Antimicrobial Properties of Chilean Native Plants: Future Aspects in Their Application in the Food Industry
Abstract
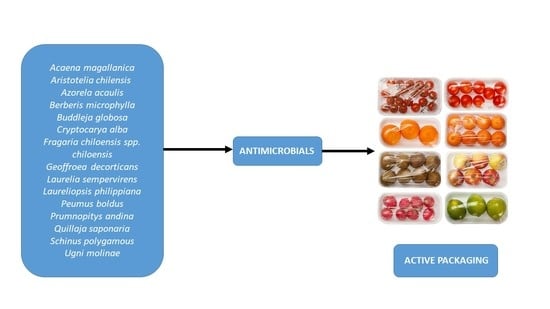
1. Introduction
2. Materials and Methods
3. Antimicrobial Properties of Chilean Native Plants
3.1. Acaena magallánica
3.2. Aristotela chilensis
3.3. Azorella acaulis
3.4. Berberis microphylla
3.5. Buddleja globosa
3.6. Cryptocarya alba
3.7. Fragaria chiloensis spp. chiloensis
3.8. Geoffroea decorticans
3.9. Laurelia sempervirens
3.10. Laureliopsis philippiana
3.11. Peumus boldus
3.12. Prumnopitys andina
3.13. Quillaja saponaria
3.14. Schinus polygama
3.15. Ugni molinae
4. Plant Metabolites Incorporated in Films and its Application in Food Industry
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koutsoumanis, K.; Tsaloumi, S.; Aspridou, Z.; Tassou, C.; Gougouli, M. Application of quantitative microbiological risk assessment (QMRA) to food spoilage: Principles and methodology. Trends Food Sci. Technol. 2021, 114, 189–197. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial biofilms in the food industry-A comprehensive review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Dong, M.; Zhang, H.; Li, L.; Wang, L. Food spoilage, bioactive food fresh-keeping films and functional edible coatings: Research status, existing problems and development trend. Trends Food Sci. Technol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Ge, H.; Wang, Y.; Zhao, X. Research on the drug resistance mechanism of foodborne pathogens. Microb. Pathog. 2022, 162, 105306. [Google Scholar] [CrossRef]
- Masi, M.; Winterhalter, M.; Pagès, J.M. Outer membrane porins. Subcell. Biochem. 2019, 92, 79–123. [Google Scholar] [CrossRef]
- Li, X.Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Wright, G.D. Bacterial resistance to antibiotics: Enzymatic degradation and modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef]
- Lambert, P.A. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005, 57, 1471–1485. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Zhang, Q.; Shen, J. Antimicrobial resistance in Campylobacter spp. Microbiol. Spectr. 2018, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Mąka, Ł.; Popowska, M. Antimicrobial resistance of Salmonella spp. isolated from food. Rocz. Panstw. Zakl. Hig. 2016, 67, 343–358. [Google Scholar] [PubMed]
- Delshadi, R.; Bahrami, A.; Assadpour, E.; Williams, L.; Jafari, S.M. Nano/microencapsulated natural antimicrobials to control the spoilage microorganisms and pathogens in different food products. Food Control 2021, 128, 108180. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A review on techniques utilized for design of controlled release food active packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 2601–2621. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Moreira-Muñoz, A. The extravagant physical geography of Chile. In Plant Geography of Chile; Springer: Dordrecht, The Netherlands, 2011; pp. 3–45. [Google Scholar]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catalogue of the vascular plants of Chile. Gayana Bot. 2018, 75, 1–430. [Google Scholar] [CrossRef]
- Arroyo, M.T.; Marquet, P.; Marticorena, C.; Simonetti, J.; Cavieres, L.; Squeo, F.; Rozzi, R.; Massardo, F. El hotspot chileno, prioridad mundial para la conservacion. Biodivers. Chile Patrim. Desafios 2008, 90–93. [Google Scholar]
- Salehi, B.; Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Delporte, C.; Barra, G.V.; Cazar Ramirez, M.-E.; López, M.D.; Ramírez Alarcón, K.; Martins, N.; et al. Ethnopharmacology, phytochemistry and biological activities of native chilean plants. Curr. Pharm. Des. 2021, 27, 953–970. [Google Scholar] [CrossRef] [PubMed]
- Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Recent developments in seafood packaging technologies. Foods 2021, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Marticorena, A.E.; Cavieres, L.A. Acaena magellanica (Lam.) Vahl (Rosaceae). Gayana. Botánica 2000, 57, 107–113. [Google Scholar] [CrossRef]
- Feresin, G.E.; Tapia, A.; López, S.N.; Zacchino, S.A. Antimicrobial activity of plants used in traditional medicine of San Juan province, Argentine. J. Ethnopharmacol. 2001, 78, 103–107. [Google Scholar] [CrossRef]
- Feresin, G.E.; Tapia, A.; Angel, G.R.; Delporte, C.; Erazo, N.B.; Schmeda-Hirschmann, G. Free radical scavengers, anti-inflammatory and analgesic activity of Acaena magellanica. J. Pharm. Pharmacol. 2002, 54, 835–844. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Campos-Requena, V.H. Antioxidant compound extraction from maqui (Aristotelia chilensis [Mol] Stuntz) berries: Optimization by response surface methodology. Antioxidants 2017, 6, 10. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensis (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef]
- Mølgaard, P.; Holler, J.G.; Asar, B.; Liberna, I.; Rosenbæk, L.B.; Jebjerg, C.P.; Jørgensen, L.; Lauritzen, J.; Guzman, A.; Adsersen, A.; et al. Antimicrobial evaluation of Huilliche plant medicine used to treat wounds. J. Ethnopharmacol. 2011, 138, 219–227. [Google Scholar] [CrossRef]
- Wickens, G.E. Llareta (Azorella Compacta, Umbelliferae): A review. Econ. Bot. 1995, 49, 207–212. [Google Scholar] [CrossRef]
- Loyola, L.A.; Bórquez, J.; Morales, G.; Araya, J.; González, J.; Neira, I.; Sagua, H.; San-Martín, A. Diterpenoids from Azorella yareta and their trichomonicidal activities. Phytochemistry 2001, 56, 177–180. [Google Scholar] [CrossRef]
- Molina-Salinas, G.M.; Bórquez, J.; Ardiles, A.; Said-Fernández, S.; Loyola, L.A.; San-Martín, A.; González-Collado, I.; Peña-Rodríguez, L.M. Antituberculosis activity of natural and semisynthetic azorellane and mulinane diterpenoids. Fitoterapia 2010, 81, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Cordero, S.; Abello, L.; Galvez, F. Corporación Chilena de la Madera, Concepción, Chile. Plantas Silvestres Comestibles y Medicinales de Chile y Otras Partes del Mundo. 2017. Available online: https://ceab-rizoma.com/wp-content/uploads/2021/01/Plantas-silvestres-comestibles-y-medicinales-de-Chile-y-otras-partes-del-mundo.pdf (accessed on 2 March 2022).
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; Von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Mardones, C.; Vergara, C.; Von Baer, D.; Gómez-Alonso, S.; Gómez, M.V.; Hermosín-Gutiérrez, I. Isolation and structural elucidation of anthocyanidin 3,7-β-O-diglucosides and caffeoyl-glucaric acids from calafate berries. J. Agric. Food Chem. 2014, 62, 6918–6925. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, L.; Mutis, A.; Díaz, J.; Urzúa, A.; Fajardo, V.; Quiroz, A. Identification of isoquinoline alkaloids from Berberis microphylla by HPLC ESI-MS/MS. Bol. Latinoam. Caribe Plantas Med. Aromát 2014, 324–335. [Google Scholar]
- Manosalva, L.; Mutis, A.; Urzúa, A.; Fajardo, V.; Quiroz, A. Antibacterial activity of alkaloid fractions from Berberis microphylla G. Forst and study of synergism with ampicillin and cephalothin. Molecules 2016, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Pitta-Alvarez, S.I.; Medina-Bolivar, F.; Alvarez, M.A.; Scambatto, A.A.; Marconi, P.L. In vitro shoot culture and antimicrobial activity of Berberis buxifolia Lam. Vitr. Cell. Dev. Biol. Plant 2008, 44, 502–507. [Google Scholar] [CrossRef]
- Muñoz, O.M.; Maya, J.D.; Ferreira, J.; Christen, P.; San Martin, J.; López-Muñoz, R.; Morello, A.; Kemmerling, U. Medicinal plants of Chile: Evaluation of their anti-Trypanosoma cruzi activity. Z. Naturforsch. C 2013, 68, 198–202. [Google Scholar] [CrossRef]
- Norman, E.M. Buddlejaceae. Flora Neotropica; The New York Botanical Garden Press: New York, NY, USA, 2000; pp. 1–224. [Google Scholar]
- Pardo, F.; Perich, F.; Villarroel, L.; Torres, R. Isolation of verbascoside, an antimicrobial constituent of Buddleja globosa leaves. J. Ethnopharmacol. 1993, 39, 221–222. [Google Scholar] [CrossRef]
- Pellegrini, M.C.; Alonso-Salces, R.M.; Umpierrez, M.L.; Rossini, C.; Fuselli, S.R. Chemical composition, antimicrobial activity, and mode of action of essential oils against Paenibacillus larvae, etiological agent of american foulbrood on Apis mellifera. Chem. Biodivers. 2017, 14, e1600382. [Google Scholar] [CrossRef]
- Mensah, A.Y.; Houghton, P.J.; Bloomfield, S.; Vlietinck, A.; Berghe, D.V. Known and novel terpenes from Buddleja globosa displaying selective antifungal activity against dermatophytes. J. Nat. Prod. 2000, 63, 1210–1213. [Google Scholar] [CrossRef]
- Debenedetti, S.; Muschietti, L.; Van Baren, C.; Clavin, M.; Broussalis, A.; Martino, V.; Houghton, P.J.; Warhurst, D.; Steele, J. In vitro antiplasmodial activity of extracts of Argentinian plants. J. Ethnopharmacol. 2002, 80, 163–166. [Google Scholar] [CrossRef]
- Touma, J.; Navarro, M.; Sepúlveda, B.; Pavon, A.; Corsini, G.; Fernández, K.; Quezada, C.; Torres, A.; Larrazabal-Fuentes, M.J.; Paredes, A.; et al. The chemical compositions of essential oils derived from Cryptocarya alba and Laurelia sempervirens possess antioxidant, antibacterial and antitumoral activity potential. Molecules 2020, 25, 5600. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Astudillo, L.; Bastida, J.; Codina, C.; De Arias, A.R.; Ferreira, M.E.; Inchaustti, A.; Yaluff, G. Cryptofolione derivatives from Cryptocarya alba fruits. J. Pharm. Pharmacol. 2010, 53, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.F.; Ladio, A. Native and exotic plants with edible fleshy fruits utilized in patagonia and their role as sources of local functional foods. BMC Complement. Med. Ther. 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, A.R.; Ellis, S.M.; Hancock, R.E.W.; Towers, G.H.N. Antibiotic screening of medicinal plants of the British Columbian native peoples. J. Ethnopharmacol. 1992, 37, 213–223. [Google Scholar] [CrossRef]
- McCutcheon, A.R.; Ellis, S.M.; Hancock, R.E.W.; Towers, G.H.N. Antifungal screening of medicinal plants of British Columbian native peoples. J. Ethnopharmacol. 1994, 44, 157–169. [Google Scholar] [CrossRef]
- Salvat, A.; Antonacci, L.; Fortunato, R.H.; Suarez, E.Y.; Godoy, H.M. Antimicrobial activity in methanolic extracts of several plant species from northern Argentina. Phytomedicine 2004, 11, 230–234. [Google Scholar] [CrossRef]
- Quiroga, E.N.; Sampietro, D.A.; Sgariglia, M.A.; Soberón, J.R.; Vattuone, M.A. Antimycotic activity of 5′-prenylisoflavanones of the plant Geoffroea decorticans, against Aspergillus species. Int. J. Food Microbiol. 2009, 132, 42–46. [Google Scholar] [CrossRef]
- Bittner, M.; Aguilera, M.A.; Hernández, V.; Arbert, C.; Becerra, J.; Casanueva, M.E. Actividad fungistática de extractos de aceites esenciales de Peumus boldus Mol., Laureliopsis philippiana (Looser) Schodde y Laurelia sempervirens (Ruiz & Pav.) Tul. (Monimiaceae chilenas). Chil. J. Agric. Res. 2009, 69, 30–37. [Google Scholar] [CrossRef]
- Ortiz, C.; Silva, G.; Moya, E.; Fischer, S.; Urbina, A.; Rodríguez, J.C. Variación estacional de la repelencia de los aceites esenciales de Monimiaceae sobre Sitophilus zeamais Motschulsky (Curculionidae). Chil. J. Agric. Anim. Sci. 2017, 33, 221–230. [Google Scholar] [CrossRef]
- Madrid, A.; Godoy, P.; González, S.; Zaror, L.; Moller, A.; Werner, E.; Cuellar, M.; Villena, J.; Montenegro, I. Chemical characterization and anti-oomycete activity of Laureliopsis philippianna essential oils against Saprolegnia parasitica and S. australis. Molecules 2015, 20, 8033–8047. [Google Scholar] [CrossRef] [PubMed]
- Pastene, E.; Parada, V.; Avello, M.; Ruiz, A.; García, A. Catechin-based procyanidins from Peumus boldus mol. aqueous extract inhibit Helicobacter pylori urease and adherence to adenocarcinoma gastric cells. Phyther. Res. 2014, 28, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Bluma, R.V.; Etcheverry, M.G. Application of essential oils in maize grain: Impact on Aspergillus section Flavi growth parameters and aflatoxin accumulation. Food Microbiol. 2008, 25, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Passone, M.A.; Etcheverry, M. Antifungal impact of volatile fractions of Peumus boldus and Lippia turbinata on Aspergillus section Flavi and residual levels of these oils in irradiated peanut. Int. J. Food Microbiol. 2014, 168–169, 17–23. [Google Scholar] [CrossRef]
- Ferrante, C.; Chiavaroli, A.; Angelini, P.; Venanzoni, R.; Flores, G.A.; Brunetti, L.; Petrucci, M.; Politi, M.; Menghini, L.; Leone, S.; et al. Phenolic content and antimicrobial and anti-Inflammatory effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics 2020, 9, 783. [Google Scholar] [CrossRef]
- Da Cruz, J.E.R.; da Costa Guerra, J.F.; de Souza Gomes, M.; de Freitas, G.R.O.; Morais, E.R. Phytochemical analysis and evaluation of antimicrobial activity of Peumus boldus, Psidium guajava, Vernonia polysphaera, Persea Americana, Eucalyptus citriodora leaf extracts and Jatropha multifida Raw Sap. Curr. Pharm. Biotechnol. 2019, 20, 433–444. [Google Scholar] [CrossRef]
- Santoro, D.; Bohannon, M.; Ahrens, K.; Navarro, C.; Gatto, H.; Marsella, R. Evaluation on the effects of 0.1% Peumus boldus leaf and Spiraea ulmaria plant extract combination on bacterial colonization in canine atopic dermatitis: A preliminary randomized, placebo controlled, double-blinded study. Res. Vet. Sci. 2018, 118, 164–170. [Google Scholar] [CrossRef]
- Morello, A.; Lipchenca, I.; Cassels, B.K.; Speisky, H.; Aldunate, J.; Repetto, Y. Trypanocidal effect of boldine and related alkaloids upon several strains of Trypanosoma cruzi. Comp. Biochem. Physiol. Pharmacol. Toxicol. Endocrinol. 1994, 107, 367–371. [Google Scholar] [CrossRef]
- Salama, I.C.; Arrais-Lima, C.; Arrais-Silva, W.W. Evaluation of boldine activity against intracellular amastigotes of Leishmania amazonensis. Korean J. Parasitol. 2017, 55, 337–340. [Google Scholar] [CrossRef][Green Version]
- Tietjen, I.; Ntie-Kang, F.; Mwimanzi, P.; Onguéné, P.A.; Scull, M.A.; Idowu, T.O.; Ogundaini, A.O.; Meva’a, L.M.; Abegaz, B.M.; Rice, C.M.; et al. Screening of the pan-african natural product library identifies ixoratannin A-2 and boldine as novel HIV-1 inhibitors. PLoS ONE 2015, 10, e0121099. [Google Scholar] [CrossRef]
- Becerra, J.; Flores, C.; Mena, J.; Aqueveque, P.; Alarcón, J.; Bittner, M.; Hernández, V.; Hoeneisen, M.; Ruiz, E.; Silva, M. Antifungal and antibacterial activity of diterpenes isolated from wood extractables of Chilean Podocarpaceae. J. Chil. Chem. Soc. 2002, 47, 151–157. [Google Scholar] [CrossRef]
- Smith, E.C.J.; Wareham, N.; Zloh, M.; Gibbons, S. 2β-Acetoxyferruginol-A new antibacterial abietane diterpene from the bark of Prumnopitys andina. Phytochem. Lett. 2008, 1, 49–53. [Google Scholar] [CrossRef]
- Reichert, C.L.; Salminen, H.; Weiss, J. Quillaja saponin characteristics and functional properties. Annu. Rev. Food Sci. Technol. 2019, 10, 43–73. [Google Scholar] [CrossRef]
- Sen, S.; Makkar, H.P.S.; Muetzel, S.; Becker, K. Effect of Quillaja saponaria saponins and Yucca schidigera plant extract on growth of Escherichia coli. Lett. Appl. Microbiol. 1998, 27, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Sewlikar, S.; D’Souza, D.H. Antimicrobial effects of Quillaja saponaria extract against Escherichia coli O157:H7 and the emerging non-O157 shiga toxin-producing E. coli. J. Food Sci. 2017, 82, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Byrd, J.A.; Cartwright, A.L.; Bailey, C.A. Hemolytic and antimicrobial activities differ among saponin-rich extracts from guar, quillaja, yucca, and soybean. Appl. Biochem. Biotechnol. 2010, 162, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Roner, M.R.; Sprayberry, J.; Spinks, M.; Dhanji, S. Antiviral activity obtained from aqueous extracts of the Chilean soapbark tree (Quillaja saponaria Molina). J. Gen. Virol. 2007, 88, 275–285. [Google Scholar] [CrossRef]
- Roner, M.R.; Tam, K.I.; Kiesling-Barrager, M. Prevention of rotavirus infections in vitro with aqueous extracts of Quillaja saponaria Molina. Future Med. Chem. 2010, 2, 1083–1097. [Google Scholar] [CrossRef]
- Tam, K.I.; Roner, M.R. Characterization of in vivo anti-rotavirus activities of saponin extracts from Quillaja saponaria Molina. Antivir. Res. 2011, 90, 231–241. [Google Scholar] [CrossRef]
- González, S.; Guerra, P.E.; Bottaro, H.; Molares, S.; Demo, M.S.; Oliva, M.M.; Zunino, M.P.; Zygadlo, J.A. Aromatic plants from Patagonia. Part I. Antimicrobial activity and chemical composition of Schinus polygamus (Cav.) Cabrera essential oil. Flavour Fragr. J. 2004, 19, 36–39. [Google Scholar] [CrossRef]
- Erazo, S.; Delporte, C.; Negrete, R.; García, R.; Zaldívar, M.; Iturra, G.; Caballero, E.; López, J.L.; Backhouse, N. Constituents and biological activities of Schinus polygamus. J. Ethnopharmacol. 2006, 107, 395–400. [Google Scholar] [CrossRef] [PubMed]
- López de Dicastillo, C.; Bustos, F.; Valenzuela, X.; López-Carballo, G.; Vilariño, J.M.; Galotto, M.J. Chilean berry Ugni molinae Turcz. fruit and leaves extracts with interesting antioxidant, antimicrobial and tyrosinase inhibitory properties. Food Res. Int. 2017, 102, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Fuentes, M.; Parra, L.; Rodriguez-Saona, C.; Seguel, I.; Ceballos, R.; Quiroz, A. Domestication in murtilla (Ugni molinae) reduced defensive flavonol levels but increased resistance against a native herbivorous insect. Environ. Entomol. 2015, 44, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G.E. Isolation and characterization of phenolic compounds and anthocyanins from murta (Ugni molinae Turcz.) fruits. Assessment of antioxidant and antibacterial activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef]
- Shene, C.; Reyes, A.K.; Villarroel, M.; Sineiro, J.; Pinelo, M.; Rubilar, M. Plant location and extraction procedure strongly alter the antimicrobial activity of murta extracts. Eur. Food Res. Technol. 2009, 228, 467–475. [Google Scholar] [CrossRef]
- Brockgreitens, J.; Abbas, A. Responsive food packaging: Recent progress and technological prospects. Compr. Rev. Food Sci. 2016, 15, 3–15. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; de Melo, N.R.; Andrade, M.; Sanches-Silva, A. Potential of migration of active compounds from protein-based films with essential oils to a food and a food simulant. Packag. Technol. Sci. 2017, 30, 791–798. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Var, I.; Uzunlu, S. Active Antimicrobial Food Packaging; IntechOpen: London, UK, 2019; p. 104. [Google Scholar]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez., J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Girardi, N.S.; García, D.; Robledo, S.N.; Passone, M.A.; Nesci, A.; Etcheverry, M. Microencapsulation of Peumus boldus oil by complex coacervation to provide peanut seeds protection against fungal pathogens. Ind. Crops Prod. 2016, 92, 93–101. [Google Scholar] [CrossRef]
- Girardi, N.S.; Passone, M.A.; García, D.; Nesci, A.; Etcheverry, M. Microencapsulation of Peumus boldus essential oil and its impact on peanut seed quality preservation. Ind. Crops Prod. 2018, 114, 108–114. [Google Scholar] [CrossRef]
- Fellenberg, M.A.; Espinoza, A.; Peña, I.; Alarcón, J. Antioxidant and bacteriostatic effects of the addition of extract of quillay polyphenols (Quillaja saponaria) in the marinade of broiler chicken. Braz. J. Poult. Sci. 2011, 13, 71–79. [Google Scholar] [CrossRef]
- Hauser, C.; Peñaloza, A.; Guarda, A.; Galotto, M.J.; Bruna, J.E.; Rodríguez, F.J. Development of an active packaging film based on a methylcellulose coating containing murta (Ugni molinae Turcz) leaf extract. Food Bioproc. Technol. 2016, 9, 298–307. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential oils: Antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 17, 65–72. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Ali, W.; Sultana, P.; Joshi, M.; Rajendran, S. A solvent induced crystallisation method to imbue bioactive ingredients of neem oil into the compact structure of poly (ethylene terephthalate) polyester. Mater. Sci. Eng. C 2016, 64, 399–406. [Google Scholar] [CrossRef]
- Karami, N.; Kamkar, A.; Shahbazi, Y.; Misaghi, A. Edible films based on chitosan-flaxseed mucilage: In vitro antimicrobial and antioxidant properties and their application on survival of food-borne pathogenic bacteria in raw minced trout fillets. Pharm. Biomed. Res. 2019, 5, 10–16. [Google Scholar] [CrossRef]
- Orsuwan, A.; Sothornvit, R. Development and characterization of banana flour film incorporated with montmorillonite and banana starch nanoparticles. Carbohydr. Polym. 2017, 174, 235–242. [Google Scholar] [CrossRef]
- Jang, S.-A.; Shin, Y.-J.; Song, K.B. Effect of rapeseed protein–gelatin film containing grapefruit seed extract on ’Maehyang’ strawberry quality. Int. J. Food Sci. 2011, 46, 620–625. [Google Scholar] [CrossRef]
- Hong, Y.H.; Lim, G.O.; Song, K.B. Physical properties of Gelidium corneumgelatin blend films containing grapefruit seed extract or green tea extract and its application in the packaging of pork loins. J. Food Sci. 2009, 74, C6–C10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Tosati, J.V.; de Oliveira, E.F.; Oliveira, J.V.; Nitin, N.; Monteiro, A.R. Light activated antimicrobial activity of turmeric residue edible coatings against crosscontamination of Listeria innocua on sausages. Food Control 2018, 84, 177–185. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingur, G.; Ozen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falco, I.; Randazzo, W.; Sanchez, G.; Lopez-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Andrade-Mahecha, M.M.; Torres-Vargas, O.L. Development of antimicrobial biocomposite films to preserve the quality of bread. Molecules 2018, 23, 212. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Rosa, G.B.; Ferreira, A.L.A.; da Rosa, C.G.; Beling, P.C.; Xavier, L.O.; Hansen, C.M.; Ferrareze, J.P.; Nunes, M.R.; Barreto, P.L.M.; et al. Bioactive food packaging based on starch, citric pectin and functionalized with Acca sellowiana waste by-product: Characterization and application in the postharvest conservation of apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Weston, R. Bioactive products fromfruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chem. 2010, 121, 923–926. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar] [CrossRef]
- Naithani, R.; Huma, L.; Holland, L.; Shukla, D.; McCormick, D.; Mehta, R.; Moriarty, R. Antiviral activity of phytochemicals: A comprehensive review. Mini-Rev. Med. Chem. 2008, 8, 1106–1133. [Google Scholar] [CrossRef]
- Madigan, M.; Martinko, J.; Bender, K.; Buckley, D.; Stahl, D. Brock Biology of Microorganisms, 14th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Yang, Y.; Zhang, T. Antimicrobial activities of tea polyphenol on phytopathogens: A Review. Molecules 2019, 24, 816. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction techniques, pharmaceutical and therapeutic potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential application of essential oils as antimicrobial preservatives in cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Marshall, M.R.; Wei, C.I. Antibacterial activity of some essential oil components against five foodborne pathogens. J. Agric. Food Chem. 2002, 43, 2839–2845. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Samoylenko, V.; Ashfaq, M.K.; Jacob, M.R.; Tekwani, B.L.; Khan, S.I.; Manly, S.P.; Joshi, V.C.; Walker, L.A.; Muhammad, I. Indolizidine, antiinfective and antiparasitic compounds from Prosopis glandulosa var. glandulosa. J. Nat. Prod. 2008, 72, 92–98. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chowdhury, B.K.; Bhattacharyya, P. Clausenol and clausenine—two carbazole alkaloids from Clausena anisata. Phytochemistry 1995, 40, 295–298. [Google Scholar] [CrossRef]
- Tavares, D.L.C.; Zanon, G.; Weber, A.D.; Neto, A.T.; Mostardeiro, C.P.; Da Cruz, I.B.M.; Oliveira, R.M.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity. PLoS ONE 2014, 9, 97000. [Google Scholar] [CrossRef]
- Nissanka, A.P.K.; Karunaratne, V.; Bandara, B.M.R.; Kumar, V.; Nakanishi, T.; Nishi, M.; Inada, A.; Tillekeratne, L.M.V.; Wijesundara, D.S.A.; Gunatilaka, A.A.L. Antimicrobial alkaloids from Zanthoxylum tetraspermum and caudatum. Phytochemistry 2001, 56, 857–861. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, Z.K.; Yang, F.M.; Sun, Q.Y.; He, H.P.; Di, Y.T.; Mu, S.Z.; Lu, Y.; Chang, Y.; Zheng, Q.T.; et al. Benzylphenethylamine alkaloids from Hosta plantaginea with inhibitory activity against tobacco mosaic virus and acetylcholinesterase. J. Nat. Prod. 2007, 70, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Fleck, J.D.; Betti, A.H.; Pereira da Silva, F.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular chemical characteristics and biological activities. Molecules 2019, 24, 171. [Google Scholar] [CrossRef]
- Glauert, A.M.; Dingle, J.T.; Lucy, J.A. Action of saponin on biological cell membranes. Nature 1962, 196, 953–955. [Google Scholar] [CrossRef]
- Arabski, M.; Wȩgierek-Ciuk, A.; Czerwonka, G.; Lankoff, A.; Kaca, W. Effects of saponins against clinical E. coli strains and eukaryotic cell line. J. Biomed. Biotechnol. 2012, 2012, 286216. [Google Scholar] [CrossRef] [PubMed]
| Plant | Target Microorganism | Polymer Used | Reported Effect/Possible Application | References |
|---|---|---|---|---|
| Acaena magallanica | Escherichia coli, Microsporum canis | NDA | NDA | [25] |
| Aristotelia chilensis | Escherichia coli, Staphylococcus aureus, Bacillus subtilis Listeria innocua, Serratia marcescens, Aeromonas hydrophila, Achromobacter denitrificans, Alcaligenes faecalis, Enterobacter gergoviae, Enterobacter amnigenus, Shewanella putrefaciens | Chitosan | Antimicrobial effect against Aeromonas hydrophila, Achromobacter denitrificans, Alcaligenes faecalis, Citrobacter freundii, Listeria innocua, Pseudomonas fluorescens, Serratia marcescens, and Shewanella putrefaciens/active packaging | [28,29,79] |
| Azorela acaulis | Mycobacterium tuberculosis Trichomonas vaginalis | NDA | NDA | [31,32] |
| Berberis microphylla | Bacillus cereus, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis Staphyloccocus aureus, Escherichia coli Trypanosoma cruzi | NDA | NDA | [28,37,39] |
| Buddleja globosa | Staphylococcus aureus, Escherichia coli Paenibacillus larvae Trichophyton rubrum, Tricophyton interdigitale, Epidermophyton floccosum Plasmodium falciparum | NDA | NDA | [41,42,43,44] |
| Cryptocarya alba | Trypanosoma cruzi, Leishmania spp. Helicobacter pylori, Staphylococcus aureus, Escherichia coli, Candida albicans | NDA | NDA | [45,46] |
| Fragaria chiloensis spp. chiloensis | Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Fusarium tricuitum, Candida albicans, Fusarium tricuictum, Microsporum cookerii, Microsporum gypseum, Saccharomyces cerevisiae, Trichoderma viridae, Trichophyton mentagrophytes Bacillus subtilis, Enterobacter aerogenes, Escherichia coli, Mycobacterium phlei, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus aureus, Salmonella Typhimurium | NDA | NDA | [48,49] |
| Geoffroea decorticans | Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella Typhimurium, Enterococcus faecium, Staphylococcus aureus Aspergillus flavus, Aspergillus parasiticus, Aspergillus nomius, Aspergillus nomius | NDA | NDA | [50,51] |
| Laurelia sempervirens | Phragmidium violaceum Helicobacter pylori, Staphylococcus aureus, Escherichia coli, Candida albicans | NDA | NDA | [29,45,52] |
| Laureliopsis philippiana | Saprolegnia spp. Escherichia coli, Staphylococcus aereus, Bacillus subtilis, Streptococcus pneumoniae, Pseudomonas aeruginose, Penicillium expansum | NDA | NDA | [29,54] |
| Peumus boldus | Helicobacter pylori Aspergillus flavus, Aspergillus parasiticus Escherichia coli, Pseudomonas aeruginosa, Salmonella typhy, Bacillus cereus, Bacillus subtilis, Staphylococcus aureus, Candida tropicalis, Candida albicans, Candida parapsilosis, Trichophyton rubrum, Trichophyton tonsurans, Trichophyton erinacei, Arthroderma crocatum, Arthroderma quadrifidum, Arthroderma gypseum, Arthroderma currey and Arthroderma insingulare Staphylococcus aureus Trypanosoma cruzi Leishmania amazonensis Human immunodeficiency virus 1 and Hepatitis C virus | Gelatin and gum arabic | Antimicrobial effect against Aspergillus and Penicillium spp./active packaging | [55,56,57,58,59,60,61,63,80,81] |
| Prumnopitys andina | Bacillus brevis, B. subtilis, E. coli, Micrococcus luteus, Providencia sp., Pseudomonas sp., Shigella sp., Staphylococcus aureus, Enterococcus faecalis, Streptococcus pyogenes Aspergillus sp., Fusarium fujikuroi, F. ciliatum, Mucor miehei, Nematospora, Propionobacterium acnes | NDA | NDA | [64,65] |
| Quillaja saponaria | Escherichia coli Escherichia coli Escherichia coli, Staphylococcus aureus, Salmonella typhimurium Rotavirus, vaccinia virus, herpes simplex virus, varicella-zoster virus, human immunodeficiency virus | NDA | Antimicrobial effect against mesophilic aerobe and total coliform/chicken marination | [68,69,70,71,72,82] |
| Schinus polygamus | Bacillus cereus, Candida albicans Escherichia coli, Klebsiella pneumoniae, Salmonella aviatum, Salmonella aeruginosa, Staphylococcus aureus, Micrococcus flavus and Bacillus subtilis | NDA | NDA | [73,74] |
| Ugni molinae | Escherichia coli, Salmonella enterica, Staphylococcus aureus S. aureus, K. pneumonia and P. aeruginose Escherichia coli, Listeria monocytogenes Penicillium expansum Pseudomonas aeruginosa | Polyethylene film/methylcellulose | Antimicrobial effect against Listeria innocua/active packaging | [29,75,77,78,83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, M.C.; Fuentes, J.A.; Atala, C.; Cuadros-Orellana, S.; Fuentes, C.; Gordillo-Fuenzalida, F. Antimicrobial Properties of Chilean Native Plants: Future Aspects in Their Application in the Food Industry. Foods 2022, 11, 1763. https://doi.org/10.3390/foods11121763
Otero MC, Fuentes JA, Atala C, Cuadros-Orellana S, Fuentes C, Gordillo-Fuenzalida F. Antimicrobial Properties of Chilean Native Plants: Future Aspects in Their Application in the Food Industry. Foods. 2022; 11(12):1763. https://doi.org/10.3390/foods11121763
Chicago/Turabian StyleOtero, María Carolina, Juan A. Fuentes, Cristian Atala, Sara Cuadros-Orellana, Camila Fuentes, and Felipe Gordillo-Fuenzalida. 2022. "Antimicrobial Properties of Chilean Native Plants: Future Aspects in Their Application in the Food Industry" Foods 11, no. 12: 1763. https://doi.org/10.3390/foods11121763
APA StyleOtero, M. C., Fuentes, J. A., Atala, C., Cuadros-Orellana, S., Fuentes, C., & Gordillo-Fuenzalida, F. (2022). Antimicrobial Properties of Chilean Native Plants: Future Aspects in Their Application in the Food Industry. Foods, 11(12), 1763. https://doi.org/10.3390/foods11121763