Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Propagation and Fermentation
2.3. Monitoring of Beer Fermentation: WL Medium and Quantitative Real-Time PCR
Primer Design and qPCR
2.4. Analysis of the Beers
2.5. Determination of Sugar Concentration, Glycerol, and Ethanol Content
2.6. Volatile Compound Analysis
2.7. Melatonin Production and Antioxidant Capacity of Beers
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results
3.1. Main Characteristics of Beers
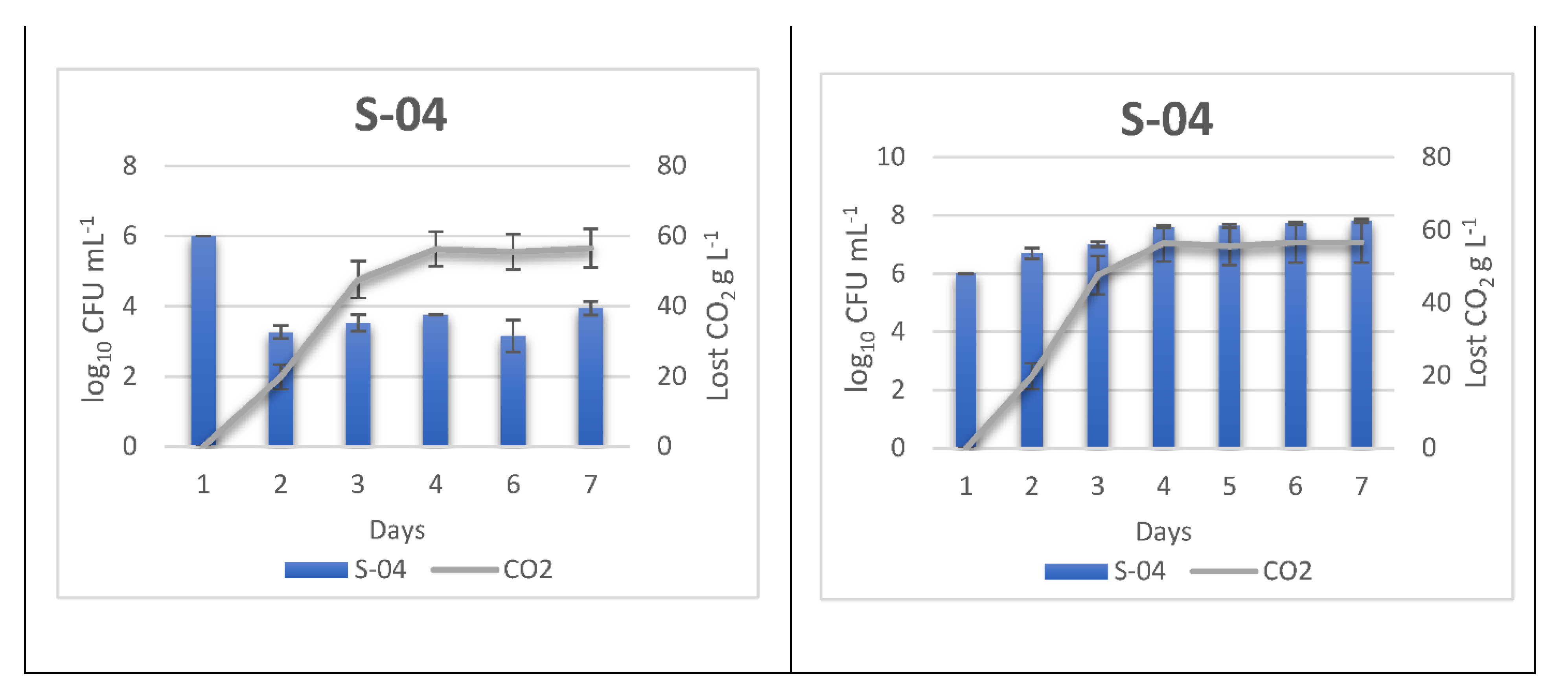
3.2. Monitoring Sequentially Inoculated Beer Fermentations
3.3. Volatile Components in Beers
3.4. Antioxidant Capacity
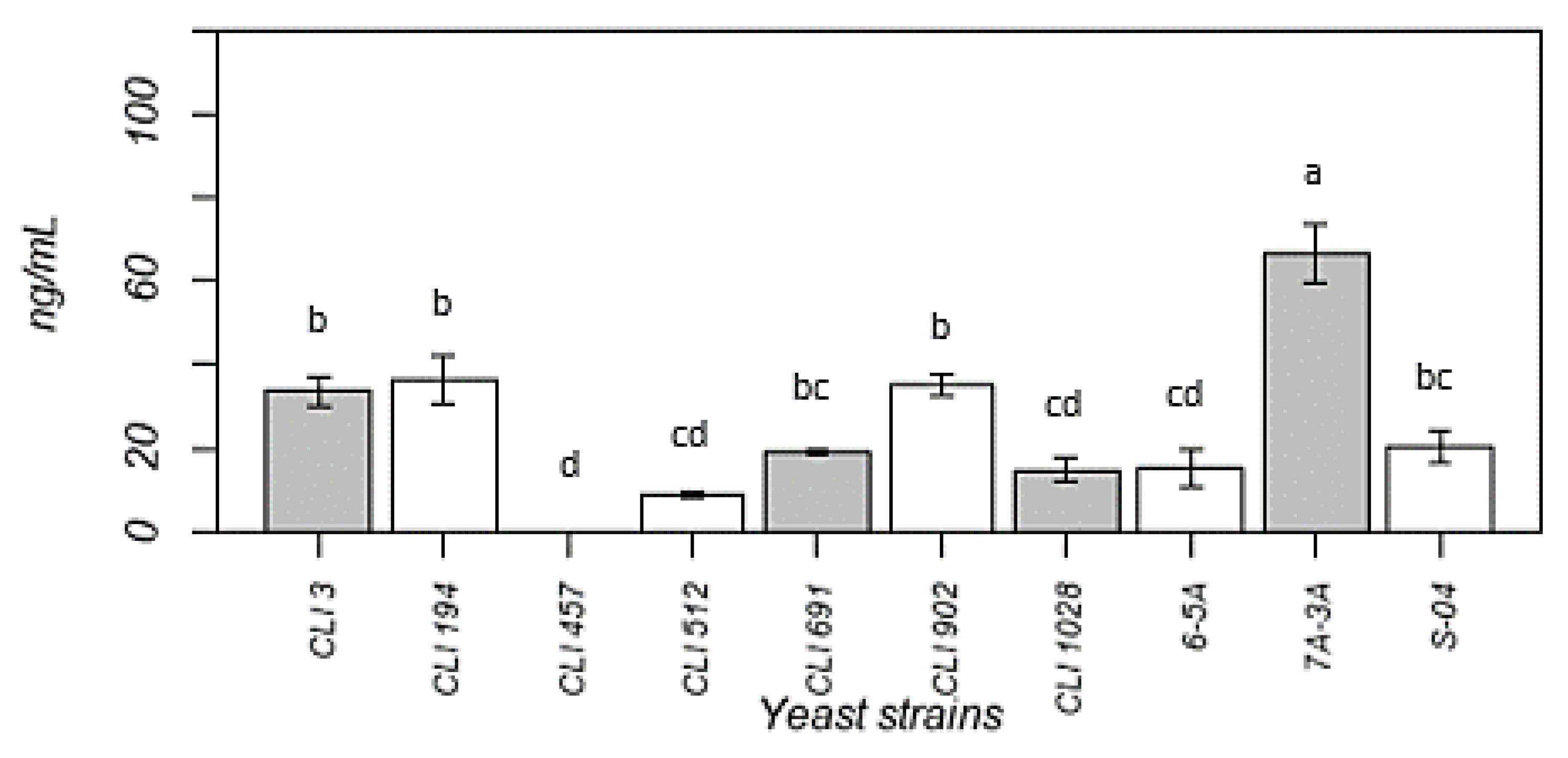
3.5. Melatonin Production
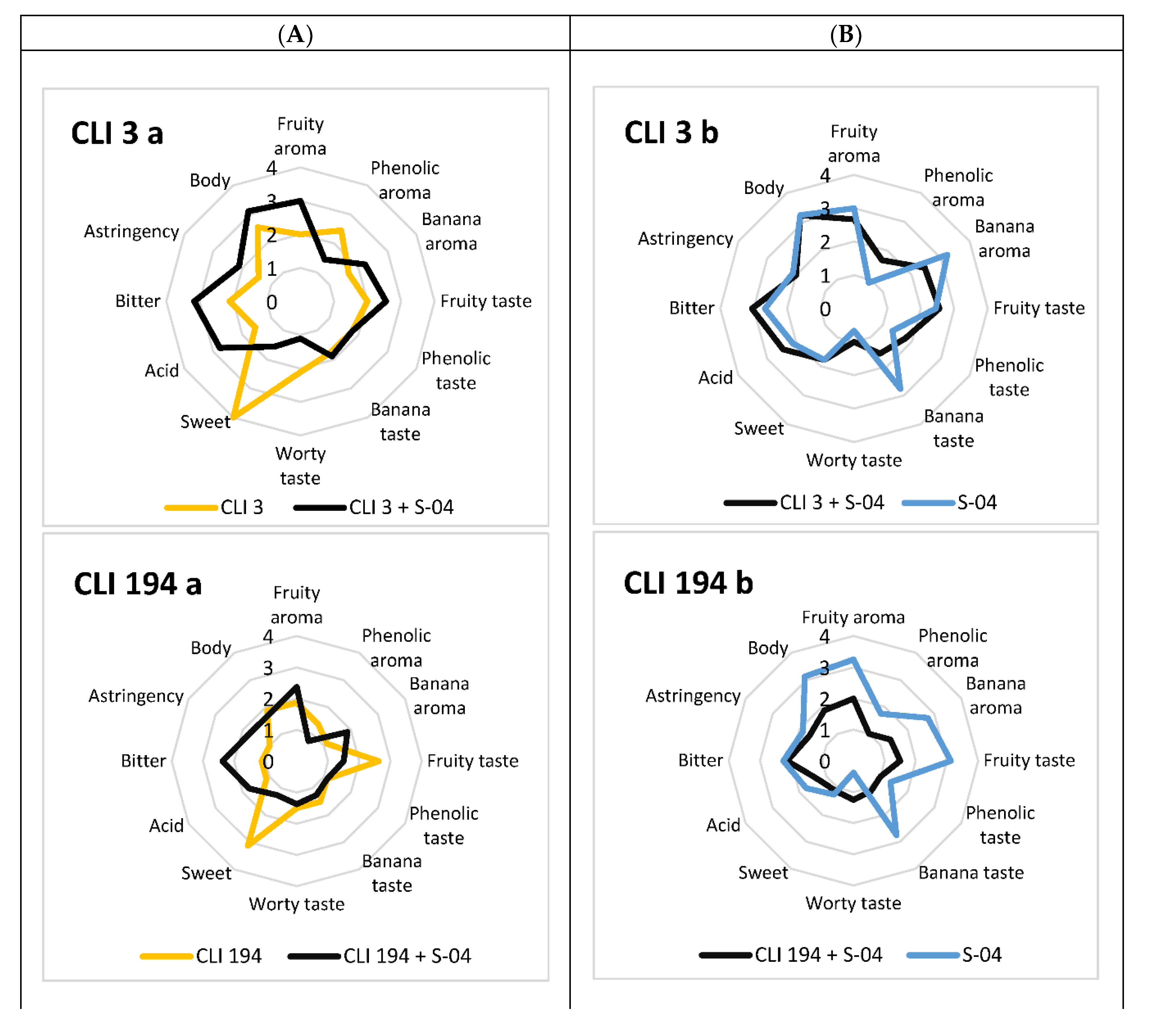
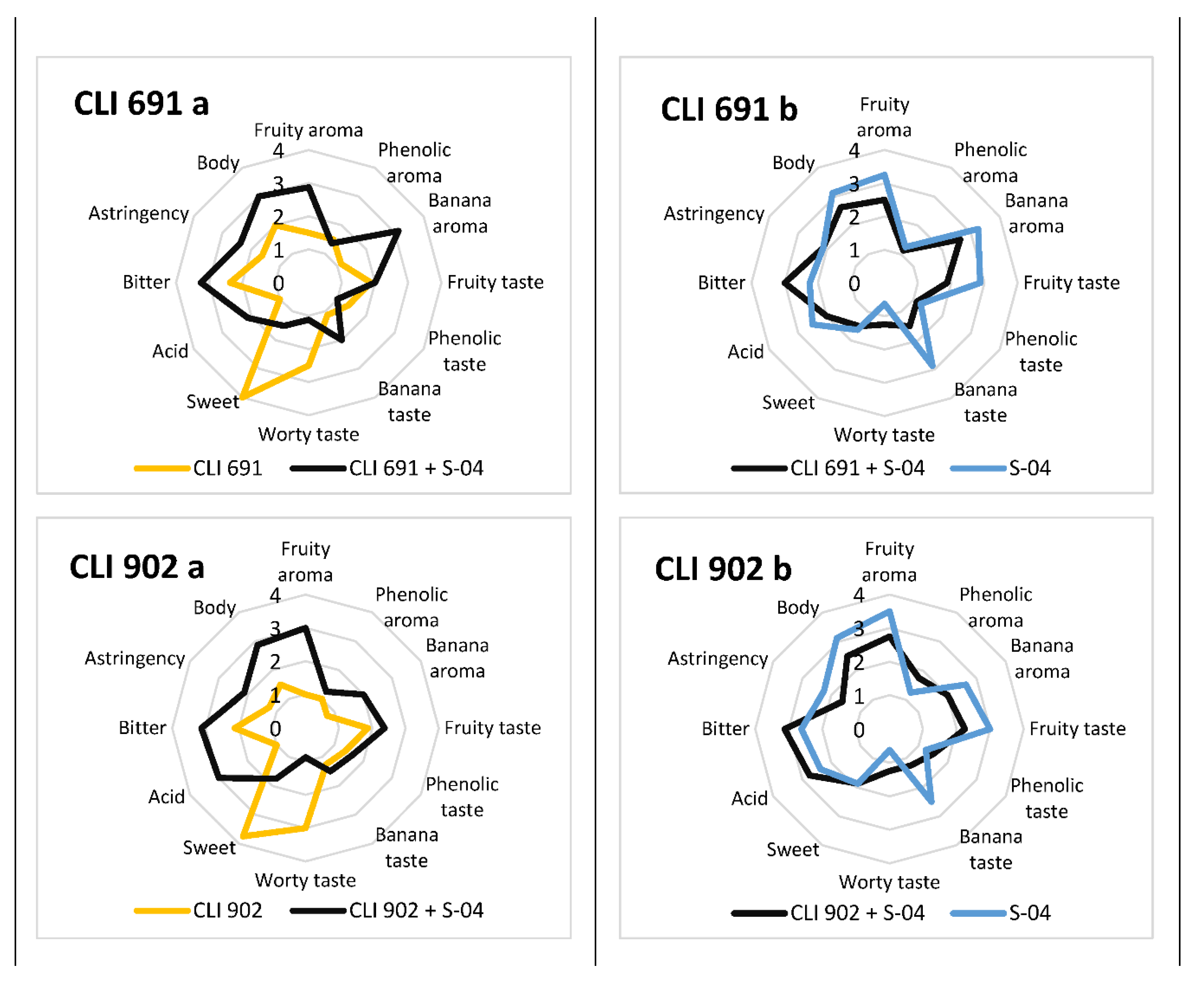
3.6. Sensory Analysis
3.7. Principal Components Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bokulich, N.A.; Bamforth, C.W.; Mills, D.A. Brewhouse-Resident Microbiota Are Responsible for Multi-Stage Fermentation of American Coolship Ale. PLoS ONE 2012, 7, e35507. [Google Scholar] [CrossRef] [PubMed]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The Yeast Saccharomyces cerevisiae—The Main Character in Beer Brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The Soul of Beer’s Aroma—A Review of Flavour-Active Esters and Higher Alcohols Produced by the Brewing Yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, G. The Production of Secondary Metabolites with Flavour Potential during Brewing and Distilling Wort Fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Anderson, H.E.; Santos, I.C.; Hildenbrand, Z.L.; Schug, K.A. A Review of the Analytical Methods Used for Beer Ingredient and Finished Product Analysis and Quality Control. Anal. Chim. Acta 2019, 1085, 1–20. [Google Scholar] [CrossRef]
- Aquilani, B.; Laureti, T.; Poponi, S.; Secondi, L. Beer Choice and Consumption Determinants When Craft Beers Are Tasted: An Exploratory Study of Consumer Preferences. Food Qual. Prefer. 2015, 41, 214–224. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could Non-Saccharomyces Yeasts Contribute on Innovative Brewing Fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Bourbon-Melo, N.; Palma, M.; Rocha, M.P.; Ferreira, A.; Bronze, M.R.; Elias, H.; Sá-Correia, I. Use of Hanseniaspora guilliermondii and Hanseniaspora opuntiae to Enhance the Aromatic Profile of Beer in Mixed-Culture Fermentation with Saccharomyces cerevisiae. Food Microbiol. 2021, 95, 103678. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the Brewing Process: A New Approach to Enhance Bioflavour and to Reduce Ethanol Content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by Non-Conventional Yeasts in Sequential Beer Fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef]
- Larroque, M.N.; Carrau, F.; Fariña, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and Non-Saccharomyces Native Yeasts on Beer Aroma Compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Kopecká, J.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for New Brewing Yeasts in the Non-Saccharomyces Sector with Torulaspora delbrueckii as Model. Yeast 2016, 33, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not Your Ordinary Yeast: Non-Saccharomyces Yeasts in Wine Production Uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Burini, J.A.; Eizaguirre, J.I.; Loviso, C.; Libkind, D. Levaduras No Convencionales Como Herramientas de Innovación y Diferenciación En La Producción de Cerveza. Rev. Argent. Microbiol. 2021, 53, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Pat. Food. Nutr. Agric. 2019, 11, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Bellut, K.; Arendt, E.K. Chance and Challenge: Non-Saccharomyces Yeasts in Nonalcoholic and Low Alcohol Beer Brewing—A Review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- De Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of New Strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to Produce Low-Alcohol Beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Faria, J.D.A.F.; Cruz, A.G. Characterization of Brazilian Lager and Brown Ale Beers Based on Color, Phenolic Compounds, and Antioxidant Activity Using Chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Moreno, H.; Calvo, J.R. Melatonin Present in Beer Contributes to Increase the Levels of Melatonin and Antioxidant Capacity of the Human Serum. Clin. Nutr. 2009, 28, 188–191. [Google Scholar] [CrossRef]
- Postigo, V.; Sánchez, A.; Cabellos, J.M.; Arroyo, T. New Approaches for the Fermentation of Beer: Non-Saccharomyces Yeasts from Wine. Fermentation 2022, 8, 280. [Google Scholar] [CrossRef]
- Montrocher, R.; Verner, M.C.; Briolay, J.; Gautier, C.; Marmeisse, R. Phylogenetic Analysis of the Saccharomyces cerevisiae Group Based on Polymorphisms of RDNA Spacer Sequences. Int. J. Syst. Bacteriol. 1998, 48, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteve-Zarzoso, B.; Belloch, C.; Uruburu, F.; Querol, A. Identification of Yeasts by RFLP Analysis of the 5.8S RRNA Gene and the Two Ribosomal Internal Transcribed Spacers. Int. J. Syst. Bacteriol. 1999, 49, 329–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL Medium to Profile Native Flora Fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar]
- Hierro, N.; Esteve-Zarzoso, B.; Mas, A.; Guillamón, J.M. Monitoring of Saccharomyces and Hanseniaspora Populations during Alcoholic Fermentation by Real-Time Quantitative PCR. FEMS Yeast Res. 2007, 7, 1340–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.; Esteve-Zarzoso, B.; Crespo, J.; Cabellos, J.M.; Arroyo, T. Yeast Monitoring of Wine Mixed or Sequential Fermentations Made by Native Strains from D.O. “Vinos de Madrid” Using Real-Time Quantitative PCR. Front. Microbiol. 2017, 8, 2520. [Google Scholar] [CrossRef] [PubMed]
- Zott, K.; Claisse, O.; Lucas, P.; Coulon, J.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Characterization of the Yeast Ecosystem in Grape Must and Wine Using Real-Time PCR. Food Microbiol. 2010, 27, 559–567. [Google Scholar] [CrossRef]
- Rawsthorne, H.; Phister, T.G. A Real-Time PCR Assay for the Enumeration and Detection of Zygosaccharomyces bailii from Wine and Fruit Juices. Int. J. Food Microbiol. 2006, 112, 1–7. [Google Scholar] [CrossRef]
- Turgeon, Z.; Jurgens, R.C.; Sierocinski, T.; Brimacombe, C.A.; Jin, Y.; Swanson, J.M.; Husnik, J.I. De Novo Hybridization Approach Expands Diversity of Industrially Applicable Lager Yeast Strains with Unique Genome Composition. Tech. Q. 2021, 58, 106–116. [Google Scholar] [CrossRef]
- Ortega, C.; López, R.; Cacho, J.; Ferreira, V. Fast Analysis of Important Wine Volatile Compounds—Development and Validation of a New Method Based on Gas Chromatographic-Flame Ionisation Detection Analysis of Dichloromethane Microextracts. J. Chromatogr. A 2001, 923, 205–214. [Google Scholar] [CrossRef]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A New Bioactive Compound in Wine. J. Food Compos. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Huang, X.; Mazza, G. Simultaneous Analysis of Serotonin, Melatonin, Piceid and Resveratrol in Fruits Using Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Hevia, D.; Mayo, J.C.; Tan, D.-X.; Rodriguez-Garcia, A.; Sainz, R.M. Melatonin Enhances Photo-Oxidation of 2′,7′-Dichlorodihydrofluorescein by an Antioxidant Reaction That Renders N1-Acetyl-N2-Formyl-5-Methoxykynuramine (AFMK). PLoS ONE 2014, 9, e109257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocadaǧli, T.; Yilmaz, C.; Gökmen, V. Determination of Melatonin and Its Isomer in Foods by Liquid Chromatography Tandem Mass Spectrometry. Food Chem. 2014, 153, 151–156. [Google Scholar] [CrossRef]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.-M.; Porumb, R.; Epure, P. Progress in Electrochemical (Bio)Sensors for Monitoring Wine Production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.6: Paired Comparison Test (IM). In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.4. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.12. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 1997. [Google Scholar]
- European Brewery Convention Analytica—EBC. Section 13 Sensory Analysis Method 13.0. In EBC Methods of Analysis; Fachverlag Hans Carl: Nürnberg, Germany, 2004. [Google Scholar]
- Eßlinger, H.M. Handbook of Brewing Processes Technology Markets-Wiley; Wiley: Weinheim, Germany, 2009; ISBN 9783527314065. [Google Scholar]
- Iattici, F.; Catallo, M.; Solieri, L. Designing New Yeasts for Craft Brewing: When Natural Biodiversity Meets Biotechnology. Beverages 2020, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Alves-Araújo, C.; Pacheco, A.; Almeida, M.J.; Spencer-Martins, I.; Leão, C.; Sousa, M.J. Sugar Utilization Patterns and Respiro-Fermentative Metabolism in the Baker’s Yeast Torulaspora delbrueckii. Microbiology 2007, 153, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.I.S.; Wahl, A.; Gombert, A.K. Aerobic Growth Physiology of Saccharomyces Cerevisiae on Sucrose Is Strain-Dependent. FEMS Yeast Res. 2021, 21, foab021. [Google Scholar] [CrossRef]
- Klein, C.J.L.; Olsson, L.; Nielsen, J. Glucose Control in Saccharomyces cerevisiae: The Role of MIG1 in Metabolic Functions. Microbiology 1998, 144, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Nikulin, J.; Vidgren, V.; Krogerus, K.; Magalhães, F.; Valkeemäki, S.; Kangas-Heiska, T.; Gibson, B. Brewing Potential of the Wild Yeast Species Saccharomyces paradoxus. Eur. Food Res. Technol. 2020, 246, 2283–2297. [Google Scholar] [CrossRef]
- Carbonetto, B.; Ramsayer, J.; Nidelet, T.; Legrand, J.; Sicard, D. Bakery Yeasts, a New Model for Studies in Ecology and Evolution. Yeast 2018, 35, 591–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, T.; Silva-Sousa, F.; Pereira, F.; Rito, T.; Soares, P.; Franco-Duarte, R.; Sousa, M.J. Biotechnological Importance of Torulaspora delbrueckii: From the Obscurity to the Spotlight. J. Fungi 2021, 7, 712. [Google Scholar] [CrossRef] [PubMed]
- Taillandier, P.; Lai, Q.P.; Julien-Ortiz, A.; Brandam, C. Interactions between Torulaspora delbrueckii and Saccharomyces cerevisiae in Wine Fermentation: Influence of Inoculation and Nitrogen Content. World J. Microbiol. Biotechnol. 2014, 30, 1959–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langstaff, S.A.; Guinard, J.-X.; Lewis, M.J. Instrumental Evaluation of the Mouthfeel of Beer and Correlation With Sensory Evaluation. J. Inst. Brew. 1991, 97, 427–433. [Google Scholar] [CrossRef]
- Parker, W.E.; Richardson, P.J. The Quantitative Determination Of Glycerol In Beer By Gas-Liquid Chromatography. J. Inst. Brew. 1970, 76, 191–198. [Google Scholar] [CrossRef]
- Shehadeh, A.; Kechagia, D.; Evangelou, A.; Tataridis, P.; Shehadeh, F. Effect of Ethanol, Glycerol, Glucose and Tartaric Acid on the Viscosity of Model Aqueous Solutions and Wine Samples. Food Chem. 2019, 300, 125191. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in Alcoholic Fermentation Processes: Role of Physiological Fitness and Microbial Interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Maharaj, Y.R.; Pillay, B. Effects of Fermentation Temperature on the Composition of Beer Volatile Compounds, Organoleptic Quality and Spent Yeast Density. Electron. J. Biotechnol. 2011, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.; Ladefoged, A.M.; Nørgaard, L. Rapid Determination of Bitterness in Beer Using Fluorescence Spectroscopy and Chemometrics. J. Inst. Brew. 2005, 111, 3–10. [Google Scholar] [CrossRef]
- Hao, J.; Speers, R.A.; Fan, H.; Deng, Y.; Dai, Z. A Review of Cyclic and Oxidative Bitter Derivatives of Alpha, Iso-Alpha and Beta-Hop Acids. J. Am. Soc. Brew. Chem. 2020, 78, 89–102. [Google Scholar] [CrossRef]
- Bamforth, C.W. Beer and Health. In Beer; Elsevier: Amsterdam, The Netherlands, 2009; pp. 229–253. ISBN 9780126692013. [Google Scholar]
- Laws, D.R.J.; McGuinness, J.D.; Rennie, H. The Losses Of Bitter Substances During Fermentation. J. Inst. Brew. 1972, 78, 314–321. [Google Scholar] [CrossRef]
- Popescu, V.; Soceanu, A.; Dobrinas, S.; Stanciu, G. A Study of Beer Bitterness Loss during the Various Stages of the Romanian Beer Production Process. J. Inst. Brew. 2013, 119, 111–115. [Google Scholar] [CrossRef]
- Wang, L.; Hong, K.; Agbaka, J.I.; Zhu, G.; Lv, C.; Ma, C. Application of UHPLC-Q/TOF-MS-Based Metabolomics Analysis for the Evaluation of Bitter-Tasting Krausen Metabolites during Beer Fermentation. J. Food Compos. Anal. 2021, 99, 103850. [Google Scholar] [CrossRef]
- Kishimoto, T.; Teramoto, S.; Fujita, A.; Yamada, O. Evaluation of Components Contributing to the International Bitterness Unit of Wort and Beer. J. Am. Soc. Brew. Chem. 2022, 80, 53–61. [Google Scholar] [CrossRef]
- Engan, S. Organoleptic Threshold Values Of Some Organic Acids In Beer. J. Inst. Brew. 1974, 80, 162–163. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological Traits of Lachancea thermotolerans Show Signs of Domestication and Allopatric Differentiation. Sci. Rep. 2018, 8, 14812. [Google Scholar] [CrossRef]
- Guido, L.F. Sulfites in Beer: Reviewing Regulation, Analysis and Role. Sci. Agric. 2016, 73, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Meilgaard, M.C. Individual Differences in Sensory Threshold for Aroma Chemicals Added to Beer. Food Qual. Prefer. 1993, 4, 153–167. [Google Scholar] [CrossRef]
- Dvořak, J.; Dostálek, P.; Štěrba, K.; Čejka, P.; Kellner, V.; Čulík, J.; Beinrohr, E. Determination of Total Sulphur Dioxide in Beer Samples by Flow-through Chronopotentiometry. J. Inst. Brew. 2006, 112, 308–313. [Google Scholar] [CrossRef]
- Meilgaard, M.; Elizondo, A.; Moya, E. A Study of Carbonyl Compounds in Beer, Part II. Flavor and Flavor Thresholds of Aldehydes and Ketones Added to Beer. Tec. Q. Master Brew. Assac. Am. 1970, 7, 143–149. [Google Scholar]
- Passoth, V.; Fredlund, E.; Druvefors, U.Ä.; Schnürer, J. Biotechnology, Physiology and Genetics of the Yeast Pichia anomala. FEMS Yeast Res. 2006, 6, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, G.M. Pichia anomala: Cell Physiology and Biotechnology Relative to Other Yeasts. Antonie Van Leeuwenhoek 2011, 99, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla, B.; Gil, J.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2018, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, F.K.; Mayer, V.W.; Scheel, I.; Resnick, M.A. Acetone, Methyl Ethyl Ketone, Ethyl Acetate, Acetonitrile and Other Polar Aprotic Solvents Are Strong Inducers of Aneuploidy in Saccharomyces cerevisiae. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1985, 149, 339–351. [Google Scholar] [CrossRef]
- Garcia-Caro, R.D.C.; Thompson, G.; Zhang, P.; Hokamp, K. Enhanced Flavour Profiles through Radicicol Induced Genomic Variation in S. Pastorianus Lager Yeast; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2022; pp. 1–24. [Google Scholar]
- Branco, P.; Kemsawasd, V.; Santos, L.; Diniz, M.; Caldeira, J.; Almeida, M.G.; Arneborg, N.; Albergaria, H. Saccharomyces cerevisiae Accumulates GAPDH-Derived Peptides on Its Cell Surface That Induce Death of Non-Saccharomyces Yeasts by Cell-to-Cell Contact. FEMS Microbiol. Ecol. 2017, 93, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy Sulphur Compounds, Higher Alcohols and Esters Production Profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii Grown as Pure and Mixed Cultures in Grape Must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of Yeast Species and Strains in Wine Flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Seixas, I.; Barbosa, C.; Mendes-Faia, A.; Güldener, U.; Tenreiro, R.; Mendes-Ferreira, A.; Mira, N.P. Genome Sequence of the Non-Conventional Wine Yeast Hanseniaspora guilliermondii UTAD222 Unveils Relevant Traits of This Species and of the Hanseniaspora Genus in the Context of Wine Fermentation. DNA Res. 2019, 26, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Engan, S. Organoleptic Threshold Values Of Some Alcohols And Esters In Beer. J. Inst. Brew. 1972, 78, 33–36. [Google Scholar] [CrossRef]
- Einfalt, D. Barley-Sorghum Craft Beer Production with Saccharomyces cerevisiae, Torulaspora delbrueckii and Metschnikowia pulcherrima Yeast Strains. Eur. Food Res. Technol. 2021, 247, 385–393. [Google Scholar] [CrossRef]
- Tan, Y.; Siebert, K.J. Quantitative Structure-Activity Relationship Modeling of Alcohol, Ester, Aldehyde, and Ketone Flavor Thresholds in Beer from Molecular Features. J. Agric. Food Chem. 2004, 52, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of Chemical and Sensory Properties during Aging of Top-Fermented Beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected Non-Saccharomyces Wine Yeasts in Controlled Multistarter Fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-Yeast Interactions Revealed by Aromatic Profile Analysis of Sauvignon Blanc Wine Fermented by Single or Co-Culture of Non-Saccharomyces and Saccharomyces Yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; De Schutter, D.; Daenen, L.; Lynch, K.; Zannini, E.; Arendt, E. Application of Non-Saccharomyces Yeasts Isolated from Kombucha in the Production of Alcohol-Free Beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.A.; Barros, A.S.; Carvalho, B.; Brandão, T.; Gil, A.M.; Ferreira, A.C.S. Evaluation of Beer Deterioration by Gas Chromatography-Mass Spectrometry/Multivariate Analysis: A Rapid Tool for Assessing Beer Composition. J. Chromatogr. A 2011, 1218, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.; Magalhães, J.; Pereira, F.B.; Macieira, F.; Domingues, L.; Oliveira, J.M. Volatile Fingerprinting Differentiates Diverse-Aged Craft Beers. LWT 2019, 108, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Blanco, C.A.; Andrés-Iglesias, C.; Montero, O. Low-Alcohol Beers: Flavor Compounds, Defects, and Improvement Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 1379–1388. [Google Scholar] [CrossRef]
- Sterckx, F.L.; Missiaen, J.; Saison, D.; Delvaux, F.R. Contribution of Monophenols to Beer Flavour Based on Flavour Thresholds, Interactions and Recombination Experiments. Food Chem. 2011, 126, 1679–1685. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Prediction of Flavor Differences between Beers from Their Chemical Composition. J. Agric. Food Chem. 1982, 30, 1009–1017. [Google Scholar] [CrossRef]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The Chemical Profiling of Fatty Acids during the Brewing Process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L.; et al. Domestication and Divergence of Saccharomyces Cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O.I. The Deoxyribose Method: A Simple “Test-Tube” Assay for Determination of Rate Constants for Reactions of Hydroxyl Radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X. Research Progress on the Antioxidant Biological Activity of Beer and Strategy for Applications. Trends Food Sci. Technol. 2021, 110, 754–764. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- Olas, B.; Bryś, M. Beer Components and Their Beneficial Effect on the Hemostasis and Cardiovascular Diseases—Truth or Falsehood. Food Chem. Toxicol. 2020, 146, 111782. [Google Scholar] [CrossRef]
- Tafulo, P.A.R.; Queirós, R.B.; Delerue-Matos, C.M.; Sales, M.G.F. Control and Comparison of the Antioxidant Capacity of Beers. Food Res. Int. 2010, 43, 1702–1709. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Postigo, V.; García, M.; Cabellos, J.M.; Arroyo, T. Wine Saccharomyces Yeasts for Beer Fermentation. Fermentation 2021, 7, 290. [Google Scholar] [CrossRef]
- Morcillo-Parra, M.Á.; Beltran, G.; Mas, A.; Torija, M.-J. Effect of Several Nutrients and Environmental Conditions on Intracellular Melatonin Synthesis in Saccharomyces cerevisiae. Microorganisms 2020, 8, 853. [Google Scholar] [CrossRef]
- Vigentini, I.; Gardana, C.; Fracassetti, D.; Gabrielli, M.; Foschino, R.; Simonetti, P.; Tirelli, A.; Iriti, M. Yeast Contribution to Melatonin, Melatonin Isomers and Tryptophan Ethyl Ester during Alcoholic Fermentation of Grape Musts. J. Pineal Res. 2015, 58, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, H.; Calvo, J.R.; Maldonado, M.D. High Levels of Melatonin Generated during the Brewing Process. J. Pineal Res. 2013, 55, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding Flavor to Beverages with Non-Conventional Yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Martin, V.; Valera, M.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on Quality and Composition of Riesling Wines Fermented by Sequential Inoculation with Non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Varela, C. The Impact of Non-Saccharomyces Yeasts in the Production of Alcoholic Beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Le Floch, A.; de Revel, G.; Barbe, J.-C. Study of Sensory Interactions among Red Wine Fruity Esters in a Model Solution. J. Agric. Food Chem. 2013, 61, 8504–8513. [Google Scholar] [CrossRef]
- Meilgaard, M. Flavor Chemistry of Beer. I. Flavor Interaction between Principal Volatiles. Master Brew. Assoc. Am. Tech. Q. 1975, 12, 107–117. [Google Scholar]
- San-Juan, F.; Ferreira, V.; Cacho, J.; Escudero, A. Quality and Aromatic Sensory Descriptors (Mainly Fresh and Dry Fruit Character) of Spanish Red Wines Can Be Predicted from Their Aroma-Active Chemical Composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Zhao, H.; Huang, M.; Sun, Y.; Zhang, J.; Sun, B. Recent Advances in the Understanding of Off-Flavors in Alcoholic Beverages: Generation, Regulation, and Challenges. J. Food Compos. Anal. 2021, 103, 104117. [Google Scholar] [CrossRef]
- Haslbeck, K.; Bub, S.; von Kamp, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Coelhan, M. The Influence of Brewing Yeast Strains on Monoterpene Alcohols and Esters Contributing to the Citrus Flavour of Beer. J. Inst. Brew. 2018, 124, 403–415. [Google Scholar] [CrossRef] [Green Version]
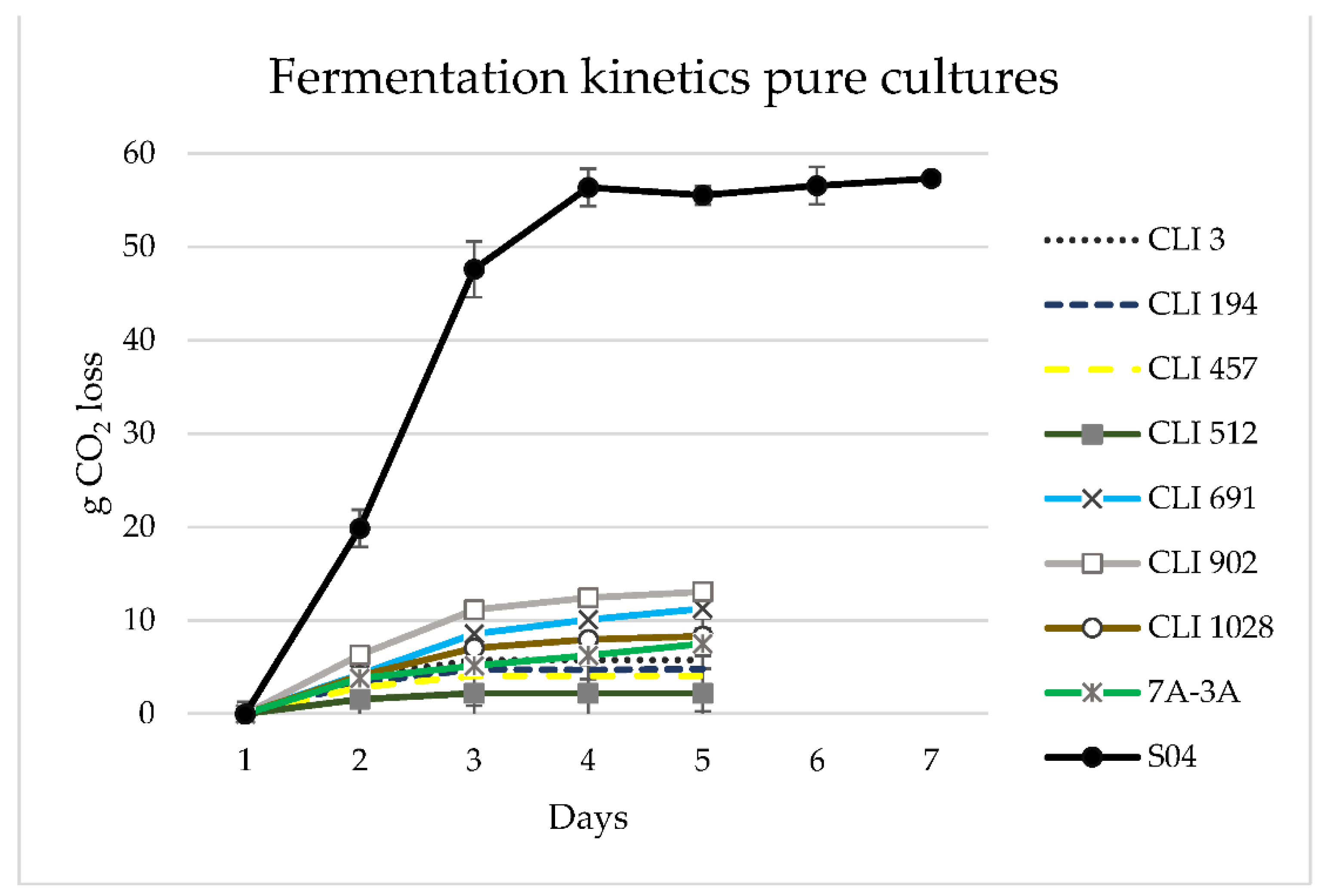
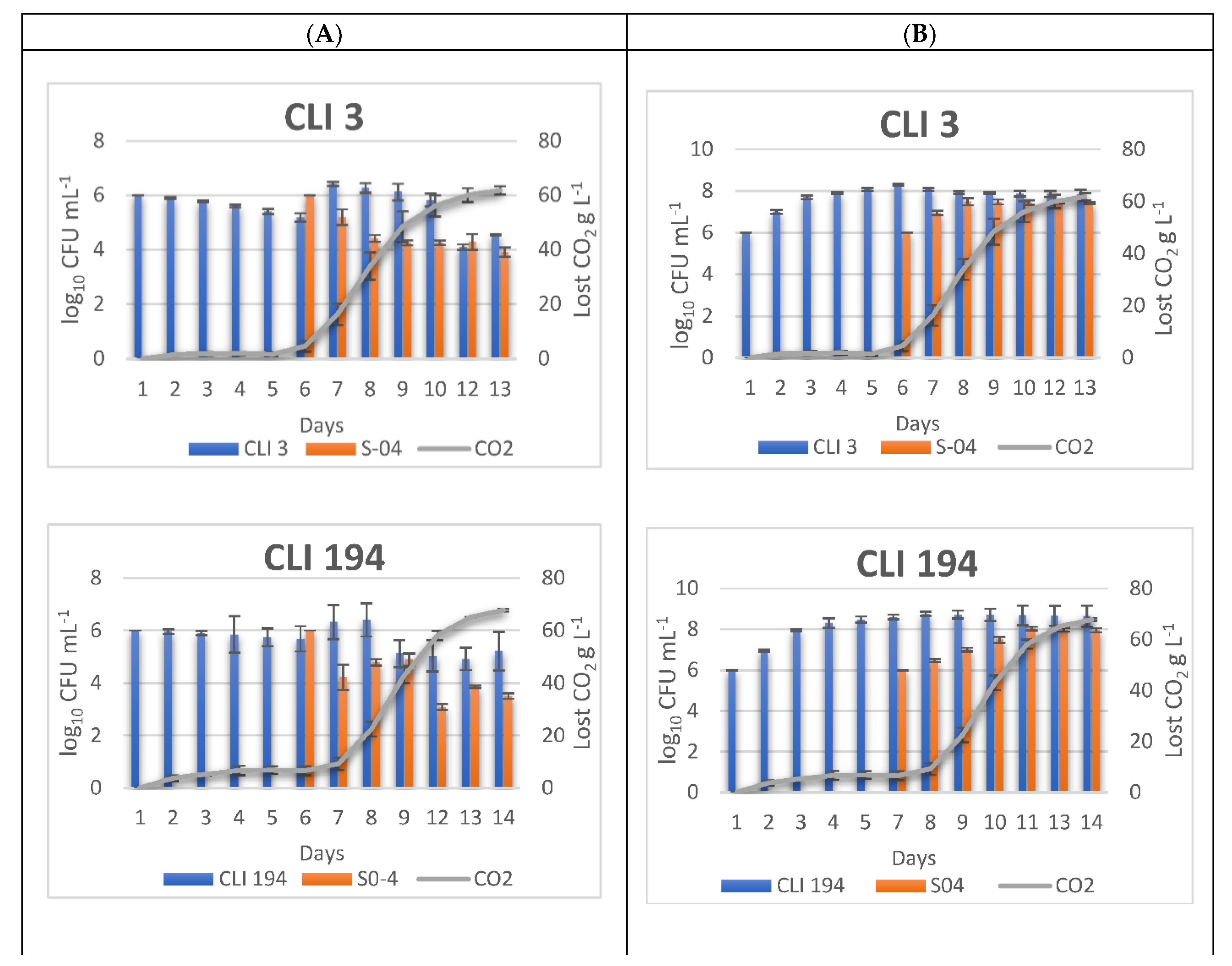
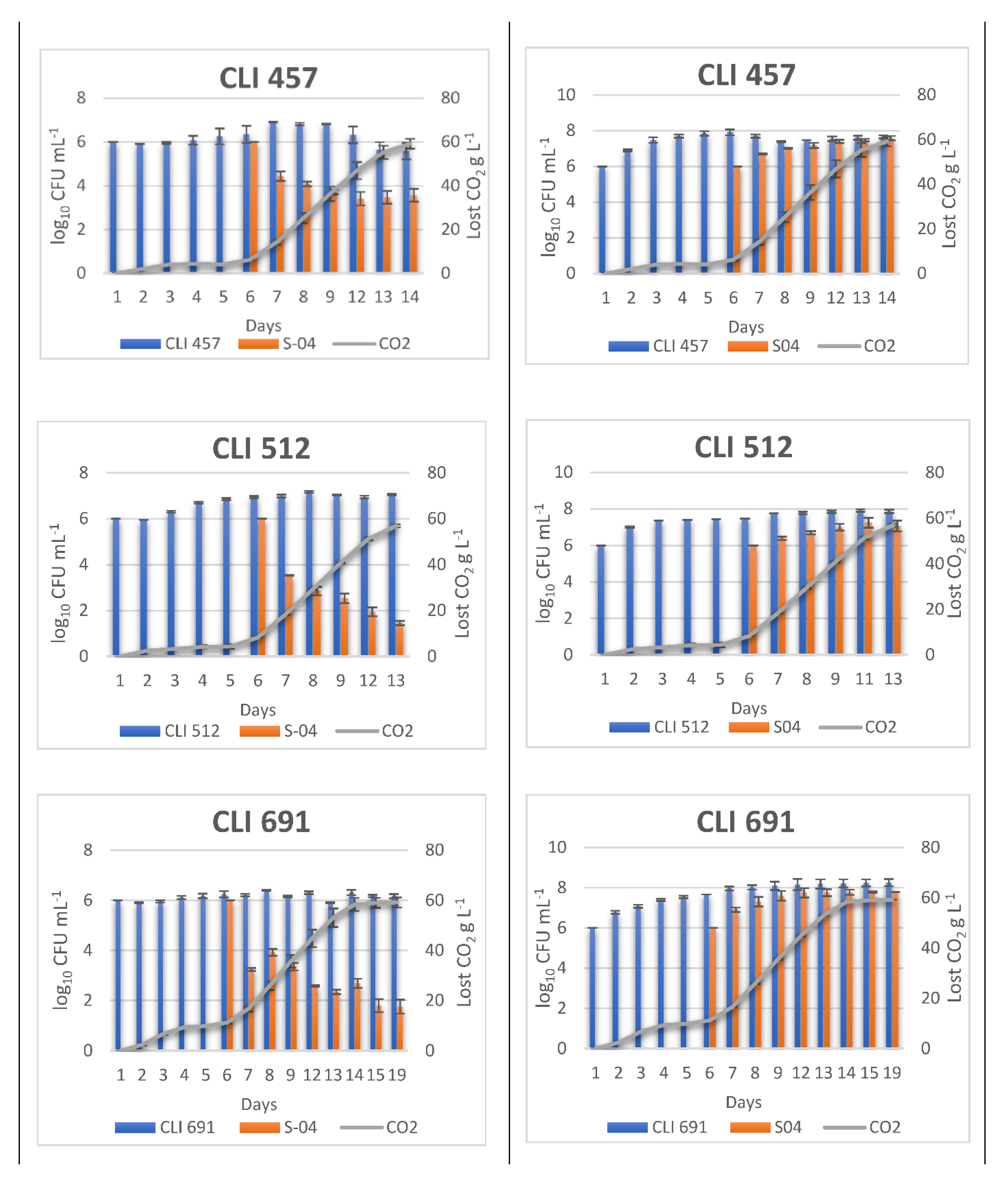
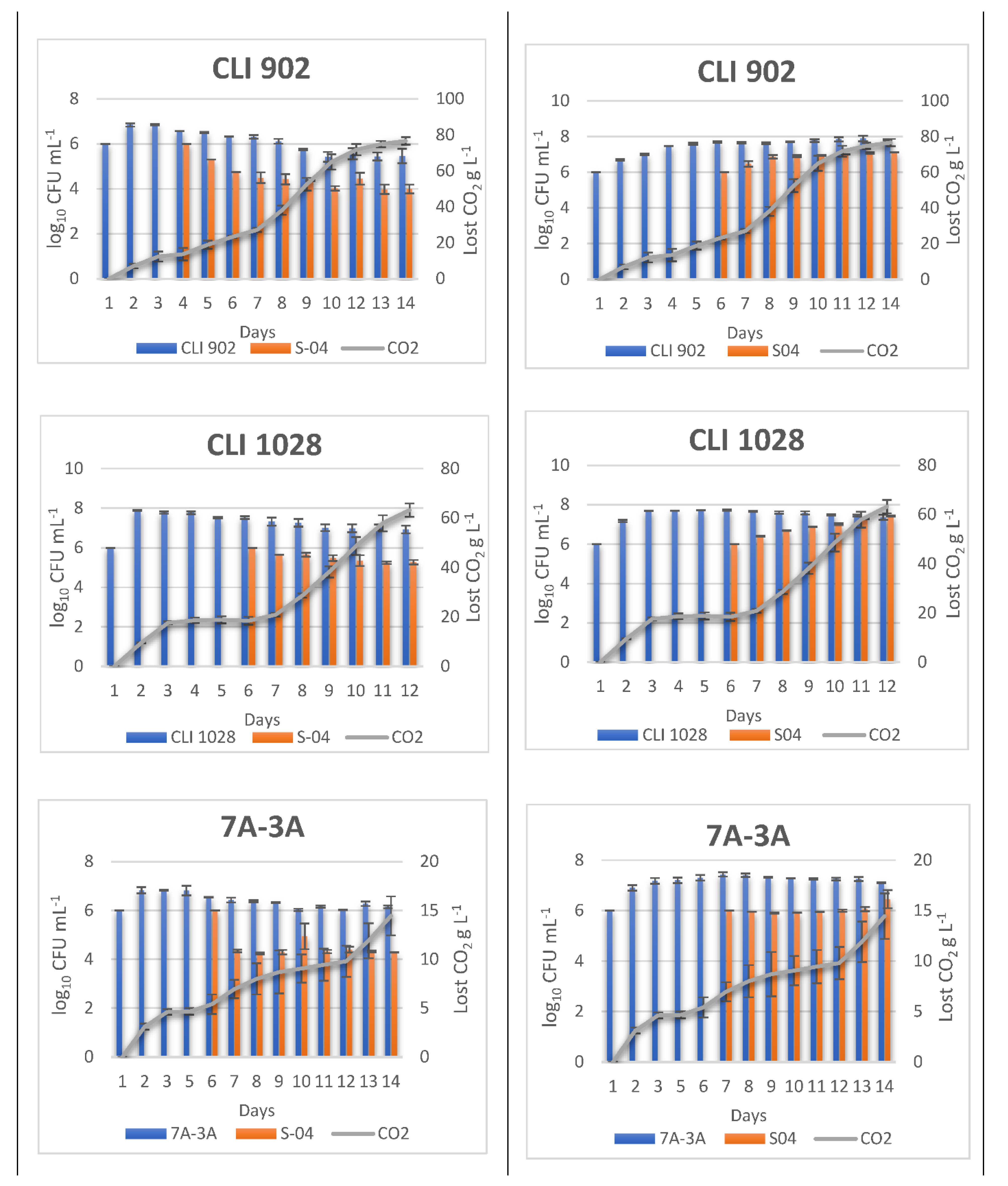

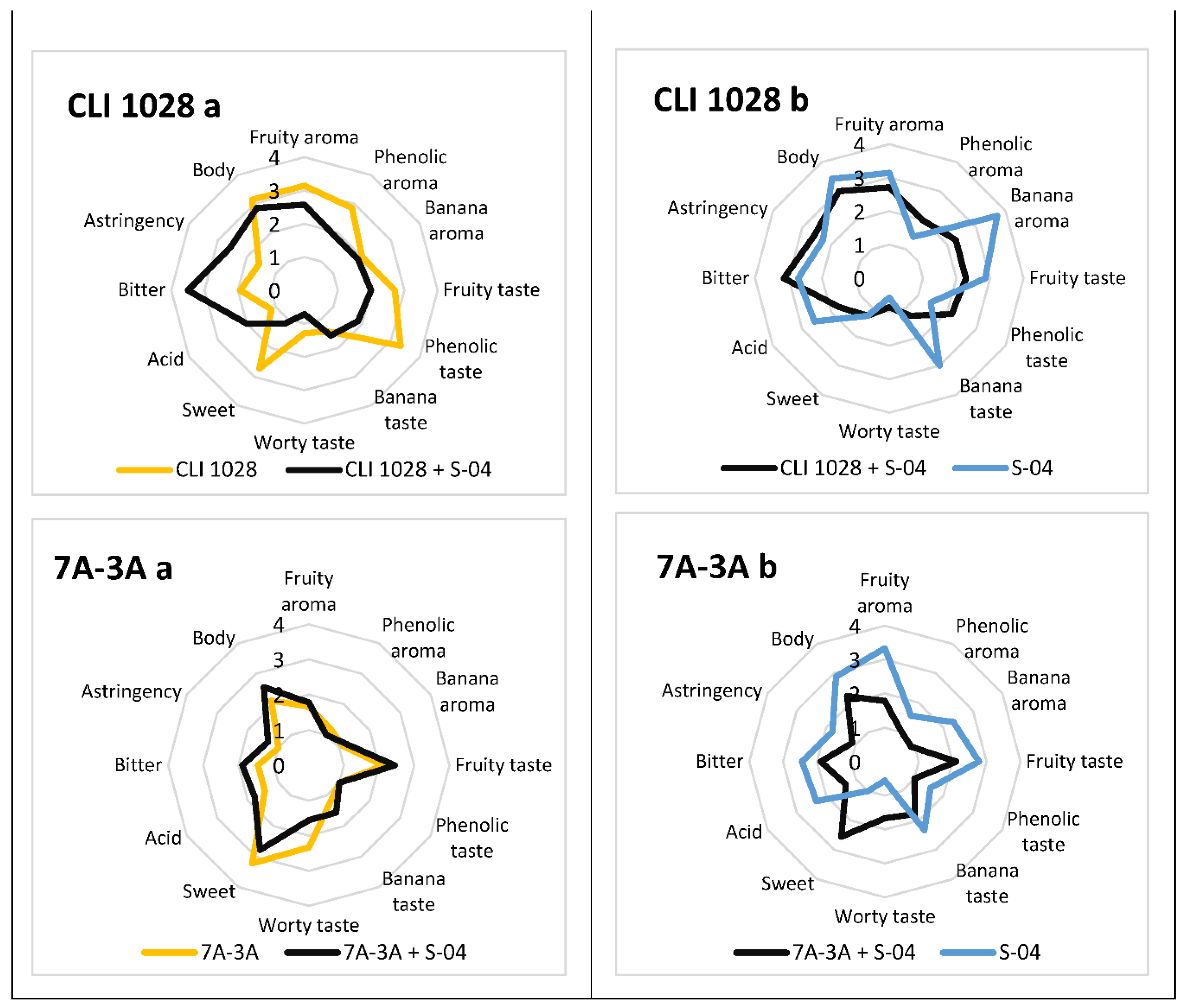
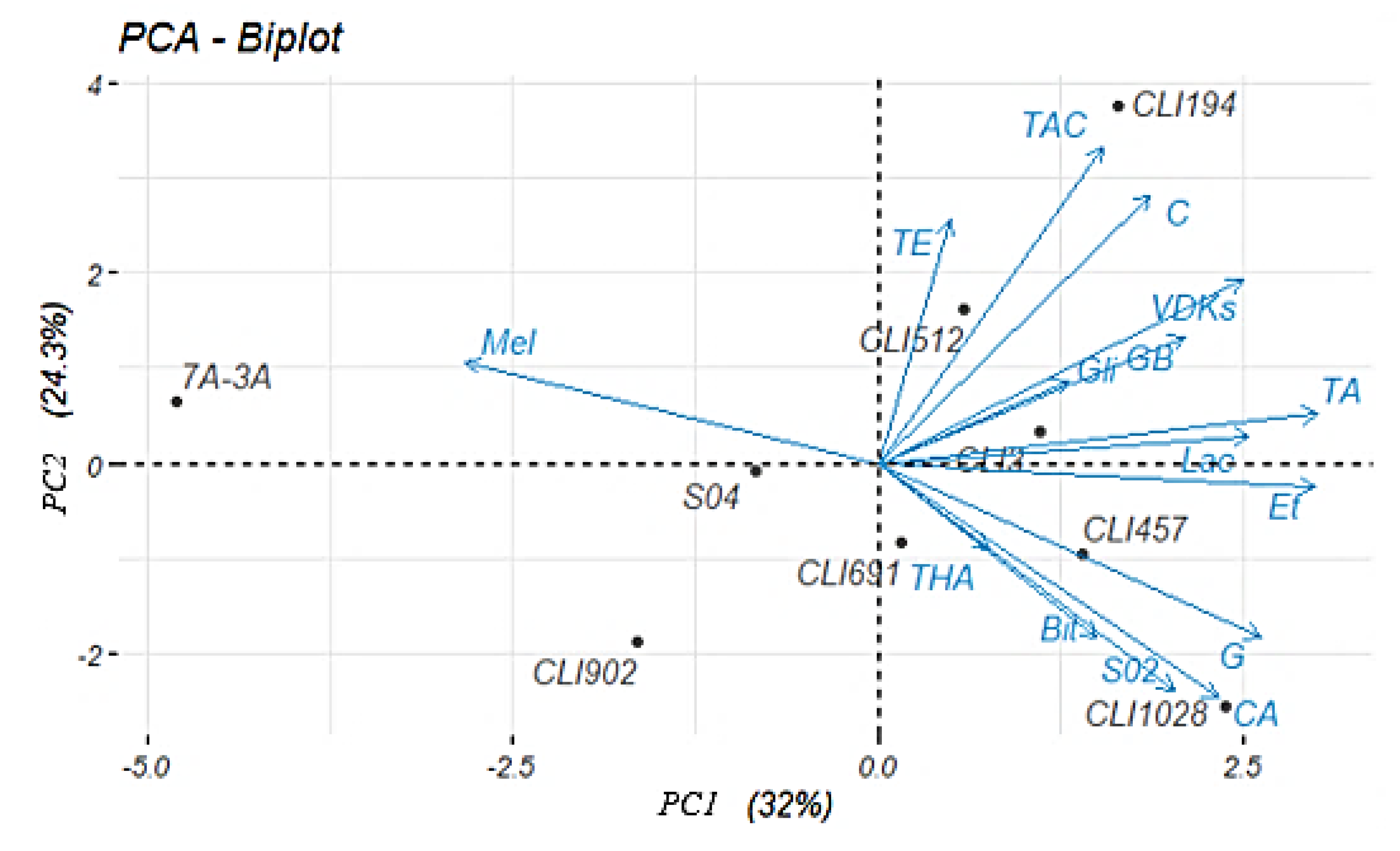
| Yeast Strain | Species |
|---|---|
| CLI 3 | Hanseniaspora vineae |
| CLI 194 | Hanseniaspora valbyensis |
| CLI 457 | Metschnikowia pulcherrima |
| CLI 512 | Hanseniaspora guilliermondii |
| CLI 691 | Zygosaccharomyces bailii |
| CLI 902 | Torulaspora delbrueckii |
| CLI 1028 | Wickerhamomyces anomalus |
| 7A-3A | Torulaspora delbrueckii |
| Yeast Strains | Primer Name | Sequence 5′–3′ | Reference |
|---|---|---|---|
| Hanseniaspora vineae (CLI 3) | CESP-F | ATCGAATTTTTGAACGCACATTG | [25] |
| HUV-R | AACCCTGAGTATCGCCCACA | ||
| Hanseniaspora valbyensis (CLI 194) | HV1-F | GCAGCTCAAAGTGGGTGGTA | This study |
| HV1-R | GAGGCGAGTGCATGCAAAAA | ||
| Metschnikowia pulcherrima (CLI 457) | MP2-F | AGACACTTAACTGGGCCAGC | [26] |
| MP2-R | GGGGTGGTGTGGAAGTAAGG | ||
| Hanseniaspora gilliermondii (CLI 512) | Hauf 2L | CCCTTTGCCTAAGGTACG | [27] |
| Hauf 2R | CGCTGTTCTCGCTGTGATG | ||
| Zygosaccharomyces bailii (CLI 691) | ZBF1 | CATGGTGTTTTGCGCC | [28] |
| ZBR1 | CGTCCGCCACGAAGTGGTAGA | ||
| Torulaspora delbrueckii (CLI 902, 7A-3A) | Tods L2 | CAAAGTCATCCAAGCCAGC | [27] |
| Tods R2 | TTCTCAAACAATCATGTTTGGTAG | ||
| Wickerhamomyces anomalus (CLI 1028) | WACLI2-F | ATTGGCGAGAGACCGATAGC | This study |
| WACLI2-R | TTGCCATCCGAATCGATGCT | ||
| Saccharomyces cerevisiae (S-04) | SC1 | AAAACTCCACAGTGTGTTG | [27] |
| SC2 | GCTTAAGTGCGCGGTCTTG |
| Yeast Strains | Residual Fermentable Sugars (g L−1) | Glycerol (g L−1) | Ethanol % (v/v) | ||||
|---|---|---|---|---|---|---|---|
| Maltotriose | Maltose | Glucose | Fructose | ||||
| CLI 3 | PC | 13.68 ± 0.00 a | 63.80 ± 0.22 a | 0.25 ± 0.02 | 1.02 ± 0.00 a | 1.21 ± 0.01 f | 0.62 ± 0.17 e |
| SC | 11.05 ± 2.33 abc | 1.16 ± 0.10 e | 0.23 ± 0.02 | 0.17 ± 0.00 b | 4.28 ± 0.40 a | 5.28 ± 0.10 b | |
| CLI 194 | PC | 13.71 ± 0.45 a | 60.91 ± 0.09 bc | 0.55 ± 0.59 | 0.77 ± 0.06 ab | 1.15 ± 0.06 f | 0.73 ± 0.01 de |
| SC | 4.37 ± 3.07 bcde | 0.96 ± 0.41 e | 0.52 ± 0.30 | 0.16 ± 0.01 b | 3.55 ±0.21 bcd | 5.58 ± 0.38 b | |
| CLI 457 | PC | 13.74 ± 0.02 a | 64.26 ± 0.23 a | 0.17 ± 0.01 | 0.24 ± 0.00 b | 1.26 ± 0.01 f | 0.62 ± 0.03 e |
| SC | 2.75 ± 1.12 cde | 1.77 ± 0.12 e | 0.17 ± 0.03 | 0.16 ± 0.00 b | 4.17 ± 0.02 a | 5.67 ± 0.17 b | |
| CLI 512 | PC | 12.03 ± 0.24 ab | 59.11 ± 1.94 c | 0.12 ± 0.01 | 0.24 ± 0.00 b | 1.12 ± 0.04 f | 0.61 ± 0.01 e |
| SC | 9.34 ± 5.43 abcd | 1.34 ± 0.44 e | 0.18 ± 0.02 | 0.18 ± 0.01 b | 3.88 ± 0.27 ab | 5.11 ± 0.25 b | |
| CLI 691 | PC | 13.01 ± 0.14 a | 63.16 ± 0.89 ab | 0.63 ± 0.01 | 0.15 ± 0.02 b | 1.34 ± 0.01 f | 0.63 ± 0.01 e |
| SC | 10.83 ± 2.16 abc | 1.63 ± 0.07 e | 0.12 ± 0.03 | 0.16 ± 0.00 b | 3.79 ± 0.09 abc | 5.23 ± 0.12 b | |
| CLI 902 | PC | 13.35 ± 0.12 a | 63.94 ± 0.46 a | 0.14 ± 0.01 | 0.26 ± 0.00 b | 1.21 ± 0.14 f | 1.24 ± 0.03 d |
| SC | 1.28 ± 0.01 e | 1.31 ± 0.04 e | 0.16 ± 0.01 | 0.14 ± 0.01 b | 3.54 ± 0.06 bcd | 5.18 ± 0.01 b | |
| CLI 1028 | PC | 15.97 ± 0.47 a | 59.06 ± 0.25 c | 0.14 ± 0.02 | 0.24 ± 0.01 b | 1.08 ±0.09 f | 1.25 ± 0.00 d |
| SC | 2.09 ± 0.06 de | 1.74 ± 0.09 e | 0.13 ± 0.00 | 0.15 ± 0.00 b | 2.57 ±0.15 e | 5.18 ± 0.23 b | |
| 7A-3A | PC | 11.58 ± 0.31 abc | 59.53 ± 0.22 c | 0.12 ± 0.00 | 0.65 ± 0.00 ab | 1.28 ± 0.03 f | 0.99 ± 0.01 de |
| SC | 11.59 ± 0.04 abc | 45.31 ± 2.43 d | 0.13 ± 0.02 | 0.22 ± 0.04 b | 2.81 ± 0.36 de | 2.15 ± 0.20 c | |
| S-04 | PC | 1.37 ± 0.04 e | 1.55 ± 0.01 e | 0.11 ± 0.01 | 0.20 ± 0.04 b | 2.89 ± 0.00 e | 5.93 ± 0.58 a |
| Yeast Strains | Colour EBC | Bitterness IBU | Lactic Acid ppm | SO2 ppm | VDKs ppm |
|---|---|---|---|---|---|
| CLI 3 | 12.5 ±0.71 abc | 30.00 ± 0.57 a | 369.50 ± 70.00 a | 1.15 ± 0.21 | 0.29 ± 0.04 abc |
| CLI 194 | 15.00 ± 0.00 a | 24.95 ± 0.64 ab | 360.00 ± 9.90 ab | ≤1.00 | 0.39 ± 0.00 a |
| CLI 457 | 12.00 ± 0.00 abc | 34.75 ± 2.05 a | 395.50 ± 14.85 a | 1.15 ± 0.21 | 0.16 ± 0.02 abc |
| CLI 512 | 13.50 ± 0.71 ab | 22.85 ± 10.11 ab | 356.50 ± 30.40 ab | ≤1.00 | 0.34 ± 0.00 ab |
| CLI 691 | 11.50 ± 0.71 bc | 34.35 ± 1.48 a | 234.00 ± 7.07 b | 1.25 ± 0.07 | 0.12 ± 0.00 bc |
| CLI 902 | 10.33 ± 0.58 c | 26.30 ± 2.36 ab | 322.00 ± 37.40 ab | ≤1.00 | 0.10 ± 0.03 c |
| CLI 1028 | 12.00 ± 0.00 abc | 35.20 ± 0.57 a | 368.50 ± 17.68 a | 1.45 ± 0.64 | 0.27 ± 0.07 abc |
| 7A-3A | 11.50 ± 0.71 bc | 26.10 ± 0.28 ab | 264.00 ± 12.73 ab | ≤1.00 | ≤0.05 c |
| S-04 | 10.00 ± 1.15 c | 16.35 ± 1.35 c | 304.25 ± 29.03 ab | 1.08 ± 0.15 | 0.32 ± 0.10 ab |
| Yeast Strains | Slope | R2 | Efficiency (%) |
|---|---|---|---|
| H. vineae (CLI 3) | −3.58 ± 0.07 | 0.997 ± 0.00 | 90.23 ± 0.02 |
| H. valbyensis (CLI 194) | −2.96 ± 0.14 | 0.993 ± 0.00 | 118.15 ± 0.08 |
| M. pulcherrima (CLI 457) | −3.35 ± 0.04 | 0.992 ± 0.00 | 98.90 ± 0.02 |
| H. gilliermondii (CLI 512) | −3.33 ± 0.03 | 0.994 ± 0.00 | 99.73 ± 0.01 |
| Z. bailii (CLI 691) | −4.04 ± 0.15 | 0.992 ± 0.00 | 77.02 ±0.04 |
| T. delbrueckii (CLI 902) | −3.83 ± 0.05 | 0.998 ± 0.00 | 82.56 ± 0.01 |
| W. anomalus (CLI 1028) | −3.62 ± 0.11 | 0.998 ± 0.00 | 89.27 ± 0.04 |
| T. delbrueckii (7A-3A) | −3.67 ± 0.05 | 0.998 ± 0.00 | 87.43 ± 0.02 |
| S. cerevisiae (S-04) | −3.56 ± 0.08 | 0.999 ± 0.00 | 91.14 ± 0.03 |
| Yeast Strains | CLI 3 | CLI 194 | CLI 457 | CLI 512 | CLI 691 | CLI 902 | CLI 1028 | 7A-3A | S-04 |
|---|---|---|---|---|---|---|---|---|---|
| H. vineae | H. valbyensis | M. pulcherrima | H. guilliermondii | Z. bailii | T. delbrueckii | W. anomalus | T. delbrueckii | S. cerevisiae | |
| Higher alcohols | |||||||||
| Isobutanol | 19.21 ± 4.84 cde | 41.46 ± 1.51 b | 60.77 ± 10.62 a | 23.23 ± 2.01 cde | 27.86 ± 4.13 cd | 43.98 ± 2.18 b | 30.46 ± 0.04 bc | 9.82 ± 1.21 e | 28.54 ± 2.29 c |
| Isoamyl alcohol | 59.17 ± 2.37 bc | 110.08 ± 4.64 a | 107.78 ± 11.95 a | 64.97 ± 9.84 bc | 87.79 ± 7.62 ab | 113.04 ± 11.44 a | 73.85 ± 5.79 bc | 55.06 ± 4.72 c | 77.62 ± 5.92 bc |
| Methionol | 2.18 ± 0.25 cd | 5.31 ± 0.62 a | 1.33 ± 0.24 cd | 2.51 ± 0.83 c | 1.14 ± 0.00 d | 5.41 ± 0.23 a | 1.16 ± 0.10 d | 3.94 ± 0.13 b | 1.07 ± 0.26 d |
| β-phenylethanol | 10.90 ± 2.64 f | 32.76 ± 2.45 cde | 21.16 ± 1.90 def | 15.88 ± 2.24 ef | 58.61 ± 1.85 ab | 67.23 ± 2.08 a | 40.86 ± 0.79 bc | 26.20 ± 2.99 cdef | 34.53 ± 8.51 cd |
| Esters | |||||||||
| Ethyl isovalerate | 0.12 ± 0.04 b | 0.19 ± 0.01 b | 0.18 ± 0.02 b | 0.13 ± 0.02 b | 0.09 ± 0.00 b | 0.17 ± 0.03 b | 0.15 ± 0.01 b | 1.11 ± 0.20 a | 0.14 ± 0.02 b |
| Ethyl butirate | 0.16 ± 0.00 abc | 0.18 ± 0.00 ab | 0.20 ± 0.02 a | 0.15 ± 0.01 abc | 0.19 ± 0.01 a | 0.15 ± 0.01 abc | 0.11 ± 0.03 c | nd | 0.14 ± 0.01 bc |
| Isoamyl acetate | 0.54 ± 0.02 bc | 0.11 ± 0.01 c | 1.19 ± 0.23 ab | 0.25 ± 0.09 c | 1.83 ± 0.03 a | 0.80 ± 0.25 bc | 0.04 ± 0.05 c | 0.41 ± 0.47 bc | 1.60 ± 0.28 a |
| Ethyl hexanoate | 0.01 ± 0.02 b | 0.05 ± 0.00 ab | 0.03 ± 0.01 ab | 0.08 ± 0.08 ab | 0.03 ± 0.01 ab | 0.16 ± 0.02 ab | 0.18 ± 0.00 a | 0.06 ± 0.01 ab | 0.17 ± 0.01 a |
| Ethyl octanoate | 0.03 ± 0.02 | 0.14 ± 0.02 | 0.03 ± 0.01 | 0.14 ± 0.01 | 0.05 ± 0.00 | 0.11 ± 0.00 | 0.19 ± 0.01 | 0.08 ± 0.00 | 0.29 ± 0.38 |
| Diethyl succinate | 0.52 ± 0.35 b | 4.60 ± 0.72 a | 0.32 ± 0.04 b | 0.050 ± 0.41 b | 1.05 ± 0.69 b | nd | nd | nd | nd |
| 2-phenylethyl acetate | 0.04 ± 0.00 b | 0.05 ± 0.01 ab | 0.04 ± 0.00 b | 0.02 ± 0.00 b | 0.10 ± 0.02 a | 0.03 ± 0.01 b | 0.01 ± 0.00 b | 0.04 ± 0.02 b | 0.02 ± 0.02 b |
| Fatty acids | |||||||||
| Butyric acid | 2.12 ± 0.19 | 1.69 ± 0.01 | 2.03 ± 0.05 | 1.01 ± 1.43 | 2.80 ± 0.00 | 1.80 ± 0.00 | 1.75 ± 0.08 | 2.26 ± 0.17 | 0.85 ± 0.98 |
| Isobutyric acid | nd | nd | nd | nd | 3.05 ± 0.34 a | nd | 1.19 ± 0.04 bc | 1.31 ± 0.03 bc | 1.38 ± 0.20 b |
| Isovaleric acid | nd | 0.19 ± 0.02 | nd | 0.00 ± 0.00 | nd | nd | 6.90 ± 0.34 | 2.85 ± 0.13 | 4.16 ± 4.83 |
| Hexanoic acid | 2.21 ± 0.14 abc | 2.97 ± 0.32 a | 2.34 ± 0.10 ab | 1.98 ± 0.92 abc | 1.68 ± 0.10 bc | 1.10 ± 0.15 cd | 1.69 ± 0.16 bc | 0.35 ± 0.10 c | 1.25 ± 0.20 cd |
| Octanoic acid | 6.93 ± 0.49 ab | 9.58 ± 0.77 a | 6.99 ± 0.09 ab | 6.60 ± 2.55 ab | 4.79 ± 0.53 bc | 3.05 ± 0.15 cd | 5.22 ± 0.73 bc | 0.49 ± 0.39 d | 4.83 ± 0.40 bc |
| Decanoic acid | 1.61 ± 0.81 ab | 1.88 ± 0.39 a | 0.21 ± 0.26 bc | 1.03 ± 0.47 abc | 0.68 ± 0.15 abc | 0.18 ± 0.02 bc | 0.82 ± 0.91 abc | 0.22 ± 0.11 bc | 0.17 ± 0.04 c |
| Ketones | |||||||||
| Diacetyl | 0.13 ± 0.03 bc | nd | 0.11 ± 0.00 c | 0.14 ± 0.02 bc | 0.12 ± 0.03 c | 0.39 ± 0.03 a | nd | 0.37 ± 0.06 ab | 0.10 ± 0.12 c |
| Acetoin | 2.66 ± 1.19 ab | 6.72 ± 0.54 a | 1.85 ± 0.58 b | 4.54 ± 3.26 ab | 2.08 ± 0.13 b | 1.19 ± 0.42 b | 1.44 ± 0.30 b | 1.13 ± 0.42 b | 1.19 ± 0.42 b |
| Phenols | |||||||||
| Guaiacol | 0.09 ± 0.01 bc | 0.11 ± 0.00 bc | 0.09 ± 0.01 bc | 0.10 ± 0.01 bc | 0.13 ± 0.00 ab | 0.12 ± 0.00 abc | 0.23 ± 0.00 a | nd | 0.12 ± 0.02 bc |
| Yeast Strains | Q1 | Q2 | QT |
|---|---|---|---|
| CLI 3 | 4.34 ± 0.35 abc | 8.93 ± 0.50 a | 13.27 ± 0.85 ab |
| CLI 194 | 3.47 ± 0.11 bc | 7.45 ± 0.14 ab | 10.92 ± 0.04 abc |
| CLI 457 | 4.44 ± 0.01 abc | 8.63 ± 0.53 ab | 13.07 ± 0.54 ab |
| CLI 512 | 3.56 ± 0.43 bc | 8.00 ± 0.21 ab | 11.56 ± 0.64 abc |
| CLI 691 | 4.58 ± 0.35 ab | 7.57 ± 0.85 ab | 12.15 ± 0.50 abc |
| CLI 902 | 4.34 ± 0.02 abc | 8.99 ± 0.76 a | 13.33 ± 0.77 ab |
| CLI 1028 | 4.80 ± 0.17 ab | 8.70 ± 0.12 ab | 13.49 ± 0.30 a |
| 7A-3A | 3.23 ± 0.13 bc | 6.40 ± 0.55 b | 9.63 ± 0.42 c |
| S-04 | 3.54 ± 0.35 bc | 7.64 ± 0.59 ab | 11.18 ± 0.94 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postigo, V.; Sanz, P.; García, M.; Arroyo, T. Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation. Foods 2022, 11, 2029. https://doi.org/10.3390/foods11142029
Postigo V, Sanz P, García M, Arroyo T. Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation. Foods. 2022; 11(14):2029. https://doi.org/10.3390/foods11142029
Chicago/Turabian StylePostigo, Vanesa, Paula Sanz, Margarita García, and Teresa Arroyo. 2022. "Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation" Foods 11, no. 14: 2029. https://doi.org/10.3390/foods11142029







