A Simple and Rapid “Signal On” Fluorescent Sensor for Detecting Mercury (II) Based on the Molecular Beacon Aptamer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. DNA Sequence of the Molecular Beacon Aptamer
2.3. Selection of the Molecular Beacon Aptamer
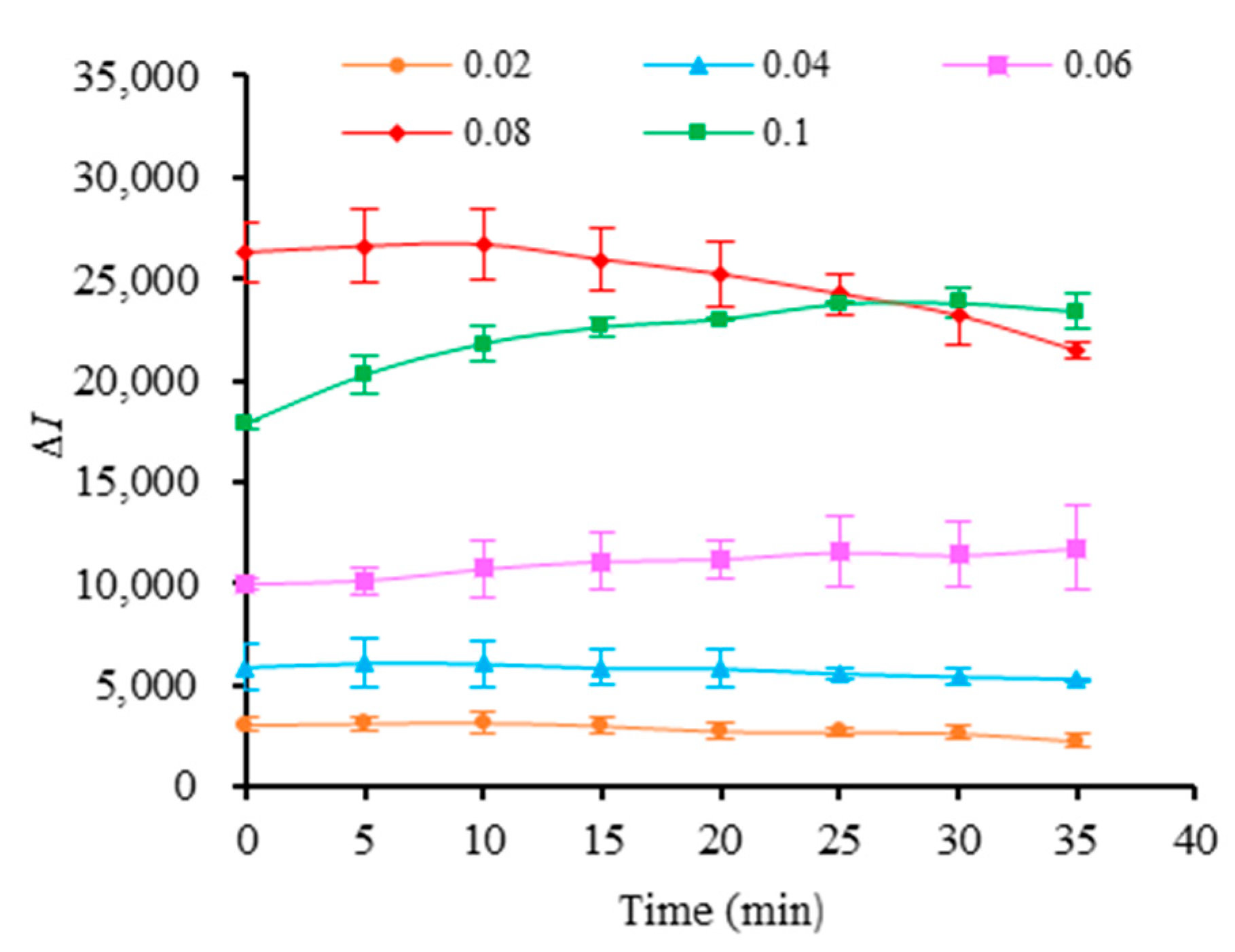
2.4. Selection of MB3 Concentration and Reaction Time
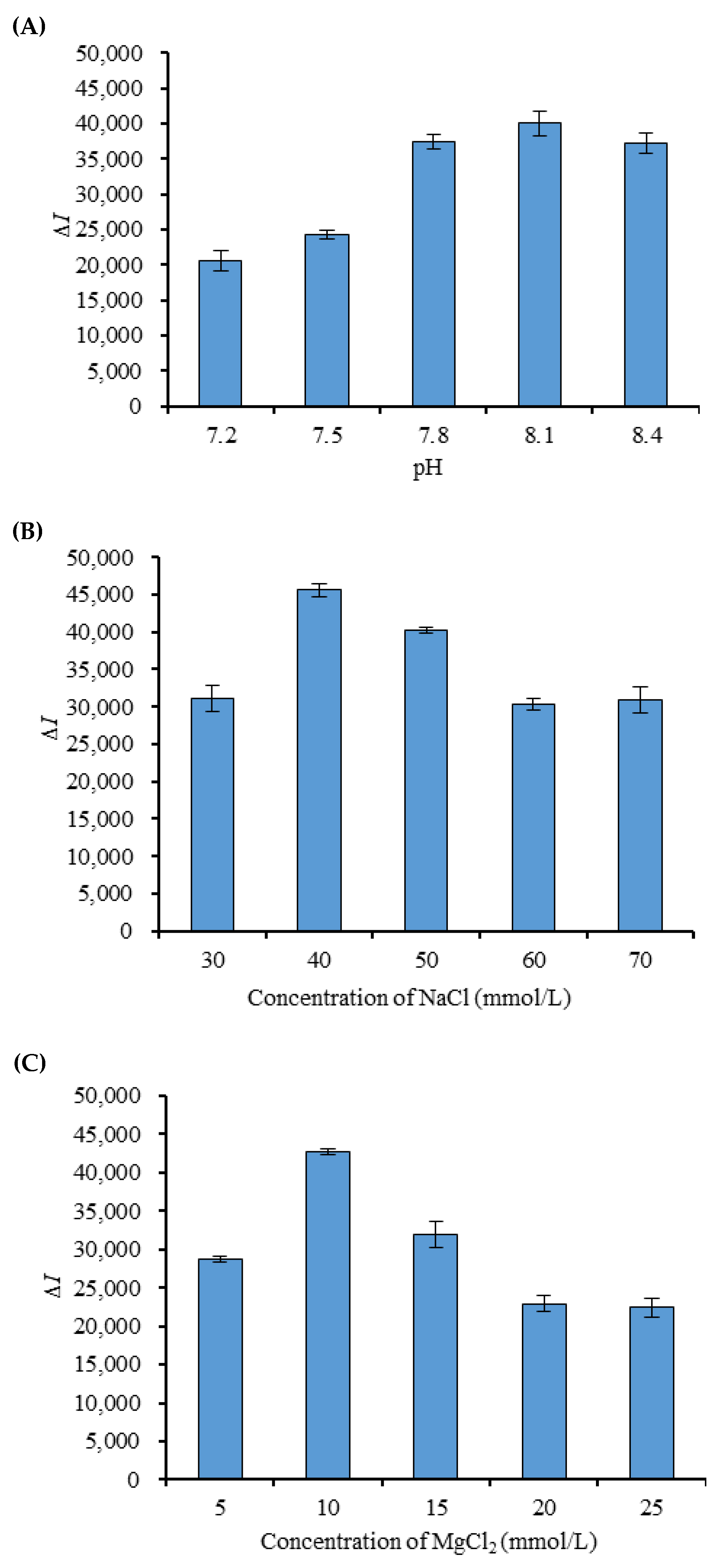
2.5. Selection of pH and Ionic Strength in the Buffer
2.6. Specificity Experiment
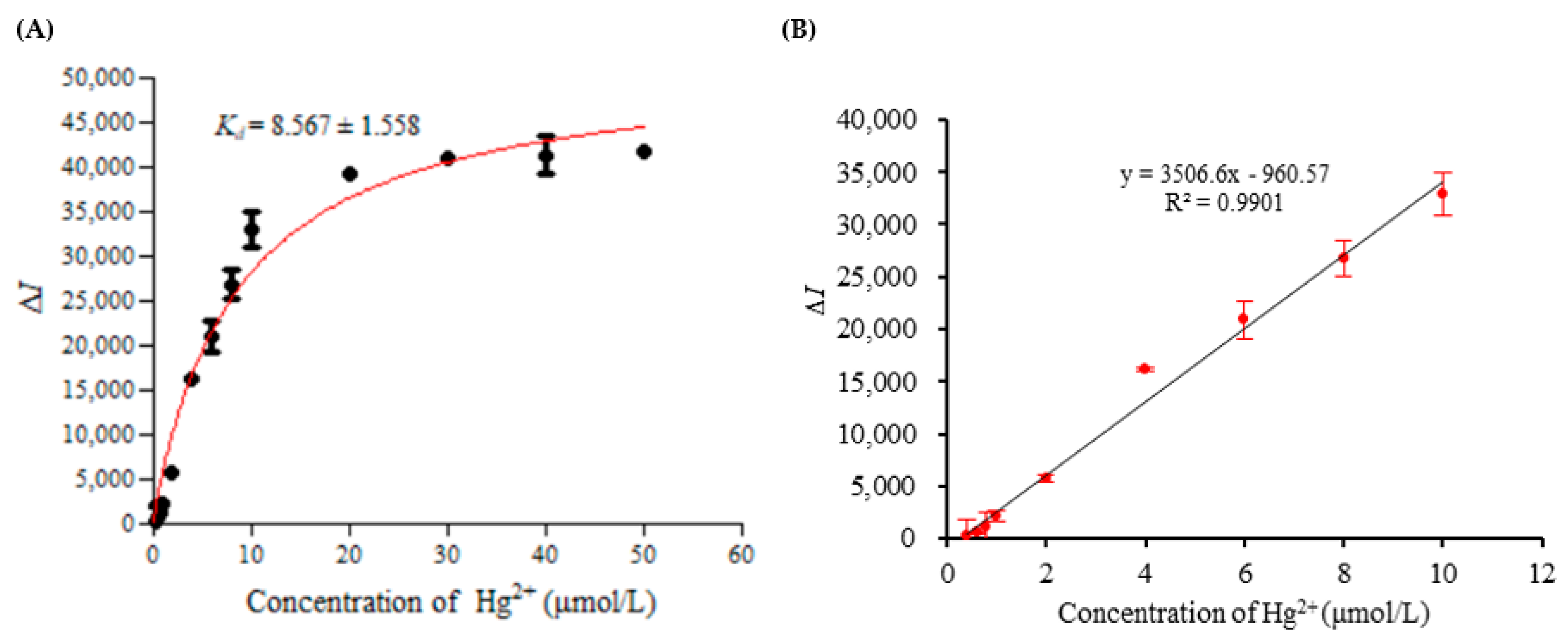
2.7. Affinity Experiment and Standard Curve
2.8. Detection Limit and Precision
2.9. Recovery Test of Hg2+ in Water Samples
3. Results
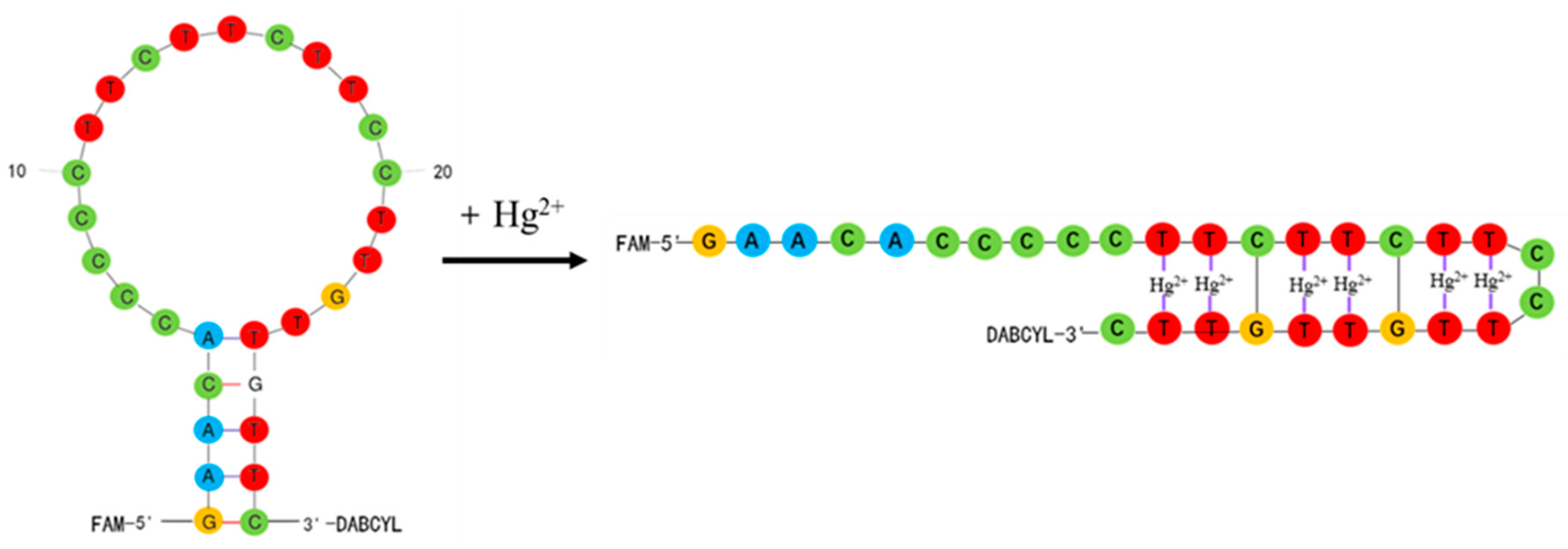
3.1. Detection Principle
3.2. Design and Verification of Molecular Beacon Aptamer
3.3. MB3 Concentration and Incubation Time
3.4. pH and Ionic Strength in the Buffer
3.5. Specificity Test
3.6. Affinity Test, Standard Curve, Detection Limit and Precision
3.7. Recovery Test of Hg2+ in Water Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Shen, H.; Hu, J.H.; Fu, Q.Q.; Yao, C.Z.; Yu, S.T.; Xiao, W.; Tang, Y. Aptamer-based fluorescence-quenching lateral flow strip for rapid detection of mercury (II) ion in water samples. Anal. Bioanal. Chem. 2017, 409, 5209–5216. [Google Scholar] [CrossRef] [PubMed]
- Isaad, J.; El Achari, A. Colorimetric and fluorescent probe based on coumarin for sequential sensing of mercury (II) and cyanide ions in aqueous solutions. J. Lumin. 2022, 243, 118668. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Yang, X.R. Colorimetric biosensing of mercury (II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens. Bioelectron. 2010, 25, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- Diviš, P.; Reichstädter, M.; Gao, Y.; Leermakers, M.; Křikala, J. Determination of Mercury in Fish Sauces by Thermal Decomposition Gold Amalgamation Atomic Absorption Spectroscopy after Preconcentration by Diffusive Gradients in Thin Films Technique. Foods 2020, 9, 1858. [Google Scholar] [CrossRef]
- Domínguez, M.A.; Grünhut, M.; Pistonesi, M.F.; Di Nezio, M.S.; Centurión, M.E. Automatic flow-batch system for cold vapor atomic absorption spectroscopy determination of mercury in honey from Argentina using online sample treatment. J. Agric. Food Chem. 2012, 60, 4812–4817. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Conti, M.E.; Ristorini, M.; Frezzini, M.A.; Papi, M.; Massimi, L.; Canepari, S. An Analytical Method for the Biomonitoring of Mercury in Bees and Beehive Products by Cold Vapor Atomic Fluorescence Spectrometry. Molecules 2021, 26, 4878. [Google Scholar] [CrossRef]
- Herrero Fernández, Z.; Estevez Álvarez, J.R.; Montero Álvarez, A.; Muñiz Ugarte, O.; Pupo González, I.; Rodríguez González, M.; Dos Santos Júnior, J.A.; Bezerra, M.; Dos Santos Junior, O.P. Metal contaminants in rice from Cuba analyzed by ICP-MS, ICP-AES and CVAAS. Food Addit. Contam. Part B Surveill. 2021, 14, 59–65. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Jing, C. Recent progress in detection of mercury using surface enhanced Raman spectroscopy—A review. J. Environ. Sci. 2016, 39, 134–143. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ye, H.; Yang, Z.; Khan, I.M.; Niazi, S.; Guo, Y.; Wang, Z.; Yang, H. Split aptamer acquisition mechanisms and current application in antibiotics detection: A short review. Crit. Rev. Food Sci. Nutr. 2022, 1–12. [Google Scholar] [CrossRef]
- Ono, A.; Togashi, H. Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew. Chem. Int. Ed. Engl. 2004, 43, 4300–4302. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Q.X.; Gao, F.; Gao, F. A high-performance aptasensor for mercury (II) based on the formation of a unique ternary structure of aptamer-Hg(2+)-neutral red. Chem. Commun. 2014, 50, 9397–9400. [Google Scholar] [CrossRef] [PubMed]
- Moutsiopoulou, A.; Broyles, D.; Dikici, E.; Daunert, S.; Deo, S.K. Molecular Aptamer Beacons and Their Applications in Sensing, Imaging, and Diagnostics. Small 2019, 15, e1902248. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Kramer, F.R. Molecular beacons: Probes that fluoresce upon hybridization. Nat. Biotechnol. 1996, 14, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Liu, N.N.; Zhang, Z.C.; Xu, D.Y.; Ma, S.; Wang, X.F.; Zhou, T.; Zhang, G.D.; Wang, F. Construction of Aptamer-Based Molecular Beacons with Varied Blocked Structures and Targeted Detection of Thrombin. Langmuir 2021, 37, 8738–8745. [Google Scholar] [CrossRef]
- Şahin, S.; Caglayan, M.O.; Üstündağ, Z. A review on nanostructure-based mercury (II) detection and monitoring focusing on aptamer and oligonucleotide biosensors. Talanta 2020, 220, 121437. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Ma, J.X.; Chen, X.D.; Xiu, F.R.; Chen, Y.T.; Lu, Y.W. Practical aptamer-based assay of heavy metal mercury ion in contaminated environmental samples: Convenience and sensitivity. Anal. Bioanal. Chem. 2020, 412, 439–448. [Google Scholar] [CrossRef]
- Berlina, A.N.; Zherdev, A.V.; Pridvorova, S.M.; Gaur, M.S.; Dzantiev, B.B. Rapid Visual Detection of Lead and Mercury via Enhanced Crosslinking Aggregation of Aptamer-Labeled Gold Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5489–5495. [Google Scholar] [CrossRef]
- Lai, B.; Wang, R.Y.; Yu, X.T.; Wang, H.T.; Wang, Z.P.; Tan, M.Q. A Highly Sensitive “on-off” Time-Resolved Phosphorescence Sensor Based on Aptamer Functionalized Magnetite Nanoparticles for Cadmium Detection in Food Samples. Foods 2020, 9, 1758. [Google Scholar] [CrossRef]
- Li, L.; Li, B.X.; Qi, Y.Y.; Jin, Y. Label-free aptamer-based colorimetric detection of mercury ions in aqueous media using unmodified gold nanoparticles as colorimetric probe. Anal. Bioanal. Chem. 2009, 393, 2051–2057. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.J.; Ding, W.Q.; Guo, S.W.; Wu, N.Q. Fluorescent aptamer-functionalized graphene oxide biosensor for label-free detection of mercury(II). Biosens. Bioelectron. 2013, 41, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.D.; Heon Lee, J.; Lu, Y. Highly sensitive “turn-on” fluorescent sensor for Hg2+ in aqueous solution based on structure-switching DNA. Chem. Commun. 2008, 45, 6005–6007. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Sun, M.; Wei, X.P.; Zhang, L.P.; Zhang, Y. An electrochemical aptamer biosensor based on “gate-controlled” effect using β-cyclodextrin for ultra-sensitive detection of trace mercury. Biosens. Bioelectron. 2015, 74, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, Z.; Li, R.H.; Yi, F.; Liang, X.C.; Cheng, S.Q.; Wang, K.; Sun, Y.; Liu, Y. Highly Emissive Multipurpose Organoplatinum(II) Metallacycles with Contrasting Mechanoresponsive Features. Inorg. Chem. 2022, 61, 2883–2891. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Yi, F.; Han, X.-L.; Li, M.; Gao, Q.; Liang, X.; Chen, Z.; Sun, Y.; Liu, Y. Targeted photodynamic therapy using a water-soluble aggregation-Induced emission photosensitizer activated by an acidic tumor microenvironment. Chem. Eng. J. 2022, 432, 134327. [Google Scholar] [CrossRef]
- Qu, H.; Csordas, A.T.; Wang, J.; Oh, S.S.; Eisenstein, M.S.; Soh, H.T. Rapid and Label-Free Strategy to Isolate Aptamers for Metal Ions. ACS Nano 2016, 10, 7558–7565. [Google Scholar] [CrossRef] [Green Version]
- Tan, D.D.; He, Y.; Xing, X.J.; Zhao, Y.; Tang, H.W.; Pang, D.W. Aptamer functionalized gold nanoparticles based fluorescent probe for the detection of mercury (II) ion in aqueous solution. Talanta 2013, 113, 26–30. [Google Scholar] [CrossRef]



| Initial Concentration of Hg2+ (μmol/L) | Standard Adding Concentration of Hg2+ (μmol /L) | Determination of Hg2+ Concentration (μmol/L) | Recovery Rate (%) |
|---|---|---|---|
| Not detected | 8 | 7.90 ± 0.5 | 98.75 |
| Not detected | 4 | 3.97 ± 0.3 | 99.25 |
| Not detected | 0.8 | 0.76 ± 0.1 | 95.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chi, E.-Z.; Zhao, X.-H.; Zhang, Q. A Simple and Rapid “Signal On” Fluorescent Sensor for Detecting Mercury (II) Based on the Molecular Beacon Aptamer. Foods 2022, 11, 1847. https://doi.org/10.3390/foods11131847
Wang L, Chi E-Z, Zhao X-H, Zhang Q. A Simple and Rapid “Signal On” Fluorescent Sensor for Detecting Mercury (II) Based on the Molecular Beacon Aptamer. Foods. 2022; 11(13):1847. https://doi.org/10.3390/foods11131847
Chicago/Turabian StyleWang, Li, En-Zhong Chi, Xin-Huai Zhao, and Qiang Zhang. 2022. "A Simple and Rapid “Signal On” Fluorescent Sensor for Detecting Mercury (II) Based on the Molecular Beacon Aptamer" Foods 11, no. 13: 1847. https://doi.org/10.3390/foods11131847





