Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae and Culture Medium
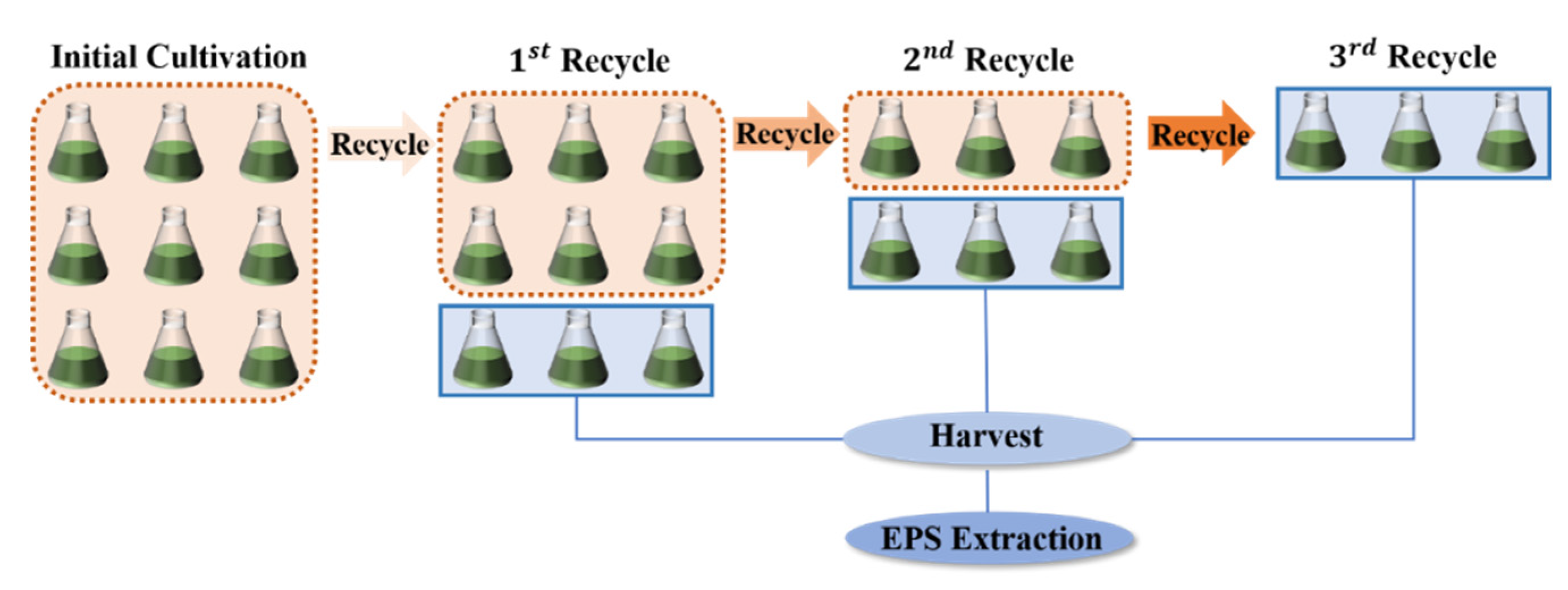
2.2. Experimental Design
2.3. Microalgae Growth Analysis
2.4. Culture Medium Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effects of Medium Recycle on the Growth and EPS Secretion of A. platensis
3.2. Effects of Initial pH on the Growth and EPS Secretion of A. platensis
3.3. Effects of Nitrogen Source and Concentration on the Growth and EPS Secretion of A. platensis
3.4. Effects of Phosphate Concentration on the Growth and EPS Secretion of A. platensis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The True Value of Spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, J.; Zheng, H.; Wu, X.; Wang, Y.; Liu, M.; Xiang, S.; Cao, L.; Ruan, R.; Liu, Y. Characterization of Additional Zinc Ions on the Growth, Biochemical Composition and Photosynthetic Performance from Spirulina Platensis. Bioresour. Technol. 2018, 269, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Costs Analysis of Microalgae Production. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 551–566. ISBN 978-0-444-64192-2. [Google Scholar] [CrossRef]
- Fanka, L.S.; da Rosa, G.M.; de Morais, M.G.; Costa, J.A.V. Outdoor Production of Biomass and Biomolecules by Spirulina (Arthrospira) and Synechococcus Cultivated with Reduced Nutrient Supply. BioEnergy Res. 2021, 15, 121–130. [Google Scholar] [CrossRef]
- Lu, Z.; Loftus, S.; Sha, J.; Wang, W.; Park, M.S.; Zhang, X.; Johnson, Z.I.; Hu, Q. Water Reuse for Sustainable Microalgae Cultivation: Current Knowledge and Future Directions. Resour. Conserv. Recycl. 2020, 161, 104975. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-Cycle Analysis on Biodiesel Production from Microalgae: Water Footprint and Nutrients Balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Dubey, B.K.; Sen, R. A comparative life cycle assessment of microalgae production by CO2 sequestration from flue gas in outdoor raceway ponds under batch and semi-continuous regime. J. Clean. Prod. 2020, 258, 120703. [Google Scholar] [CrossRef]
- Depraetere, O.; Pierre, G.; Noppe, W.; Vandamme, D.; Foubert, I.; Michaud, P.; Muylaert, K. Influence of Culture Medium Recycling on the Performance of Arthrospira Platensis Cultures. Algal Res. 2015, 10, 48–54. [Google Scholar] [CrossRef]
- Vonshak, A. (Ed.) Spirulina Platensis Arthrospira: Physiology, Cell-Biology and Biotechnology; CRC Press: London, UK, 2014; ISBN 978-0-429-07994-8. [Google Scholar]
- Loftus, S.E.; Johnson, Z.I. Cross-Study Analysis of Factors Affecting Algae Cultivation in Recycled Medium for Biofuel Production. Algal Res. 2017, 24, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, R.A.; Hussein, M.H.; Elhadary, A.M.A.; Abuelmagd, M.A. Extruded Polysaccharide/Protein Matrix from Arthrospira Platensis Cultures Mediated Silver Nanoparticles Biosynthesis and Capping. Appl. Nanosci. 2020, 10, 3839–3855. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of Exopolysaccharides Produced by Microalgae with Antitumor Activity on Human Colon Cancer Cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Yao, M.; Wang, L.; Li, Y.; Gong, Y.; Hu, Q. Effect of Recycling the Culture Medium on Biodiversity and Population Dynamics of Bio-Contaminants in Spirulina Platensis Mass Culture Systems. Algal Res. 2019, 44, 101718. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Doumandji, A.; HadjSadok, A.; Ben Ouada, H.; Nasri, M.; Jridi, M. Enhancement of Extracellular Polymeric Substances (EPS) Production in Spirulina (Arthrospira sp.) by Two-Step Cultivation Process and Partial Characterization of Their Polysaccharidic Moiety. Int. J. Biol. Macromol. 2017, 105, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, Extraction and Characterization of Microalgal and Cyanobacterial Exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Filali Mouhim, R.; Cornet, J.-F.; Fontane, T.; Fournet, B.; Dubertret, G. Production, Isolation and Preliminary Characterization of the Exopolysaccharide of the Cyanobacterium Spirulina Platensis. Biotechnol. Lett. 1993, 15, 567–572. [Google Scholar] [CrossRef]
- Silva, M.B.F.; Azero, E.G.; Teixeira, C.M.L.L.; Andrade, C.T. Influence of Culture Conditions on the Production of Extracellular Polymeric Substances (EPS) by Arthrospira Platensis. Bioresour. Bioprocess. 2020, 7, 47. [Google Scholar] [CrossRef]
- Zhang, T.; Gong, H.; Wen, X.; Lu, C. Salt Stress Induces a Decrease in Excitation Energy Transfer from Phycobilisomes to Photosystem II but an Increase to Photosystem I in the Cyanobacterium Spirulina Platensis. J. Plant Physiol. 2010, 167, 951–958. [Google Scholar] [CrossRef]
- Brezeștean, I.; Bocăneală, M.; Gherman, A.M.R.; Porav, S.A.; Kacsó, I.; Rakosy-Tican, E.; Dina, N.E. Spectroscopic Investigation of Exopolysaccharides Purified from Arthrospira Platensis Cultures as Potential Bioresources. J. Mol. Struct. 2021, 1246, 131228. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The Potential of Microalgae and Their Biopolymers as Structuring Ingredients in Food: A Review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Lee, S.Y.; Stuckey, D.C. Separation and biosynthesis of value-added compounds from food-processing wastewater: Towards sustainable wastewater resource recovery. J. Clean. Prod. 2022, 357, 131975. [Google Scholar] [CrossRef]
- Ogbonda, K.H.; Aminigo, R.E.; Abu, G.O. Influence of Temperature and pH on Biomass Production and Protein Biosynthesis in a Putative Spirulina sp. Bioresour. Technol. 2007, 98, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, Z.; Wang, Y.; Wensel, P.; Sommerfeld, M.; Hu, Q. Recycling Nannochloropsis Oceanica Culture Media and Growth Inhibitors Characterization. Algal Res. 2016, 20, 282–290. [Google Scholar] [CrossRef]
- Chen, C.Y.; Durbin, E.G. Effects of pH on the Growth and Carbon Uptake of Marine Phytoplankton. Mar. Ecol. Prog. Ser. 1994, 109, 83–94. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, L.; Zhang, Q.; Liu, Y.; Xiang, S.; Liu, T.; Ruan, R. Effect of Chlortetracycline on the Growth and Intracellular Components of Spirulina Platensis and Its Biodegradation Pathway. J. Hazard. Mater. 2021, 413, 125310. [Google Scholar] [CrossRef] [PubMed]
- Arahou, F.; Hassikou, R.; Arahou, M.; Rhazi, L.; Wahby, I. Influence of Culture Conditions on Arthrospira Platensis Growth and Valorization of Biomass as Input for Sustainable Agriculture. Aquac. Int. 2021, 29, 2009–2020. [Google Scholar] [CrossRef]
- de Jesus, C.S.; de Jesus Assis, D.; Rodriguez, M.B.; Menezes Filho, J.A.; Costa, J.A.V.; de Souza Ferreira, E.; Druzian, J.I. Pilot-Scale Isolation and Characterization of Extracellular Polymeric Substances (EPS) from Cell-Free Medium of Spirulina Sp.LEB-18 Cultures under Outdoor Conditions. Int. J. Biol. Macromol. 2019, 124, 1106–1114. [Google Scholar] [CrossRef]
- Chen, C.Y.; Kao, P.C.; Tan, C.H.; Show, P.L.; Cheah, W.Y.; Lee, W.-L.; Ling, T.C.; Chang, J.-S. Using an Innovative PH-Stat CO2 Feeding Strategy to Enhance Cell Growth and C-Phycocyanin Production from Spirulina Platensis. Biochem. Eng. J. 2016, 112, 78–85. [Google Scholar] [CrossRef]
- Xu, C.H.; Nejidat, A.; Belkin, S.; Boussiba, S. Isolation and Characterization of the Plasma Membrane by Two-Phase Partitioning from the Alkalophilic Cyanobacterium Spirulina Platensis. Plant Cell Physiol. 1994, 35, 737–741. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.-M.; Chang, W.S.; Sim, S.J. Development of Large-Scale and Economic pH Control System for Outdoor Cultivation of Microalgae Haematococcus Pluvialis Using Industrial Flue Gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef]
- Shi, W.; Li, S.; Li, G.; Wang, W.; Chen, Q.; Li, Y.; Ling, X. Investigation of Main Factors Affecting the Growth Rate of Spirulina. Optik 2016, 127, 6688–6694. [Google Scholar] [CrossRef]
- Richmond, A.; Grobbelaar, J.U. Factors Affecting the Output Rate of Spirulina Platensis with Reference to Mass Cultivation. Biomass 1986, 10, 253–264. [Google Scholar] [CrossRef]
- Pogoryelov, D.; Sudhir, P.R.; Kovács, L.; Gombos, Z.; Brown, I.; Garab, G. Sodium Dependency of the Photosynthetic Electron Transport in the Alkaliphilic Cyanobacterium Arthrospira Platensis. J. Bioenerg. Biomembr. 2003, 35, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yu, R.; Liu, A.; Liu, J.; Zeng, W.; Liu, X.; Qiu, G. Effect of pH Values on Extracellular Protein and Polysaccharide Secretions of Acidithiobacillus Ferrooxidans during Chalcopyrite Bioleaching. Trans. Nonferrous Met. Soc. China 2017, 27, 406–412. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Zhai, J.; Wei, H.; Wang, Q. Effect of Ammonium Nitrogen on Microalgal Growth, Biochemical Composition and Photosynthetic Performance in Mixotrophic Cultivation. Bioresour. Technol. 2019, 273, 368–376. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Growth Kinetics and Quantification of Carbohydrate, Protein, Lipids, and Chlorophyll of Spirulina Platensis under Aqueous Conditions Using Different Carbon and Nitrogen Sources. Bioresour. Technol. 2022, 346, 126456. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Vandamme, D.; Muylaert, K. Ammonia Inhibition on Arthrospira Platensis in Relation to the Initial Biomass Density and pH. Bioresour. Technol. 2014, 166, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Phélippé, M.; Gonçalves, O.; Thouand, G.; Cogne, G.; Laroche, C. Characterization of the Polysaccharides Chemical Diversity of the Cyanobacteria Arthrospira Platensis. Algal Res. 2019, 38, 101426. [Google Scholar] [CrossRef]
- De Philippis, R. Exocellular Polysaccharides from Cyanobacteria and Their Possible Applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Bafana, A. Characterization and Optimization of Production of Exopolysaccharide from Chlamydomonas Reinhardtii. Carbohydr. Polym. 2013, 95, 746–752. [Google Scholar] [CrossRef]
- Soanen, N.; Da Silva, E.; Gardarin, C.; Michaud, P.; Laroche, C. Improvement of Exopolysaccharide Production by Porphyridium Marinum. Bioresour. Technol. 2016, 213, 231–238. [Google Scholar] [CrossRef]
- De Philippis, R.; Margheri, M.C.; Pelosi, E.; Ventura, S. Exopolysaccharide Production by a Unicellular Cyanobacterium Isolated from a Hypersaline Habitat. J. Appl. Phycol. 1993, 5, 387–394. [Google Scholar] [CrossRef]
- Li, Q.; Fu, L.; Wang, Y.; Zhou, D.; Rittmann, B.E. Excessive Phosphorus Caused Inhibition and Cell Damage during Heterotrophic Growth of Chlorella Regularis. Bioresour. Technol. 2018, 268, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Sudo, H.; Burgess, J.G.; Takemasa, H.; Nakamura, N.; Matsunaga, T. Sulfated Exopolysaccharide Production by the Halophilic Cyanobacterium Aphanocapsa Halophytia. Curr. Microbiol. 1995, 30, 219–222. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.; Malik, A. Two-Stage Cultivation of Microalgae for Production of High-Value Compounds and Biofuels: A Review. Algal Res. 2021, 57, 102353. [Google Scholar] [CrossRef]





| Treatment | NaNO3 (g/L) | NH4Cl (g/L) | N Concentration (N g/L) |
|---|---|---|---|
| 1 | 0.00 | - | 0.00 |
| 2 | 0.50 | - | 0.08 |
| 3 | 2.50 | - | 0.41 |
| 4 | 5.00 | - | 0.82 |
| 5 | 25.00 | - | 4.12 |
| 6 | - | 0.32 | 0.08 |
| 7 | - | 1.57 | 0.41 |
| 8 | - | 3.16 | 0.82 |
| 9 | - | 15.74 | 4.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, Y.; Zhou, T.; Cao, L.; Cai, Y.; Wang, Y.; Cui, X.; Yan, H.; Ruan, R.; Zhang, Q. Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides. Foods 2022, 11, 2020. https://doi.org/10.3390/foods11142020
Li Z, Liu Y, Zhou T, Cao L, Cai Y, Wang Y, Cui X, Yan H, Ruan R, Zhang Q. Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides. Foods. 2022; 11(14):2020. https://doi.org/10.3390/foods11142020
Chicago/Turabian StyleLi, Zihan, Yuhuan Liu, Ting Zhou, Leipeng Cao, Yihui Cai, Yunpu Wang, Xian Cui, Hongbin Yan, Roger Ruan, and Qi Zhang. 2022. "Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides" Foods 11, no. 14: 2020. https://doi.org/10.3390/foods11142020
APA StyleLi, Z., Liu, Y., Zhou, T., Cao, L., Cai, Y., Wang, Y., Cui, X., Yan, H., Ruan, R., & Zhang, Q. (2022). Effects of Culture Conditions on the Performance of Arthrospira platensis and Its Production of Exopolysaccharides. Foods, 11(14), 2020. https://doi.org/10.3390/foods11142020










