Hydrothermal Conversion of Food Waste to Carbonaceous Solid Fuel—A Review of Recent Developments
Abstract
:1. Introduction
2. Methodology
3. Food Waste
3.1. Availability
3.2. Physicochemical Characteristics for Energy Applications
4. Fundamentals of Hydrothermal Carbonization Process
4.1. HTC Reactor Pre-Heating and Reaction Time
4.2. Reactant Media
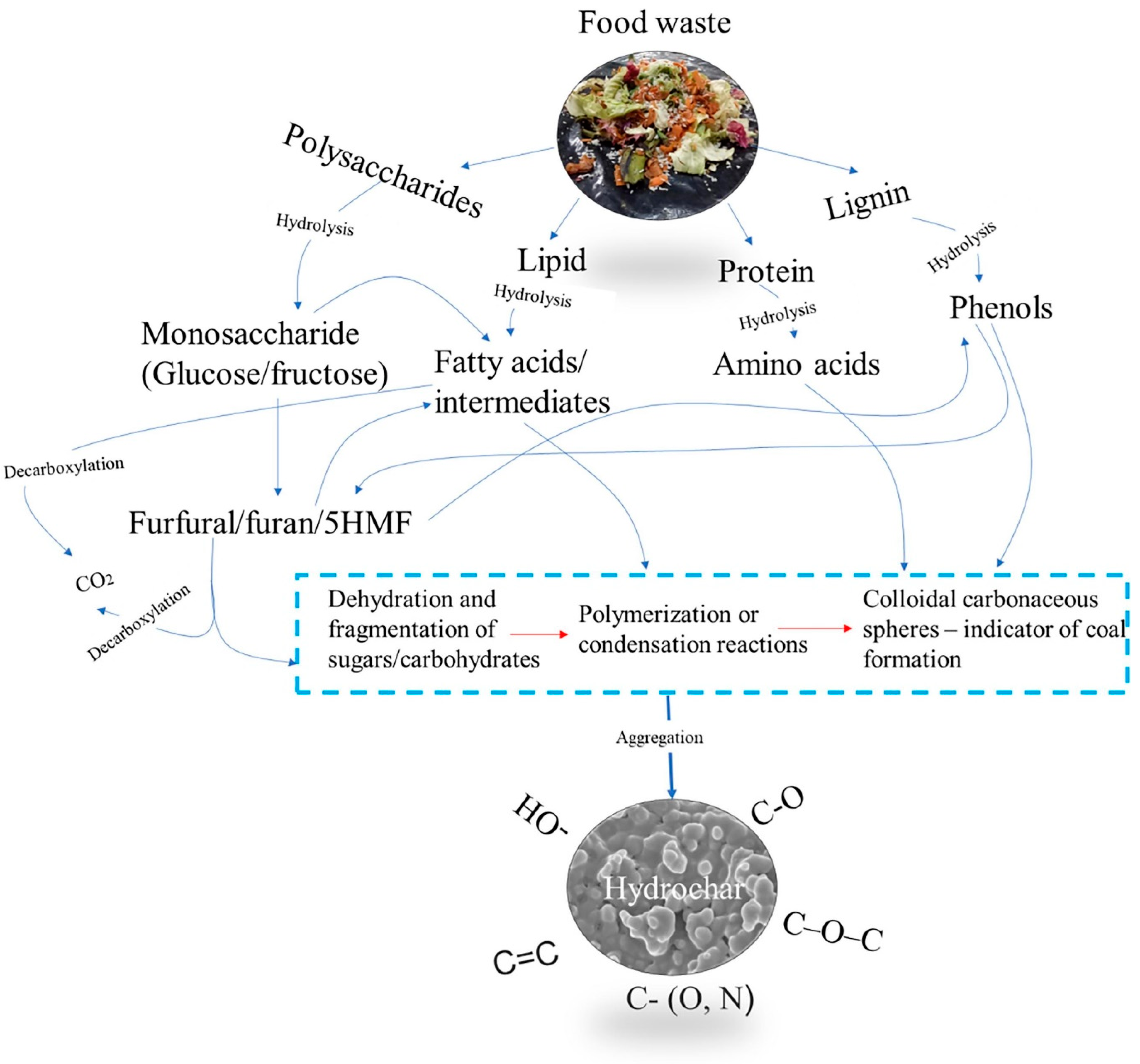
4.3. HTC Reaction and Chemistry
4.4. HTC Reactor Setup
5. Hydrothermal Carbonization of Food Waste for Char Production
5.1. Effect of Process Parameters
5.2. Temperature
5.3. Contact Time
5.4. Water–Biomass Ratio
5.5. Pressure
5.6. Heating Rate
5.7. Properties of the Produced Hydrochars
5.8. Combustion Kinetics of the Produced Char from HTC
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingrao, C.; Faccilongo, N.; Di Gioia, L.; Messineo, A. Food waste recovery into energy in a circular economy perspective: A comprehensive review of aspects related to plant operation and environmental assessment. J. Clean. Prod. 2018, 184, 869–892. [Google Scholar] [CrossRef]
- Mannarino, G.; Sarrion, A.; Diaz, E.; Gori, R.; De, M.A.; Mohedano, A.F. Improved energy recovery from food waste through hydrothermal carbonization and anaerobic digestion. Waste Manag. 2022, 142, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mendoza Martinez, C.L.; Sermyagina, E.; Saari, J.; Silva de Jesus, M.; Cardoso, M.; Matheus de Almeida, G.; Vakkilainen, E. Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Kwapinska, M.; Leahy, J.J.; Kwapinski, W. The effect of temperature, residence time, and water-sludge ratio on hydrothermal carbonization of DAF dairy sludge. J. Environ. Chem. Eng. 2020, 8, 103599. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, J.; Ying, Z.; Ji, S.; Feng, Y.; Wang, B.; Dou, B. Thermochemical conversion of sewage sludge-derived hydrochars: Volatiles release and char gasification kinetics. J. Anal. Appl. Pyrolysis 2021, 156, 105138. [Google Scholar] [CrossRef]
- Tsarpali, M.; Arora, N.; Kuhn, J.N.; Philippidis, G.P. Beneficial use of the aqueous phase generated during hydrothermal carbonization of algae as nutrient source for algae cultivation. Algal Res. 2021, 60, 102485. [Google Scholar] [CrossRef]
- Venna, S.; Bhakta, H.; Prasad, P.H.; Chowdhury, S.; Dubey, K. Landfill leachate as an alternative moisture source for hydrothermal carbonization of municipal solid wastes to solid biofuels. Bioresour. Technol. 2021, 320, 124410. [Google Scholar] [CrossRef]
- Derqui, B.; Fernandez, V.; Fayos, T. Towards more sustainable food systems. Addressing food waste at school canteens. Appetite 2018, 129, 1–11. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; García, I.L.; Sáez-Bastante, J.; Pinzi, S.; Koutinas, A.A.; Dorado, M.P. Food waste from restaurant sector—Characterization for biorefinery approach. Bioresour. Technol. 2020, 301, 122779. [Google Scholar] [CrossRef]
- Gupta, D.; Mahajani, S.M.; Garg, A. Investigation on hydrochar and macromolecules recovery opportunities from food waste after hydrothermal carbonization. Sci. Total Environ. 2020, 749, 142294. [Google Scholar] [CrossRef]
- Azaare, L.; Commeh, M.K.; Smith, A.M.; Kemausuor, F. Co-hydrothermal carbonization of pineapple and watermelon peels: Effects of process parameters on hydrochar yield and energy content. Bioresour. Technol. Rep. 2021, 15, 100720. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Zhang, Q.; Wang, C.; Zhan, H.; Ma, L. A review on the utilization of industrial biowaste via hydrothermal carbonization. Renew. Sustain. Energy Rev. 2022, 154, 111877. [Google Scholar] [CrossRef]
- Melikoglu, M. Reutilisation of food wastes for generating fuels and value added products: A global review. Environ. Technol. Innov. 2020, 19, 101040. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of organic wastes to carbonaceous solid fuel—A review of mechanisms and process parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Herzberg, R.; Schmidt, T.G.; Schneider, F. Characteristics and determinants of domestic food waste: A representative diary study across Germany. Sustainability 2020, 12, 4702. [Google Scholar] [CrossRef]
- Silvennoinen, K.; Nisonen, S.; Pietiläinen, O. Food waste case study and monitoring developing in Finnish food services. Waste Manag. 2019, 97, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Herrero, I.; Hoehn, D.; Margallo, M.; Laso, J.; Bala, A.; Batlle-Bayer, L.; Fullana, P.; Vazquez-Rowe, I.; Gonzalez, M.J.; Durá, M.J.; et al. On the estimation of potential food waste reduction to support sustainable production and consumption policies. Food Policy 2018, 80, 24–38. [Google Scholar] [CrossRef]
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of food waste per product group along the food supply chain in the European Union: A mass flow analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Liu, G.; Cheng, S. Rural household food waste characteristics and driving factors in China. Resour. Conserv. Recycl. 2021, 164, 105209. [Google Scholar] [CrossRef]
- Abdelaal, A.H.; McKay, G.; Mackey, H.R. Food waste from a university campus in the Middle East: Drivers, composition, and resource recovery potential. Waste Manag. 2019, 98, 14–20. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, Z.; Ge, C.; Liu, W.; Tang, Y.; Zhuang, X.; Qiu, R. Synergistic effect of hydrothermal co-carbonization of sewage sludge with fruit and agricultural wastes on hydrochar fuel quality and combustion behavior. Waste Manag. 2019, 100, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Abu-Thabit, N.Y.; Judeh, A.A.; Hakeem, A.S.; Ul-Hamid, A.; Umar, Y.; Ahmad, A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2020, 155, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Kadlimatti, H.M.; Mohan, B.R.; Saidutta, M.B. Bio-oil from microwave assisted pyrolysis of food waste-optimization using response surface methodology. Biomass Bioenergy 2019, 123, 25–33. [Google Scholar] [CrossRef]
- Pahla, G.; Ntuli, F.; Muzenda, E. Torrefaction of landfill food waste for possible application in biomass co-firing. Waste Manag. 2018, 71, 512–520. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.; Lee, J. Co-pyrolysis for the valorization of food waste and oriental herbal medicine byproduct. J. Anal. Appl. Pyrolysis 2021, 154, 105016. [Google Scholar] [CrossRef]
- Sharma, H.B.; Vanapalli, K.R.; Samal, B.; Cheela, V.R.S.; Dubey, B.K.; Bhattacharya, J. Circular economy approach in solid waste management system to achieve UN-SDGs: Solutions for post-COVID recovery. Sci. Total Environ. 2021, 800, 149605. [Google Scholar] [CrossRef]
- Suárez, L.; Benavente-Ferraces, I.; Plaza, C.; de Pascual-Teresa, S.; Suárez-Ruiz, I.; Centeno, T.A. Hydrothermal carbonization as a sustainable strategy for integral valorisation of apple waste. Bioresour. Technol. 2020, 309, 123395. [Google Scholar] [CrossRef]
- Theppitak, S.; Hungwe, D.; Ding, L.; Xin, D.; Yu, G.; Yoshikawa, K. Comparison on solid biofuel production from wet and dry carbonization processes of food wastes. Appl. Energy 2020, 272, 115264. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, Y.Y.; Liu, H.C.; Chen, T.C.; Hung, C.H.; Chen, C.H.; Ong, H.C. A comprehensive analysis of food waste derived liquefaction bio-oil properties for industrial application. Appl. Energy 2019, 237, 283–291. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, S. Steam gasification with torrefaction as pretreatment to enhance syngas production from mixed food waste. J. Environ. Chem. Eng. 2021, 9, 104722. [Google Scholar] [CrossRef]
- Su, H.; Zhou, X.; Zheng, R.; Zhou, Z.; Zhang, Y.; Zhu, G.; Yu, C.; Hantoko, D.; Yan, M. Hydrothermal carbonization of food waste after oil extraction pre-treatment: Study on hydrochar fuel characteristics, combustion behavior, and removal behavior of sodium and potassium. Sci. Total Environ. 2021, 754, 142192. [Google Scholar] [CrossRef] [PubMed]
- Kurose, R.; Ikeda, M.; Makino, H.; Kimoto, M.; Miyazaki, T. Pulverized coal combustion characteristics of high-fuel-ratio coals. Fuel 2004, 83, 1777–1785. [Google Scholar] [CrossRef]
- Li, H.; Jiang, L.B.; Li, C.Z.; Liang, J.; Yuan, X.Z.; Xiao, Z.H.; Xiao, Z.H.; Wang, H. Co-pelletization of sewage sludge and biomass: The energy input and properties of pellets. Fuel Process. Technol. 2015, 132, 55–61. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Ohm, T.-I.; Chae, J.-S.; Kim, J.-K.; Oh, S.-C. Study on the characteristics of biomass for co-combustion in coal power plant. J. Mater. Cycles Waste Manag. 2014, 17, 249–257. [Google Scholar] [CrossRef]
- Hegde, S.; Lodge, J.S.; Trabold, T.A. Characteristics of food processing wastes and their use in sustainable alcohol production. Renew. Sustain. Energy Rev. 2018, 81, 510–523. [Google Scholar] [CrossRef]
- Pecorini, I.; Baldi, F.; Carnevale, E.A.; Corti, A. Biochemical methane potential tests of different autoclaved and microwaved lignocellulosic organic fractions of municipal solid waste. Waste Manag. 2016, 56, 143–150. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, S.; Bharadwaj, N.; Verma, R. Low temperature steam gasification to produce hydrogen rich gas from kitchen food waste: Influence of steam flow rate and temperature. Int. J. Hydrogen Energy 2020, 45, 20843–20850. [Google Scholar] [CrossRef]
- Pagliaccia, P.; Gallipoli, A.; Gianico, A.; Gironi, F.; Montecchio, D.; Pastore, C.; di Bitonto, L.; Braguglia, C.M. Variability of food waste chemical composition: Impact of thermal pre-treatment on lignocellulosic matrix and anaerobic biodegradability. J. Environ. Manag. 2019, 236, 100–107. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Wan, Z.; Jing, F.; Li, Z.; Chen, J.; Tsang, D.C.W. Tailored design of food waste hydrochar for efficient adsorption and catalytic degradation of refractory organic contaminant. J. Clean. Prod. 2021, 310, 127482. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, T.; Zhu, Y.; Peng, C.; Wang, B.; Li, X.; Li, C.; Zeng, G. Production of fuel pellets via hydrothermal carbonization of food waste using molasses as a binder. Waste Manag. 2018, 77, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sangare, D.; Chartier, A.; Moscosa-santillan, M. Kinetic studies of hydrothermal carbonization of avocado stone and analysis of the polycyclic aromatic hydrocarbon contents in the hydrochars produced. Fuel 2022, 316, 123163. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Nelles, M. Symbiotic relationship between hydrothermal carbonization technology and anaerobic digestion for food waste in China. Bioresour. Technol. 2018, 260, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Sub- and Supercritical Water Technology for Biofuels; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Olszewski, M.P.; Wang, X.; Pfersich, J.; Sebastian, V.; Manyà, J.; Hedin, N.; Kruse, A. Assessment of the effects of process water recirculation on the surface chemistry and morphology of hydrochar. Renew. Energy 2020, 155, 1173–1180. [Google Scholar] [CrossRef]
- Lin, H.; Li, Q.; Zhang, S.; Zhang, L.; Hu, G.; Hu, X. Involvement of the organics in aqueous phase of bio-oil in hydrothermal carbonization of lignin. Bioresour. Technol. 2022, 351, 127055. [Google Scholar] [CrossRef]
- Li, L.; Hale, M.; Olsen, P.; Berge, N.D. Using liquid waste streams as the moisture source during the hydrothermal carbonization of municipal solid wastes. Waste Manag. 2014, 34, 2185–2195. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T. Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste. Bioresour. Technol. 2018, 247, 182–189. [Google Scholar] [CrossRef]
- Alvarez-murillo, A.; Libra, J.A.; Ro, K.S. Theoretical framework for estimating design reactor pressure for water-based hydrothermal carbonization (HTC) systems. Therm. Sci. Eng. Prog. 2022, 30, 101241. [Google Scholar] [CrossRef]
- Fakkaew, K.; Koottatep, T.; Polprasert, C. Effects of hydrolysis and carbonization reactions on hydrochar production. Bioresour. Technol. 2015, 192, 328–334. [Google Scholar] [CrossRef]
- Volpe, M.; Picone, A.; Codignole, F.; Chelang, M.; Volpe, R.; Messineo, A. Potential pitfalls on the scalability of laboratory-based research for hydrothermal carbonization. Fuel 2022, 315, 123189. [Google Scholar] [CrossRef]
- Benavente, V.; Calabuig, E.; Fullana, A. Upgrading of moist agro-industrial wastes by hydrothermal. J. Anal. Appl. Pyrolysis 2015, 113, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Moscosa-santillan, M.; San, D.; Potosí, L.; Nava, A.; Universitaria, Z.; Luis, S. Hydrodynamics, heat transfer and kinetics reaction of CFD modeling of a batch stirred reactor under hydrothermal carbonization conditions. Energy 2021, 219, 119635. [Google Scholar] [CrossRef]
- Deng, C.; Kang, X.; Lin, R.; Murphy, J.D. Microwave assisted low-temperature hydrothermal treatment of solid anaerobic digestate for optimising hydrochar and energy recovery. Chem. Eng. J. 2021, 395, 124999. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Li, Y.; Nelles, M. The influence of hydrothermal operation on the surface properties of kitchen waste-derived hydrochar: Biogas upgrading. J. Clean. Prod. 2020, 259, 121020. [Google Scholar] [CrossRef]
- Gupta, D.; Mahajani, S.M.; Garg, A. Hydrothermal carbonization of household wet waste—Characterization of hydrochar and process wastewater stream. Bioresour. Technol. 2021, 342, 125972. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Y.; Song, Y.; Xu, A.; Zhu, G.; Jiang, J.; Hantoko, D. Comprehensive experimental study on energy conversion of household kitchen waste via integrated hydrothermal carbonization and supercritical water gasification. Energy 2022, 242, 123054. [Google Scholar] [CrossRef]
- Motavaf, B.; Dean, R.A.; Nicolas, J.; Savage, P.E. Hydrothermal carbonization of simulated food waste for recovery of fatty acids and nutrients. Bioresour. Technol. 2021, 341, 125872. [Google Scholar] [CrossRef]
- Akarsu, K.; Duman, G.; Yilmazer, A.; Keskin, T.; Azbar, N.; Yanik, J. Sustainable valorization of food wastes into solid fuel by hydrothermal carbonization. Bioresour. Technol. 2019, 292, 121959. [Google Scholar] [CrossRef]
- Sarrion, A.; Diaz, E.; Rubia, M.A.; De Mohedano, A.F. Fate of nutrients during hydrothermal treatment of food waste. Bioresour. Technol. 2021, 342, 125954. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M.; Goldfarb, J.L.; Maag, A.R. Effect of solvent and feedstock selection on primary and secondary chars produced via hydrothermal carbonization of food wastes. Bioresour. Technol. 2022, 348, 126799. [Google Scholar] [CrossRef]
- Xie, X.; Peng, C.; Song, X.; Peng, N.; Gai, C. Pyrolysis kinetics of the hydrothermal carbons derived from microwave-assisted hydrothermal carbonization of food waste digestate. Energy 2022, 245, 123269. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, D.; Saldaña, M.D.A. Hydrolysis of sweet blue lupin hull using subcritical water technology. Bioresour. Technol. 2015, 194, 75–82. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Qian, J.; Zhao, Y.; Wang, T.; Zhai, Y. Biowaste hydrothermal carbonization for hydrochar valorization: Skeleton structure, conversion pathways and clean biofuel applications. Bioresour. Technol. 2021, 324, 124686. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Weldon, R.; Lynam, J.G. Hydrothermal carbonization of coffee silverskins. Biocatal. Agric. Biotechnol. 2021, 36, 102145. [Google Scholar] [CrossRef]
- Al Afif, R.; Anayah, S.S.; Pfeifer, C. Batch pyrolysis of cotton stalks for evaluation of biochar energy potential. Renew. Energy 2020, 147, 2250–2258. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Yao, Z. Conversion of sweet potato waste to solid fuel via hydrothermal carbonization. Bioresour. Technol. 2018, 249, 900–907. [Google Scholar] [CrossRef]
- Guo, S.; Dong, X.; Wu, T.; Zhu, C. Influence of reaction conditions and feedstock on hydrochar properties. Energy Convers. Manag. 2016, 123, 95–103. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. Eur. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba-rec, I.; Szymańska-Chargot, M.; Szyma, M. Upgrading of green waste into carbon-rich solid biofuel by hydrothermal carbonization: The effect of process parameters on hydrochar derived from acacia. Energy 2020, 202, 117717. [Google Scholar] [CrossRef]
- Murillo, H.A. Bioresource Technology Valorization of oat husk by hydrothermal carbonization: Optimization of process parameters and anaerobic digestion of spent liquors. Bioresour. Technol. 2022, 343, 126112. [Google Scholar] [CrossRef] [PubMed]
- Ischia, G.; Fiori, L.; Gao, L.; Goldfarb, J.L. Valorizing municipal solid waste via integrating hydrothermal carbonization and downstream extraction for biofuel production. J. Clean. Prod. 2021, 289, 125781. [Google Scholar] [CrossRef]
- Sobek, S.; Tran, Q.K.; Junga, R.; Werle, S. Hydrothermal carbonization of the waste straw: A study of the biomass transient heating behavior and solid products combustion kinetics. Fuel 2022, 314, 122725. [Google Scholar] [CrossRef]
- Das, P.; Chandramohan, V.P.; Mathimani, T.; Pugazhendhi, A. A comprehensive review on the factors affecting thermochemical conversion efficiency of algal biomass to energy. Sci. Total Environ. 2021, 766, 144213. [Google Scholar] [CrossRef]
- Lachos-perez, D.; Paulo, C.; Abaide, E.R.; Zabot, G.L.; De Castilhos, F. Hydrothermal carbonization and Liquefaction: Differences, progress, challenges, and opportunities. Bioresour. Technol. 2022, 343, 126084. [Google Scholar] [CrossRef] [PubMed]
- Macdermid-watts, K.; Adewakun, E.; Abhi, T.D.; Pradhan, R.; Dutta, A. Hydrothermal carbonization valorization as an alternative application for. J. Environ. Chem. Eng. 2021, 9, 105431. [Google Scholar] [CrossRef]
- Sayğılı, H.; Sayğılı, G.A. A sustainable generated hydrochar from pomegranate residues for remediation of process water contaminated with Cu(II) ions. Adv. Powder Technol. 2021, 32, 4814–4824. [Google Scholar] [CrossRef]
- Olszewski, M.P.; Nicolae, S.A.; Arauzo, P.J.; Titirici, M.; Kruse, A. Wet and dry ? In fl uence of hydrothermal carbonization on the pyrolysis of spent grains. J. Clean. Prod. 2020, 260, 121101. [Google Scholar] [CrossRef]
- Alwahabi, Z.T.; Kwong, C.W.; Nguyen, D.; Zhao, W. Effect of hydrothermal carbonisation temperature on the ignition properties of grape marc hydrochar fuels. Fuel 2022, 313, 122668. [Google Scholar] [CrossRef]
- Sultana, A.I.; Saha, N.; Reza, M.T. Upcycling simulated food wastes into superactivated hydrochar for remarkable hydrogen storage. J. Anal. Appl. Pyrolysis 2021, 159, 105322. [Google Scholar] [CrossRef]
- Qi, J.; Fan, C.; Wu, H.; Li, S. Structure evolution of lignite char in step pyrolysis and its combustion reactivity. Fuel 2022, 315, 123256. [Google Scholar] [CrossRef]
- Richards, A.P.; Haycock, D.; Frandsen, J.; Fletcher, T.H. A review of coal heating value correlations with application to coal char, tar, and other fuels. Fuel 2021, 283, 118942. [Google Scholar] [CrossRef]
- Wilk, M.; Śliz, M.; Gajek, M. The effects of hydrothermal carbonization operating parameters on high-value hydrochar derived from beet pulp. Renew. Energy 2021, 177, 216–228. [Google Scholar] [CrossRef]
- Kojić, M.M.; Petrović, J.T.; Petrović, M.S.; Stanković, S.M.; Porobić, S.J.; Marinović-Cincović, M.T.; Mihajlović, M.L. Hydrothermal carbonization of spent mushroom substrate: Physicochemical characterization, combustion behavior, kinetic and thermodynamic study. J. Anal. Appl. Pyrolysis 2021, 155, 105028. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Vanapalli, K.R.; Cheela, V.R.S.; Venna, S.; Dubey, B. Study on the process wastewater reuse and valorisation during hydrothermal co-carbonization of food and yard waste. Sci. Total Environ. 2022, 806, 150748. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, D.; Liang, L.; Hu, X.; Ling, M.; Zhou, Z.; Fu, L.; Liu, Z.; Feng, Q. Co-hydrothermal dechlorination of PVC plastic and bagasse: Hydrochar combustion characteristics and gas emission behavior. Process Saf. Environ. Prot. 2022, 161, 88–99. [Google Scholar] [CrossRef]
- Khan, M.A.; Hameed, B.H.; Raza Siddiqui, M.; Alothman, Z.A.; Alsohaimi, I.H. Physicochemical properties and combustion kinetics of food waste derived hydrochars. J. King Saud Univ. Sci. 2022, 34, 101941. [Google Scholar] [CrossRef]
- Islam, M.A.; Kabir, G.; Asif, M.; Hameed, B.H. Combustion kinetics of hydrochar produced from hydrothermal carbonisation of Karanj (Pongamia pinnata) fruit hulls via thermogravimetric analysis. Bioresour. Technol. 2015, 194, 14–20. [Google Scholar] [CrossRef]
- Mo, Q.; Liao, J.; Chang, L.; Han, Y.; Chaffee, A.L.; Bao, W. Study on combustion performance of hydrothermally dewatered lignite by thermal analysis technique. Fuel 2021, 285, 119217. [Google Scholar] [CrossRef]

| Category | Food Waste | C (%) | HC (%) | L (%) | Moisture Content (%. wet) | Ash (%) | HHV (MJ/kg) | O/C | Fuel Ratio (FC/VM) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| - | Mixed | - | - | - | 62.2 | 5.41 | 19.76 | 1.02 | 1.12 | [24] |
| - | Mixed | 2.0 | 1.2 | 0.1 | 3.30 | 10.3 | - | 0.65 | 0.21 | [25] |
| - | Mixed | - | - | - | - | 5.68 | 10.54 | 1.06 | 0.21 | [26] |
| Plant | Apple | - | - | - | - | 2.30 | - | 0.97 | 0.23 | [27] |
| Animal-based | Chicken | - | - | - | - | 2.37 | 25.32 | 0.41 | 0.27 | [28] |
| Vegetal | Cabbage | - | - | - | - | 2.10 | 17.77 | 0.89 | 0.12 | [28] |
| Carbohydrate-rich | Rice | - | - | - | - | 0.29 | 18.33 | 1.05 | 0.16 | [28] |
| Vegetal | Mixed | - | - | - | - | 11.4 | 16.7 | 1.00 | 0.014 | [20] |
| - | Mixed | - | - | - | 74.0 | 1.15 | 22.74 | 0.71 | - | [29] |
| - | Mixed | 36.63 | 1.12 | 15.61 | 9.60 | 3.62 | 16.07 | 0.98 | 0.17 | [30] |
| - | De-Oiled food | 3.12 | 22.76 | 2.68 | 1.59 | 13.01 | 19.16 | 0.548 | 0.064 | [31] |
| Food Substrates Components | HTC Optimum Conditions | Key Property of Hydro Char Targeted | Value | Reference |
|---|---|---|---|---|
| Lettuce/taro/watermelon peel | Lettuce (180–240 °C) 180 rpm for 3 h | 2,4-D adsorption | 88.4. mg/g | [40] |
| Discarded vegetables and meats, potatoes and less fruit peels and eggshells. | 225 °C, 4.5 h | Methane yield | 19% | [55] |
| Cooked meat, vegetables, rice, noodles, fruit peels, vegetable parts, and condiments, paper cups, and woody chopsticks | 230–260 °C, 8 h | Compressive strength Impact resistance index | 2.37 MPa 10 | [41] |
| Mainly of fruit and vegetables | 200 °C, 1 h | H/C O/C | 1.41 0.52 | [2] |
| Cooked FW (as received) without addition of water | 200 °C, 1 h, 2 L | Heating value | ~30 MJ/kg | [10] |
| Cooked rice, chicken, fruit and vegetable peels, lentils, and bread | 200 °C, 1–8 h, 2 L | Heating value | ~27 MJ/kg | [56] |
| Household kitchen waste | 300 °C. 1.25 h, 0.5 L 35 MPa | Heating value | 20.63 MJ/kg | [57] |
| Food waste (51.4 wt.% carbohydrates, 15.7 wt.% lipids, and 27.5 wt.% proteins) | 180–220 °C, 0.25–0.5 h | Fatty acid retention Net fat recovery | 78% ~50% | [58] |
| Real kitchen waste | 260 °C, 1 h, 0.5 L, 4 °C/min, 100 rpm | Ammonium concentration | 929.75 mg/L | [48] |
| Municipality food waste | 200–300 °C, 1 h, 1 L 30 bar, N2 gas, 600 rpm. | Carbon content Heating value | 39–73% 15–31 MJ/kg | [59] |
| Defrosted feedstock | 170–230 °C,1 h, 4 L 1.5 kg, 3 °C/min | Heating value Ash Fixed Carbon | 18.6–26.2 MJ/kg <7.0% <45% | [60] |
| Retail-level food waste | 250 °C, 1 h, 1 L 0.55–0.58 MPa, 400 rpm. | Hydrochar partitionability Best solvent | 50% Ethanol | [61] |
| Waste | Sample | Proximate Composition | Ultimate Composition (wt.%) | Energy Properties | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VM | FC | Ash | C | H | N | S | O | HHV (MJ/kg) | Fuel Ratio | EY (%) | |||
| Raw food waste | HC-230 | 56.2 | 29.5 | 14.3 | 54.8 | 6.1 | 2.3 | 0.2 | 23.7 | 23.7 | - | - | [2] |
| Municipal waste | HC-200 | 51.0 | 43.6 | 5.4 | 58.21 | 5.15 | 3.01 | 0.14 | 28.08 | 23.3 | - | 57.1 | [59] |
| Lettuce waste | HC-220 | - | - | 8.7 | 63.3 | 7.21 | 3.41 | - | 26.1 | - | - | - | [40] |
| Watermelon peel | HC-220 | - | - | 2.5 | 62.3 | 6.26 | 3.09 | - | 28.4 | - | - | - | |
| Taro | HC-220 | - | - | 0.5 | 68.6 | 5.30 | 2.09 | - | 24.0 | - | - | - | |
| Pineapple peel waste | HC-200 | 59.4 | 38.9 | 1.7 | 61.1 | 5.3 | - | - | 30.9 | 25.1 | 0.65 | 62.8 | [51] |
| Orange peel waste | HC-200 | 60.8 | 37.5 | 1.7 | 60.7 | 5.2 | - | - | 31.3 | 24.8 | 0.62 | 63.3 | |
| Tangerine peel waste | HC-220 | 59.5 | 38.4 | 2.1 | 61.6 | 5.3 | - | - | 29.3 | 25.5 | 0.65 | 60.7 | |
| Corn Fibre | HC-220 | 65.40 | 7.84 | 2.53 | 65.40 | 7.84 | 2.53 | 0.21 | 23.78 | - | 0.12 | 27.26 | [77] |
| Pomegranate residue | HC-220 | - | - | - | 56.14 | 6.06 | 1.54 | 0.45 | 35.85 | 21.27 | - | 68.79 | [78] |
| Brewer’s spent grain | HC-220 | 64.04 | 31.10 | 4.86 | 65.90 | 6.48 | 5.13 | 0.09 | 17.54 | 27.04 | 0.49 | [79] | |
| Grape marc | HC-220 | 58.3 | 39.0 | 2.7 | 51.7 | 6.5 | 1.5 | - | 40.3 | 21.3 | 0.69 | - | [80] |
| Kitchen waste | HC-225 | - | - | 1.22 | 70.98 | 7.05 | 3.74 | - | 16.53 | 32.19 | - | - | [55] |
| Household wet waste | HC-200 | 91.4 | 5.9 | 2.7 | 58.4 | 6.4 | 2.8 | - | 29.7 | 22.7 | 0.06 | [56] | |
| Simulated food waste | HC-220 | 56.4 | 38.4 | 5.2 | 60.9 | 5.2 | 6.0 | - | 22.7 | - | 0.68 | - | [81] |
| Cabbage (raw) | HC-220 | 61.2 | 38.5 | 0.32 | 62.8 | 5.33 | 2.95 | 0.42 | 28.18 | 25.28 | 0.62 | - | [28] |
| Rice (cooked/dried) | HC-220 | 50.2 | 49.3 | 0.52 | 65.6 | 4.9 | 2.03 | 0.55 | 26.4 | 25.96 | 0.98 | - | |
| Chicken | HC-220 | 94.8 | 4.2 | 1.02 | 66.1 | 9.9 | 6.46 | 0.3 | 16.22 | 32.97 | 0.04 | - | |
| Food Waste Substrate | TGA Reactor Model | Sample Weight (mg) | Temp. Range (°C) | β (°C/min) | Φ (mL/min) | Reference |
|---|---|---|---|---|---|---|
| Beet pulp | Netzsch STA 449 F3 Jupiter | 10 | ≤700 | 10, 20, 30 | 40 | [84] |
| Spent mushroom | Setaram Setsys Evolution 1750 | 7 | 25–1000 | 5, 10, 20 | 16 | [85] |
| Sweet potato | TGA, METTLER TOLEDO | 8 ± 0.5 | 100–800 | 20 | 100 | [68] |
| Oil extracted food waste | Discovery SDT 650 | - | 25–950 | 10 | 100 | [31] |
| Food/yard waste | Perkin Elmer Pyris Diamond | - | ≤900 | 12 | 100 | [86] |
| PVC and bagasse | - | 30–900 | 20 | 100 | [87] | |
| Mixed food waste | STA 449 F5 Jupiter | 10 ± 0.5 | 50–900 | 10 | - | [88] |
| Household wet waste | Shimadzu DTG-60 TGA | 10–15 | ≤950 | 10 | 100 | [56] |
| Feedstock | Hydrochar Code | Kinetic Modelling Approach | Stages | Activation Energy, E (kJ/mol) | Reaction Order, n | Frequency Factor, A (1/s) | Reference |
|---|---|---|---|---|---|---|---|
| Mixed food waste | HTC220 | Arrhenius | 2 | Stage 1: 25.47 Stage 2: 16.52 | 3.0 0.9 | 11.36 × 10−2 7.37 × 10−2 | [88] |
| PVCR was co-treated with bagasse | HC-P-S | - | 2 | Stage 1: 86.07 Stage 2: 47.62 | - | - | [87] |
| Spent mushroom substrate | SMS-180/260 | Flynn-Wall-Ozawa (FWO) and Kissinger-Akahira-Sunose (KAS) | 3 | 81.76 (FWO) and 75.22 (KAS) for SMS180 and 91.99 (FWO) and 85.71 (KAS) for SMS260 | - | - | [85] |
| Sweet potato | 220–60 | Coats–Redfern integral | 2 | Stage 1: 211.94 Stage 2: 181.65 | 7.58 × 1017 2.40 × 109 | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Hameed, B.H.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.H. Hydrothermal Conversion of Food Waste to Carbonaceous Solid Fuel—A Review of Recent Developments. Foods 2022, 11, 4036. https://doi.org/10.3390/foods11244036
Khan MA, Hameed BH, Siddiqui MR, Alothman ZA, Alsohaimi IH. Hydrothermal Conversion of Food Waste to Carbonaceous Solid Fuel—A Review of Recent Developments. Foods. 2022; 11(24):4036. https://doi.org/10.3390/foods11244036
Chicago/Turabian StyleKhan, Moonis Ali, Bassim H. Hameed, Masoom Raza Siddiqui, Zeid A. Alothman, and Ibrahim H. Alsohaimi. 2022. "Hydrothermal Conversion of Food Waste to Carbonaceous Solid Fuel—A Review of Recent Developments" Foods 11, no. 24: 4036. https://doi.org/10.3390/foods11244036
APA StyleKhan, M. A., Hameed, B. H., Siddiqui, M. R., Alothman, Z. A., & Alsohaimi, I. H. (2022). Hydrothermal Conversion of Food Waste to Carbonaceous Solid Fuel—A Review of Recent Developments. Foods, 11(24), 4036. https://doi.org/10.3390/foods11244036











