harwood Academic Publishers GmbH: Amsterdam, The Netherlands, 1993; pp. 77–164. [Google Scholar]
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.; Ogutu, C.; Ntini, C.; Zhang, X.; Han, Y. Assessment of organic acid accumulation and its related genes in peach. Food Chem. 2020, 334. [Google Scholar] [CrossRef]
- Bizzio, L.N.; Tieman, D.; Munoz, P.R. Branched-Chain Volatiles in Fruit: A Molecular Perspective. Front. Plant Sci. 2022, 12. [Google Scholar] [CrossRef]
- Walker, R.P.; Battistelli, A.; Moscatello, S.; Chen, Z.-H.; Leegood, R.C.; Famiani, F. Metabolism of the seed and endocarp of cherry (Prunus avium L.) during development. Plant Physiol. Biochem. 2011, 49, 923–930. [Google Scholar] [CrossRef]

| Variety | Type | Flesh Colour | |
|---|---|---|---|
| 1. | Jinxia | nectarine | yellow |
| 2. | Yuhua 3 | peach | white |
| 3. | Tropic Prince | peach | yellow |
| 4. | Dahezaosheng | peach | white |
| 5. | Yixianhong | peach | white |
| 6. | Chengxiang | peach | yellow |
| 7. | NJN76 | nectarine | white |
| 8. | Sazizaosheng | peach | white |
| 9. | Hongrou1 | peach | red |
| 10. | Touxinhong | peach | red |
| Varieties | Sucrose | Glucose | Fructose | Sorbitol | Quinic Acid | Malic Acid | Citric Acid | Total Acids |
|---|---|---|---|---|---|---|---|---|
| 1. Jinxia | 43.98 a | 12.06 b | 15.27 e | 3.70 b | 1.90 cd | 2.43 e | 0.58 g | 4.91 f |
| 2. Yuhua3 | 32.67 c | 10.47 d | 13.57 cd | 1.10 f | 1.57 bc | 2.28 f | 2.11 c | 5.96 de |
| 3. Tropic Prince | 25.67 e | 8.50 e | 12.09 b | 2.06 d | 1.98 cd | 5.15 c | 3.30 a | 10.43 b |
| 4. Dahezaosheng | 23.82 f | 8.52 e | 11.65 ab | 1.27 f | 2.04 cd | 3.58 d | 2.11 c | 7.72 c |
| 5. Yixianhong | 32.91 c | 14.13 a | 12.97 bc | 4.24 a | 4.23 a | 7.74 a | 1.45 e | 13.42 a |
| 6. Chengxiang | 23.63 f | 7.59 f | 10.43 a | 1.12 f | 1.09 e | 2.82 e | 1.28 ef | 5.20 ef |
| 7. NJN76 | 40.63 b | 11.54 b | 14.87 de | 2.90 c | 1.63 d | 6.46 b | 2.36 b | 10.45 b |
| 8. Shazizaosheng | 34.46 c | 9.22 e | 12.54 bc | 1.76 e | 1.07 e | 1.78 f | 0.38 h | 3.22 g |
| 9. Hongrou1 | 30.10 d | 11.02 c | 14.51 de | 3.75 b | 3.82 a | 4.88 c | 1.71 d | 10.40 b |
| 10. Touxinhong | 39.15 b | 14.07 a | 17.76 f | 2.70 c | 2.62 b | 2.76 e | 1.15 f | 6.53 d |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Amino Acid (µg/g) | α-Ketoglutarate | Shikimate (Aromatic) | Pyruvate | Aspartate | 3-phosphoglycerate | Others | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pro | Arg | Glu | Gln | His | Phe | Trp | Tyr | Leu | Val | Ala | Asp | Asn | Thr | Ile | Met | Lys | Ser | Gly | Orn | GABA | Total | |
| 1. Jinxia | 65.26 a | 1.60 b | 54.17 e | 24.62 de | 4.23 d | 10.15 b | 0.49 c | 4.00 b | 3.20 d | 13.72 d | 20.19 d | 108.78 d | 2566.68 b | 28.08 c | 5.65 d | 0.21 | 5.52 b | 45.78 c | 1.70 b | 0.47 ab | 20.16 a | 2984.66 b |
| 2. Yuhua3 | 52.02 b | 1.26 c | 145.03 a | 27.32 cde | 5.16 c | 11.12 a | 0.98 b | 4.12 b | 3.93 c | 22.76 b | 24.74 c | 240.56 a | 1126.78 de | 33.20 b | 10.85 b | N.A | 3.28 d | 77.13 a | N.A | 0.08 c | 12.18 b | 1802.50 c |
| 3. Tropic Prince | 41.72 c | 1.02 d | 97.07 b | 111.91 a | 9.26 a | 7.86 d | 1.52 a | 7.34 a | 3.04 d | 25.59 a | 15.09 e | 148.13 b | 3279.15 a | 43.21 a | 13.27 a | N.A | 2.08 e | 77.60 a | 4.04 a | 0.29 ab | 12.42 b | 3901.58 a |
| 4. Dahezaosheng | 7.89 fg | 0.65 e | 85.17 c | 29.05 cd | 1.75 f | 5.06 e | 0.13 d | 3.09 c | 1.26 f | 9.24 e | 15.18 e | 84.76 e | 1234.31 cd | 12.52 e | 4.11 e | N.A | 2.18 e | 26.46 e | N.A | 0.11 bc | 12.52 b | 1535.43 d |
| 5. Yixianhong | 10.97 def | 2.54 a | 82.48 cd | 22.62 e | 7.78 b | 3.02 f | N.A | 3.86 b | 6.08 a | 13.84 d | 8.50 f | 124.93 c | 2517.30 b | 16.07 d | 8.18 c | N.A | 5.27 b | 33.24 d | N.A | 0.31 ab | 6.21 c | 2873.20 b |
| 6. Chengxiang | 12.46 d | 1.04 d | 76.12 d | 106.03 a | 4.52 d | 9.26 c | 0.55 c | 7.77 a | 5.14 b | 21.13 c | 63.14 a | 83.98 e | 1318.34 c | 16.05 d | 5.75 d | 0.90 | 9.56 a | 61.89 b | 3.79 a | 0.54 a | 4.39 c | 1812.34 c |
| 7. NJN76 | 14.22 d | 0.96 d | 56.41 e | 37.48 b | 1.29 g | 2.91 f | N.A | 1.37 d | 1.16 f | 4.37 g | 12.69 e | 69.99 f | 1061.27 e | 8.21 f | 1.03 g | N.A | 1.72 ef | 16.34 f | N.A | N.A | 4.74 c | 1296.18 e |
| 8. Shazizaosheng | 8.69 efg | 0.74 e | 30.06 f | 33.33 bc | 0.91 g | 2.44 f | N.A | 1.81 d | 1.84 e | 7.14 f | 36.95 b | 60.08 g | 907.75 f | 11.35 e | 2.63 f | N.A | 4.08 c | 25.98 e | N.A | N.A | 14.04 b | 1149.83 f |
| 9. Hongrou1 | 5.64 g | N.A | 30.97 f | 13.15 f | 2.02 f | 0.42 g | N.A | 0.34 e | 0.09 h | 1.64 h | 4.59 g | 33.26 h | 611.16 g | 5.80 g | 0.41 g | N.A | 0.36 g | 12.53 g | N.A | N.A | 0.85 d | 723.22 g |
| 10. Touxinhong | 12.10 de | 0.26 f | 24.61 f | 6.72 g | 3.32 e | 0.60 g | N.A | 0.51 e | 0.68 g | 1.57 h | 2.73 g | 30.95 h | 559.60 g | 5.29 g | 0.72 g | N.A | 1.37 f | 10.58 g | N.A | N.A | 1.10 d | 662.70 g |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Variety | 1. Jinxia | 2. Yuhua3 | 3. Tropic Prince | 4. Dahezaosheng | 5. Yixianhong | 6. Chengxiang | 7. NJN76 | 8. Shazizaosheng | 9. Hongrou1 | 10. Touxinhong |
|---|---|---|---|---|---|---|---|---|---|---|
| Val | 0.35 | 0.58 | 0.66 | 0.24 | 0.35 | 0.54 | 0.11 | 0.18 | 0.04 | 0.04 |
| Met | 0.04 | 0 | 0 | 0 | 0 | 0.15 | 0 | 0 | 0 | 0 |
| Lys | 0.12 | 0.07 | 0.05 | 0.05 | 0.12 | 0.21 | 0.04 | 0.09 | 0.01 | 0.03 |
| Ile | 0.19 | 0.36 | 0.44 | 0.14 | 0.27 | 0.19 | 0.03 | 0.09 | 0.01 | 0.02 |
| Leu | 0.05 | 0.07 | 0.05 | 0.02 | 0.10 | 0.09 | 0.02 | 0.03 | 0.00 | 0.01 |
| Phe + Tyr | 0.64 | 0.69 | 0.69 | 0.37 | 0.31 | 0.77 | 0.19 | 0.19 | 0.03 | 0.05 |
| Thr | 1.22 | 1.44 | 1.88 | 0.54 | 0.70 | 0.70 | 0.36 | 0.49 | 0.25 | 0.23 |
| His | 0.28 | 0.34 | 0.62 | 0.12 | 0.52 | 0.30 | 0.09 | 0.06 | 0.13 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Zhao, B.; Cai, Z.; Yan, J.; Ma, R.; Yu, M. Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods 2022, 11, 1718. https://doi.org/10.3390/foods11121718
Sun M, Zhao B, Cai Z, Yan J, Ma R, Yu M. Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods. 2022; 11(12):1718. https://doi.org/10.3390/foods11121718
Chicago/Turabian StyleSun, Meng, Bintao Zhao, Zhixiang Cai, Juan Yan, Ruijuan Ma, and Mingliang Yu. 2022. "Amino Acid Profiles in Peach (Prunus persica L.) Fruit" Foods 11, no. 12: 1718. https://doi.org/10.3390/foods11121718
APA StyleSun, M., Zhao, B., Cai, Z., Yan, J., Ma, R., & Yu, M. (2022). Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods, 11(12), 1718. https://doi.org/10.3390/foods11121718







