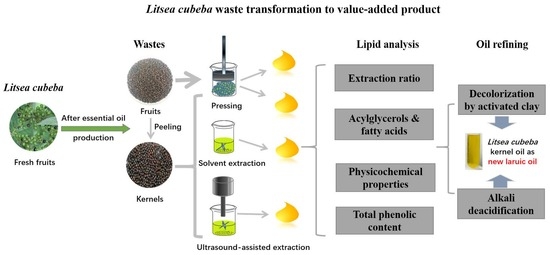
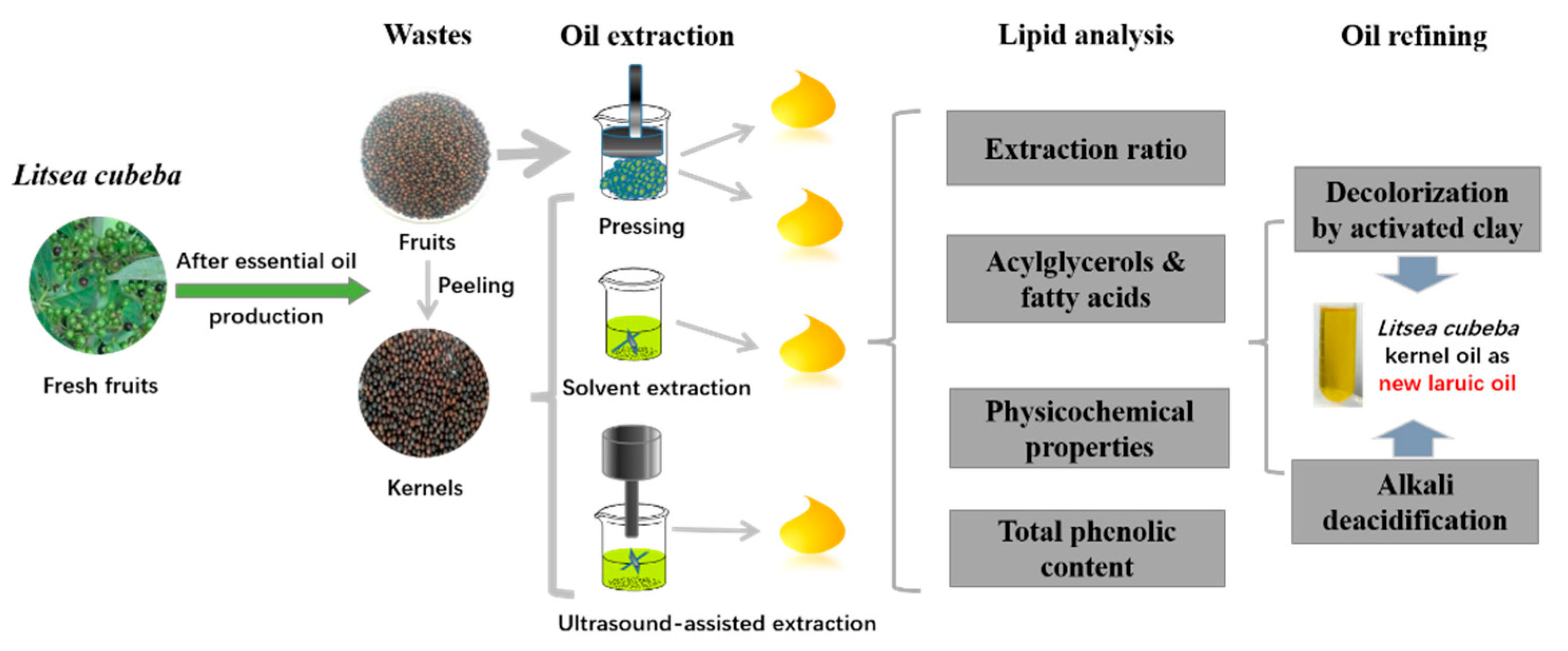
Sustainable Valorization of Litsea cubeba (Lour.) Pers. Residue as the New Lauric Oil Source Using Alternative Green Extraction and Refining Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Matertials
2.2. Moisture and Oil Content Measurement
2.3. Oil Extractions
2.3.1. Conventional Extractions
2.3.2. Ultrasound-Assisted Extraction (UAE)
2.4. Lipid Analysis
2.4.1. Determination of Physicochemical Properties
2.4.2. Acylglycerol and Fatty Acid Composition Analysis
2.4.3. Total Phenolic Content
2.5. Oil Refining
2.5.1. Decolorization by Activated Clay
2.5.2. Alkali Deacidification
2.6. Statistical Analysis
3. Results and Discussions
3.1. Optimization of UAE Parameters
3.2. The Effect of Extraction Methods on the Extraction Rate of L. cubeba Kernel and Fruit Oils
3.3. Analysis of LC Kernel and Fruit Oils Extracted by Different Methods
3.3.1. Acylglycerol and Fatty Acid Composition
3.3.2. Physicochemical Properties
3.4. Refining of Pressed L. cubeba Fruit and Kernel Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, O.; Zhou, J.W.; Ban, D.M. Analysis of volatile oil from different parts of Litsea cubeba. J. Chin. Med. Mater. 2010, 33, 1417–1419. [Google Scholar]
- Cheng, C.L.; Hong, G.B. Optimization of extraction process for bioactive compounds from Litsea cubeba fruits. Korean J. Chem. Eng. 2018, 35, 187–194. [Google Scholar] [CrossRef]
- Qiu, Y.; Yu, Y.; Lan, P.; Wang, Y.; Li, Y. An overview on total valorization of Litsea cubeba as a new woody oil plant resource toward a zero-waste biorefinery. Molecules 2021, 26, 3948. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zheng, L.H.; Zhao, K.; Chen, Y.; Yi, Z. Insecticidal activities of constituents of Litsea cubeba fruit extracts effective against the maize weevil (Coleoptera: Curculionidae). J. Insect Sci. 2017, 17, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Chen, Y.; Wang, Y. Evaluation of the yields and chemical compositions of the essential oils of different Litsea cubeba varieties. J. Essent. Oil Bear. Plants 2016, 19, 1888–1902. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, Z.; Wang, Y.; Li, Y. The effect of alternative solvents to n-hexane on the green extraction of Litsea cubeba kernel oils as new oil sources. Ind. Crop. Prod. 2018, 126, 340–346. [Google Scholar] [CrossRef]
- Cai, Z.; Zhuang, X.; Yang, X.; Huang, F.; Wang, Y.; Li, Y. Litsea cubeba kernel oil as a promising new medium-chain saturated fatty acid feedstock for biolubricant base oil synthesis. Ind. Crop. Prod. 2021, 167, 113564. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, Y.; Li, C.; Huang, X.; Jang, G.; Zhou, W.; Dai, Q. Study on nutrient and energy utilization rate of rooster and duck on Litsea cubeba residue. Chin. J. Anim. Sci. 2020, 56, 131–134. [Google Scholar]
- Ullah, H.I.; Dickson, R.; Mancini, R.; Malanca, A.A.; Pinelo, M.; Mansouri, S.S. An integrated sustainable biorefinery concept towards achieving zero-waste production. J. Clean. Prod. 2022, 336, 130137. [Google Scholar] [CrossRef]
- Martínez, M.L.; Mattea, M.A.; Maestri, D.M. Pressing and supercritical carbon dioxide extraction of walnut oil. J. Food Eng. 2008, 88, 399–404. [Google Scholar] [CrossRef]
- Willems, P.; Njm, K.; De Haan, A.B. Hydraulic pressing of oilseeds: Experimental determination and modeling of yield and pressing rates. J. Food Eng. 2008, 89, 8–16. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Rahal, N.B.; Barba, F.J.; Barth, D.; Chevalor, I. Supercritical CO2 extraction of oil, fatty acids and flavonolignans from milk thistle seeds: Evaluation of their antioxidant and cytotoxic activities in Caco-2 cells. Food Chem. Toxicol. 2015, 83, 275–282. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Cd 3d-63; AOCS: Urbana, IL, USA, 2009. [Google Scholar]
- AOCS. AOCS Official Method Cd 8-53; AOCS: Urbana, IL, USA, 2003. [Google Scholar]
- AOCS. AOCS Official Method Cd 3-25; AOCS: Urbana, IL, USA, 2010. [Google Scholar]
- Chen, Q.; Li, Y.; Fu, J.; Ma, X.; Teng, Y.; Wang, Y. Production of diacylglycerol-enriched oils by enzymatic interesterification and molecular distillation using soybean oil and distilled saturated monoacylglycerol. Eur. J. Lipid Sci. Technol. 2017, 119, 1600332. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Cc 13a-43; AOCS: Urbana, IL, USA, 2009. [Google Scholar]
- Sung, G.B.; Soon, K.Y. Isolation and identification of postharvest spoilage fungi from mulberry fruit in Korea. Korean J. Environ. Agric. 2018, 37, 221–228. [Google Scholar]
- Wang, L.; Wu, M.; Liu, H.; Ma, Y.; Wang, X.; Qin, G. Subcritical fluid extraction of Chinese quince seed: Optimization and product characterization. Molecules 2017, 22, 528. [Google Scholar] [CrossRef] [Green Version]
- Khadhraoui, B.; Fabiano-Tixier, A.S.; Petitcolas, E.; Robinet, P.; Imbert, R.; El Maataoui, M.; Chemat, F. Microscopic imaging as a tool to target spatial and temporal extraction of bioactive compounds through ultrasound intensification. Ultrason. Sonochem. 2019, 53, 214–225. [Google Scholar] [CrossRef]
- Sicaire, A.; Abert Vian, M.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016, 31, 319–329. [Google Scholar] [CrossRef]
- Jaski, J.M.; Abrantes, K.K.B.; Zanqui, A.B.; Stevanato, N.; de Silva, C.; Barao, C.E.; Bonfim-Rocha, L.; Cardozo-Filho, L. Simultaneous extraction of sunflower oil and active compounds from olive leaves using pressurized propane. Curr. Opin. Food Sci. 2022, 5, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, A.M.; Tellah, S.; Capocasale, M.; Zappia, C.; Latati, M.; Badiani, M.; Ounane, S.M. Seed oil from ten Algerian peanut landraces for edible use and biodiesel production. J. Oleo Sci. 2016, 65, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, B.; Luo, T.; Chen, F.; Wang, M.; Zheng, L.; Li, J.; Deng, Z. Medium- and long-chain triglycerides attenuate lipid accumulation and regulate the expression of proteins related to lipid metabolism in oleic acid-induced lipid deposition in human hepatic LO2 cells. J. Funct. Foods 2021, 78, 104354. [Google Scholar] [CrossRef]
- Yabuuchi, Y.; Matsushita, Y.; Otsuka, H.; Fukamachi, K.; Kobayashi, Y. Effects of supplemental lauric acid-rich oils in high-grain diet on in vitro rumen fermentation. Anim. Sci. J. 2006, 77, 300–307. [Google Scholar] [CrossRef]
- Petkova, Z.; Stefanova, G.; Girova, T.; Antova, G.; Stoyanova, M.; Damianova, S.; Gochev, V.; Stoyanova, A.; Zheljazkov, V.D. Phytochemical investigations of laurel fruits (Laurus nobilis). Nat. Prod. Commun. 2019, 14, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Saucedo, M.R.; Jiménez-Ruiz, E.I.; Rodríguez-Carpena, J.G.; Ragazzo-Sánchez, J.A.; Ulloa, J.A.; Ramírez-Ramírez, J.C.; Gastón-Peña, C.R.; Bautista-Rosales, P.U. Properties of the avocado oil extracted using centrifugation and ultrasound-assisted methods. Food Sci. Biotechnol. 2021, 30, 1051–1061. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M. Physicochemical composition of tomato seed oil for an edible use: The effect of cultivar. Int. Food Res. J. 2016, 23, 583–591. [Google Scholar]
- Giuffrè, A.M.; Zappia, C.; Capocasale, M. Effects of high temperatures and duration of heating on olive oil properties for food use and biodiesel production. J. Am. Oil Chem. Soc. 2017, 94, 819–830. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C.; Poiana, M. Influence of high temperature and duration of heating on the sunflower seed oil properties for food use and bio-diesel production. J. Oleo Sci. 2017, 66, 1193–1205. [Google Scholar] [CrossRef] [Green Version]
- Giuffrè, A.M.; Capocasale, M.; Zappia, C.; Sicari, V.; Pellicanò, T.M.; Poiana, M.; Panzera, G. Tomato seed oil for biodiesel production. Eur. J. Lipid Sci. Technol. 2016, 118, 640–650. [Google Scholar] [CrossRef]
- Karakaya, S.; Şimşek, Ş. Changes in total polar compounds, peroxide value, total phenols and antioxidant activity of various oils used in deep fat frying. J. Am. Oil Chem. Soc. 2011, 88, 1361–1366. [Google Scholar] [CrossRef]
- Naebi, M.; Torbati, M.; Azadmard-Damirchi, S.; Siabi, S.; Savage, G.P. Changes in physicochemical properties of cold press extracted oil from Balangu (Lallemantia peltata) seeds during storage. J. Food Compos. Anal. 2022, 107, 104358. [Google Scholar] [CrossRef]
- CX-STAN 210-1999; Codex Standard for Named Vegetable Oils. Alimentarius Codex: Rome, Italy, 2003; Volume 210, pp. 5–13.





| Litsea cubeba | Kernel | Fruit | |||
|---|---|---|---|---|---|
| Conventional Solvent Extraction | Ultrasound-Assisted Extraction | Cold Pressing | Cold Pressing | Virgin Coconut Oil | |
| Acylglycerols (%) | |||||
| TAG | 86.55 ± 0.09 a | 86.08 ± 0.06 a | 89.10 ± 0.10 a | 69.44 ± 1.50 b | 94.52 ± 0.28 |
| C10C12C12 | 31.66 ± 0.05 a | 31.45 ± 0.03 a | 29.69 ± 0.05 b | 21.60 ± 0.24 c | 17.07 ± 0.11 |
| C12C12C12 | 31.50 ± 0.05 b | 31.31 ± 0.17 b | 33.14 ± 0.01 a | 24.17 ± 0.58 c | 19.91 ± 0.01 |
| DAG | 10.00 ± 0.40 a | 10.38 ± 0.04 a | 7.77 ± 0.03 b | 6.41 ± 0.01 c | 13.92 ± 0.07 |
| MAG | 0.73 ± 0.19 ab | 0.83 ± 0.01 a | 0.32 ± 0.11 b | 0.83 ± 0.04 a | - |
| FFA | 2.72 ± 0.31 b | 2.71 ± 0.02 b | 2.81 ± 0.17 b | 23.32 ± 1.55 a | 0.08 ± 0.03 |
| Fatty acids (%) | |||||
| Saturated | |||||
| Octanoic acid (C8:0) | - | - | - | - | 5.07 ± 0.02 |
| Capric acid (C10:0) | 15.28 ± 2.68 a | 13.37 ± 1.40 a | 11.26 ± 0.82 a | 7.03 ± 0.51 b | 5.54 ± 0.11 |
| Lauric acid (C12:0) | 55.34 ± 3.15 a | 57.16 ± 0.20 a | 57.39 ± 0.84 a | 31.92 ± 1.20 b | 50.60 ± 0.19 |
| Myristic acid (C14:0) | 2.97 ± 0.77 a | 3.15 ± 0.59 a | 2.47 ± 0.42 a | 1.71 ± 0.31 a | 19.88 ± 0.43 |
| Palmitic acid (C16:0) | 2.68 ± 0.27 b | 2.54 ± 0.22 b | 3.61 ± 0.52 b | 14.55 ± 1.36 a | 9.18 ± 0.30 |
| Stearic acid (C18:0) | - | - | - | - | 4.13 ± 0.74 |
| Mono-unsaturated | |||||
| Caproleic acid (C10:1) | 1.09 ± 0.37 ab | 0.49 ± 0.05 b | 1.40 ± 0.52 a | 0.72 ± 0.21 ab | - |
| Lauroleic acid (C12:1) | 6.62 ± 0.29 a | 7.05 ± 0.62 a | 5.89 ± 1.07 a | 3.69 ± 0.31 b | - |
| Myristoleic acid (C14:1) | 1.21 ± 0.06 a | 1.36 ± 0.31 a | 1.30 ± 0.38 a | 1.15 ± 0.59 a | - |
| Palmitoleic acid (C16:1) | - | - | - | 1.77 ± 0.30 | - |
| Oleic acid (C18:1) | 9.59 ± 1.07 b | 9.57 ± 0.48 b | 10.90 ± 0.69 b | 19.07 ± 1.31 a | 5.61 ± 0.05 |
| Poly-unsaturated | |||||
| Linoleic acid (C18:2) | 5.23 ± 0.45 b | 5.31 ± 0.62 b | 5.78 ± 0.20 b | 18.40 ± 0.52 a | - |
| ∑SFAs | 76.27 ± 1.29 a | 76.21 ± 0.94 a | 74.73 ± 1.05 a | 55.21 ± 1.81 b | 94.39 ± 0.05 |
| ∑UFAs | 23.74 ± 1.46 b | 23.79 ± 1.44 b | 25.27 ± 1.06 b | 44.80 ± 2.17 a | 5.61 ± 0.05 |
| ∑EFAs | 5.23 ± 0.45 b | 5.31 ± 0.62 b | 5.78 ± 0.20 b | 18.40 ± 0.52 a | - |
| Litsea cubeba | Kernel | Fruit | |||
|---|---|---|---|---|---|
| Conventional Solvent Extraction | Ultrasound-Assisted Extraction | Cold Pressing | Cold Pressing | Virgin Coconut Oil | |
| Density (g/cm3) | 0.92 ± 0.00 b | 0.92 ± 0.00 b | 0.94 ± 0.01 a | 0.95 ± 0.00 a | 0.91 ± 0.01 |
| Refractive index (20 °C) | 1.46 ± 0.00 c | 1.46 ± 0.00 c | 1.47 ± 0.00 b | 1.48 ± 0.00 a | 1.43 ± 0.00 |
| Acid value (mg KOH/g) | 9.93 ± 0.16 c | 9.62 ± 0.09 c | 12.13 ± 0.35 b | 58.76 ± 0.39 a | 0.43 ± 0.02 |
| Peroxide value (meq O2/kg) | 1.31 ± 0.02 a | 1.32 ± 0.25 a | 0.54 ± 0.01 b | 0.18 ± 0.02 c | 0.11 ± 0.01 |
| Saponification value (mg KOH/g) | 297.55 ± 5.81 a | 295.90 ± 4.67 a | 287.71 ± 6.13 a | 268.42 ± 5.35 b | 299.64 ± 4.74 |
| Melting point (°C) | 28.60 ± 0.27 a | 28.29 ± 0.18 a | 28.49 ± 0.11 a | 23.40 ± 0.53 b | 25.17 ± 0.64 |
| Crystallization onset temperature (°C) | −1.73 ± 0.24 ab | −1.69 ± 0.14 a | −2.40 ± 0.06 b | −1.29 ± 0.33 a | 3.81 ± 0.40 |
| Total △Hc (J/g) | 54.14 ± 4.57 a | 54.56 ± 2.73 a | 53.41 ± 4.92 a | 25.50 ± 0.95 b | 39.10 ± 1.58 |
| Color (Lovibond units) | |||||
| a* (red/green value) | 6 | 6.1 | 10.9 | 6 | |
| b* (yellow/blue value) | 40 | 40 | 70 | 60 | |
| L* (brightness value) | - | - | - | 13 | |
| Total phenolic content (mg GAE/100 g) | 43.22 ± 1.94 c | 43.37 ± 1.77 c | 72.79 ± 1.04 b | 176.14 ± 4.81 a | 15.91 ± 3.85 d |
| The Effect of Decoloration Using Activated Clay on the Acid Value | |||||
|---|---|---|---|---|---|
| Litsea cubeba | Centrifugation | 5% | 10% | 15% | 20% |
| Fruit oil | 58.76 ± 0.39 c | 58.02 ± 0.05 c | 58.97 ± 0.28 bc | 60.09 ± 0.41 ab | 60.60 ± 0.41 a |
| Kernel oil | 11.68 ± 0.15 a | 11.60 ± 0.11 a | 11.55 ± 0.03 ab | 11.20 ± 0.07 c | 11.35 ± 0.10 bc |
| Basic physicochemical properties of Litsea cubeba kernel oils | |||||
| Refining methods | Acid value (mg KOH/g) | Peroxide value (meq O2/kg) | Total phenolic content(mg GAE/100 g) | ||
| Centrifugation | 11.68 ± 0.15 a | 0.64 ± 0.18 c | 81.29 ± 5.06 a | ||
| 5% | 11.60 ± 0.11 a | 1.27 ± 0.44 ab | 49.50 ± 3.97 b | ||
| 10% | 11.55 ± 0.03 ab | 1.82 ± 0.30 a | 36.67 ± 2.37 c | ||
| 15% | 11.20 ± 0.07 c | 1.72 ± 0.09 a | 22.73 ± 5.61 d | ||
| 20% | 11.35 ± 0.10 bc | 1.51 ± 0.03 ab | 17.21 ± 5.03 d | ||
| Deacidification | 0.86 ± 0.01 e | 0.93 ± 0.15 bc | 36.17 ± 6.01 c | ||
| Deacidification + 5% | 1.53 ± 0.10 d | 1.71 ± 0.05 a | 19.00 ± 4.04 d | ||
| Deacidification + 10% | 1.57 ± 0.06 d | 1.57 ± 0.05 a | 11.10 ± 1.26 d | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhuang, X.; Wu, X.; Qiu, C.; Wang, Y. Sustainable Valorization of Litsea cubeba (Lour.) Pers. Residue as the New Lauric Oil Source Using Alternative Green Extraction and Refining Methods. Foods 2022, 11, 2047. https://doi.org/10.3390/foods11142047
Li Y, Zhuang X, Wu X, Qiu C, Wang Y. Sustainable Valorization of Litsea cubeba (Lour.) Pers. Residue as the New Lauric Oil Source Using Alternative Green Extraction and Refining Methods. Foods. 2022; 11(14):2047. https://doi.org/10.3390/foods11142047
Chicago/Turabian StyleLi, Ying, Xiaoci Zhuang, Xinrui Wu, Chaoying Qiu, and Yong Wang. 2022. "Sustainable Valorization of Litsea cubeba (Lour.) Pers. Residue as the New Lauric Oil Source Using Alternative Green Extraction and Refining Methods" Foods 11, no. 14: 2047. https://doi.org/10.3390/foods11142047
APA StyleLi, Y., Zhuang, X., Wu, X., Qiu, C., & Wang, Y. (2022). Sustainable Valorization of Litsea cubeba (Lour.) Pers. Residue as the New Lauric Oil Source Using Alternative Green Extraction and Refining Methods. Foods, 11(14), 2047. https://doi.org/10.3390/foods11142047