Physical and 3D Printing Properties of Arrowroot Starch Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel Permeation Chromatography (GPC) Measurements
2.3. X-ray Diffractometry (XRD) Measurement
- Ic = the integrated area of the crystalline phase;
- Ia = the integrated area of the amorphous phase.
2.4. Scanning Electron Microscopy (SEM) Measurement
2.5. 13C NMR and 13C CP/MAS NMR Measurements
2.6. Differential Scanning Calorimetry (DSC) Measurement
2.7. Preparation of AS Solutions and Gels
2.8. Rheological Properties of AS Solutions and Gels
2.9. The 3D Printing of AS Gels
2.10. Statistical Analysis
3. Results and Discussion
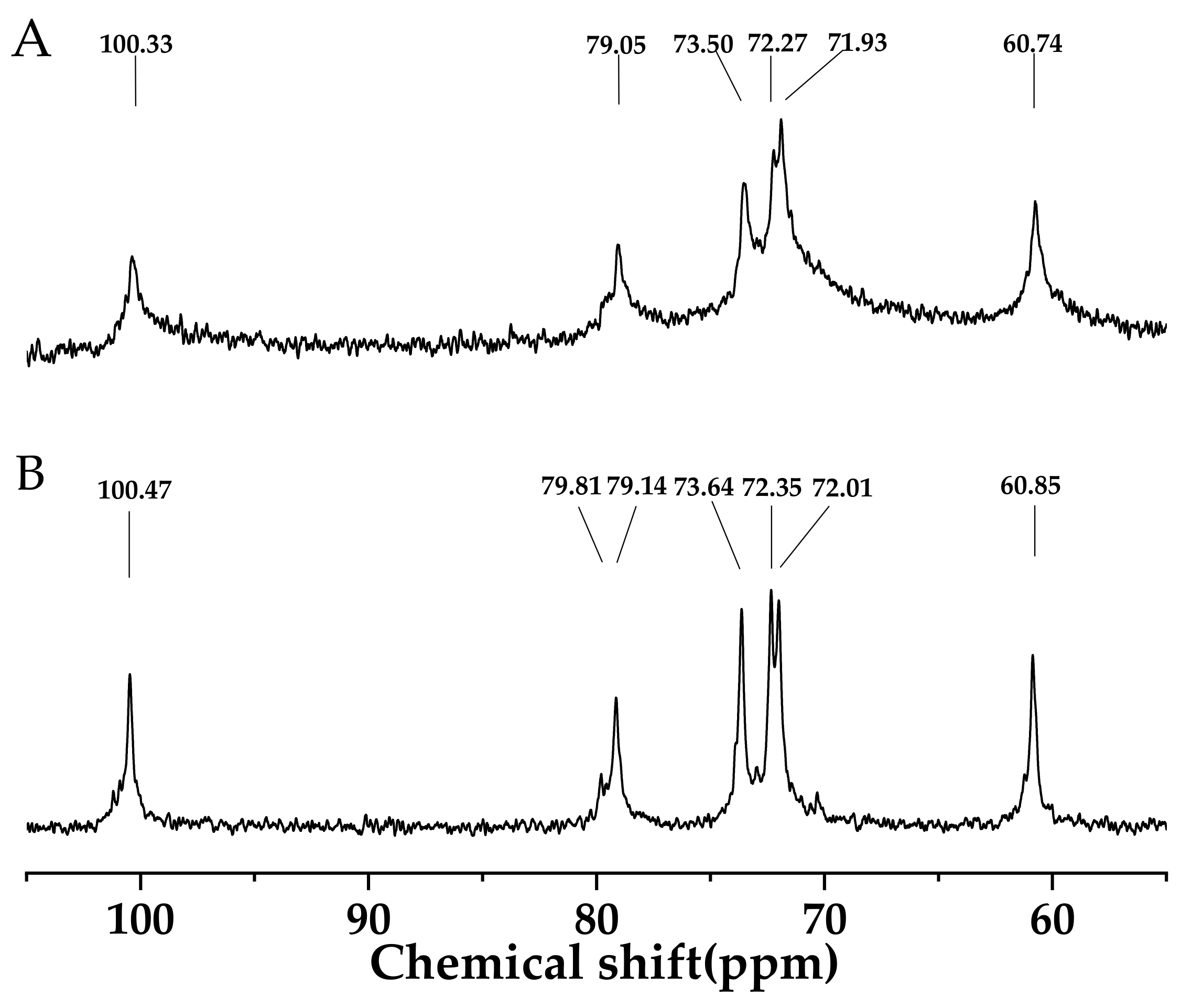
3.1. 13C NMR Analyses
3.2. Scanning Electron Microscopy (SEM) Analysis
3.3. X-ray Diffraction Analysis
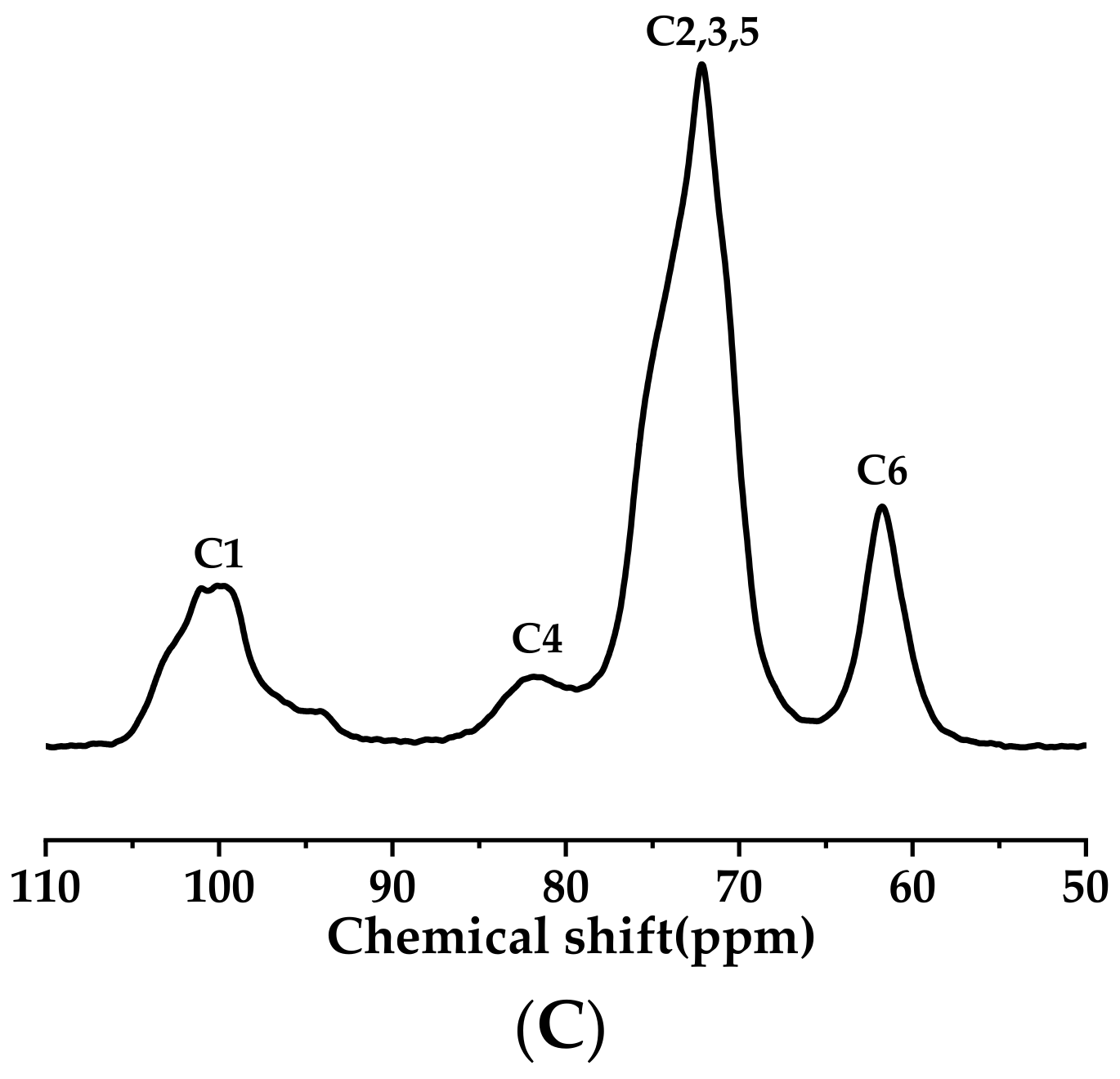
3.4. 13C CP/MAS NMR Analysis
3.5. Rheological Properties of AS Solutions and Gels
3.5.1. Overlap Concentration of AS Solution
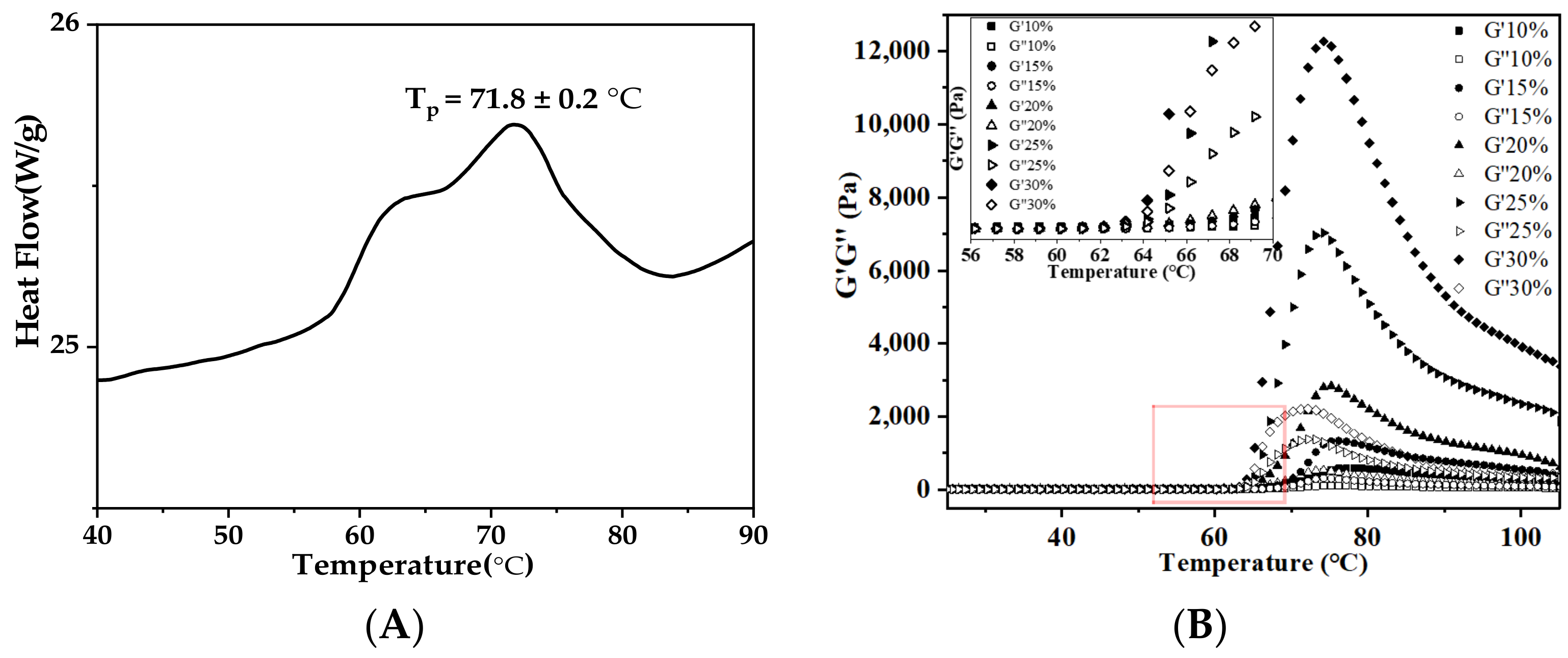
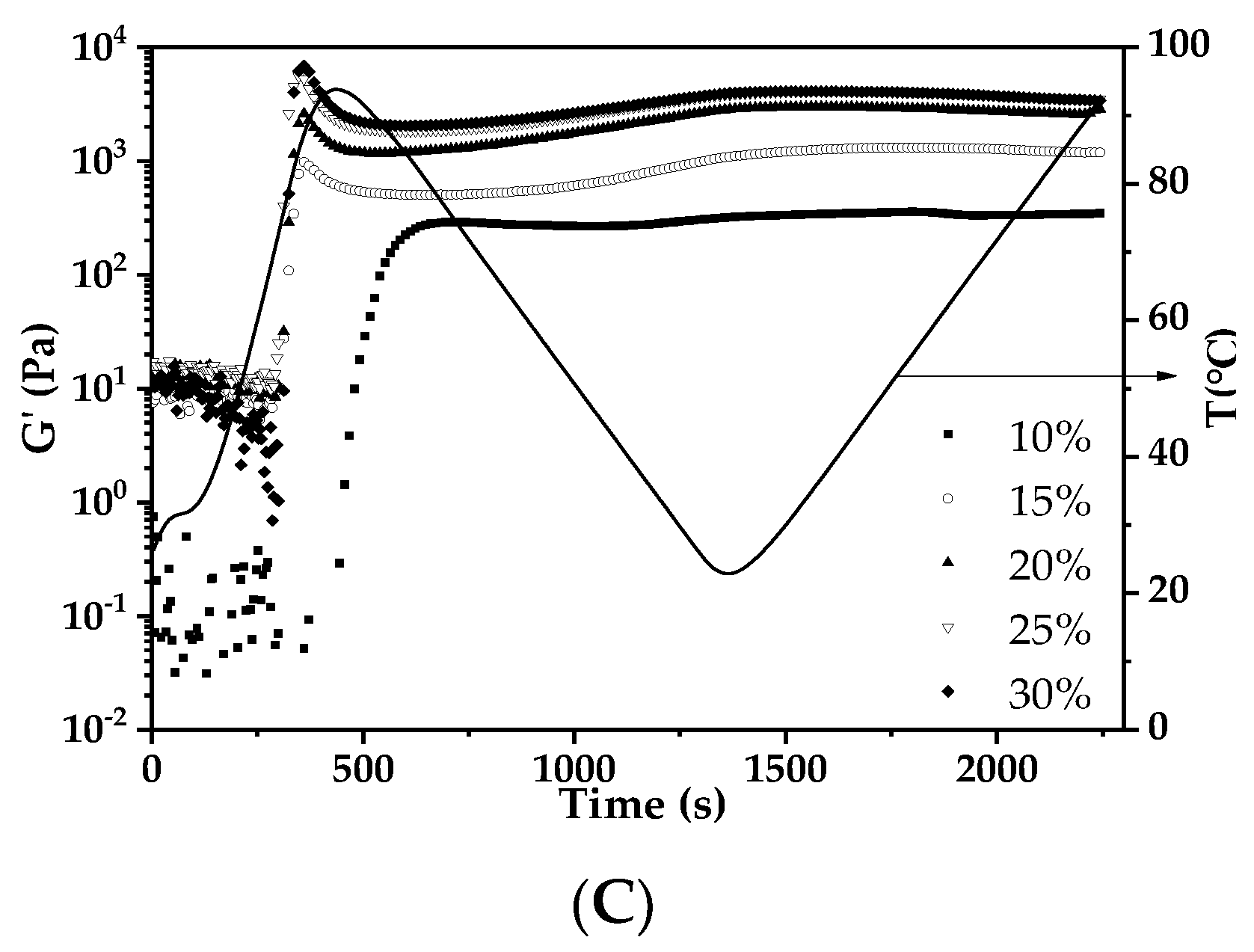
3.5.2. Thermal and Rheological Properties of AS Gels
3.6. The 3D Printing of AS Gels
3.6.1. Effect of Nozzle Size
3.6.2. Effect of Printing/Extrusion Speed
3.6.3. Effect of Temperature
3.6.4. Effect of AS Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lailli, V.; Bachri, M.S.; Widyaningsih, W. The gastroprotective effects of arrowroot tuber starch (Maranta arundinacea L.) on ethanol-induced gastric damages in rats. Pharmaciana 2020, 10, 35. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Characterization and in vitro digestibility of non-conventional starches from guinea arrowroot and La Armuña lentils as potential food sources for special diet regimens. Starch-Stärke 2018, 70, 1700124. [Google Scholar] [CrossRef]
- Nachal, N.; Moses, J.A.; Karthik, P.; Anandharamakrishnan, C. Applications of 3D Printing in Food Processing. Food Eng. Rev. 2019, 11, 123–141. [Google Scholar] [CrossRef]
- Ji, S.; Xu, T.; Li, Y.; Li, H.; Zhong, Y.; Lu, B. Effect of starch molecular structure on precision and texture properties of 3D printed products. Food Hydrocoll. 2022, 125, 107387. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Ubeyitogullari, A. Fabrication of Porous Spherical Beads from Corn Starch by Using a 3D Food Printing System. Foods 2022, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Moorthy, S.N. Physicochemical and functional properties of tropical tuber starches: A review. Starch-Stärke 2002, 54, 559–592. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, J.; Liu, R.; Xing, Y.; Jiang, H. 3D printing performance of gels from wheat starch, flour and whole meal. Food Chem. 2021, 356, 129546. [Google Scholar] [CrossRef]
- Zeng, X.; Li, T.; Zhu, J.; Chen, L.; Zheng, B. Printability improvement of rice starch gel via catechin and procyanidin in hot extrusion 3D printing. Food Hydrocoll. 2021, 121, 106997. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Q.; Li, H.; Zhou, Q.; Meng, W. Effects of Pea Protein on the Properties of Potato Starch-Based 3D Printing Materials. Int. J. Food Eng. 2018, 14, 3. [Google Scholar] [CrossRef]
- Huang, G.; Liu, J.; Jin, W.; Wei, Z.; Ho, C.-T.; Zhao, S.; Zhang, K.; Huang, Q. Formation of Nanocomplexes between Carboxymethyl Inulin and Bovine Serum Albumin via pH-Induced Electrostatic Interaction. Molecules 2019, 24, 3056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, C.; Cai, J.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Toward a better understanding of starch–monoglyceride–protein interactions. J. Agric. Food Chem. 2018, 66, 13253–13259. [Google Scholar] [CrossRef] [PubMed]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Sherwani, S.F.K.; Yusuf, J.; Ilyas, R.A. Recent developments in sustainable arrowroot (Maranta arundinacea Linn) starch biopolymers, fibres, biopolymer composites and their potential industrial applications: A review. J. Mater. Res. Technol. 2021, 13, 1191–1219. [Google Scholar] [CrossRef]
- Mutungi, C.; Passauer, L.; Onyango, C.; Jaros, D.; Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 2012, 87, 598–606. [Google Scholar] [CrossRef]
- Valérie, V.; Pieter, V.; Jeroen, L.; Bart, N. Development of a coaxial extrusion deposition for 3D printing of customizable pectin-based food simulant. J. Food Eng. 2018, 225, 42–52. [Google Scholar] [CrossRef]
- Radhika, T.; Moses, J.A.; Anandharamakrishnan, C. 3D Extrusion Printability of Rice Starch and Optimization of Process Variables. Food Bioprocess Technol. 2020, 13, 1048–1062. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Li, Y.; Huang, Q. Assembly of Pickering emulsions using milled starch particles with different amylose/amylopectin ratios. Food Hydrocoll. 2018, 84, 47–57. [Google Scholar] [CrossRef]
- Franklin, M.E.E.; Pushpadass, H.A.; Kumar, B.; Kulkarni, S.; Muthurayappa, M.; Kandasamy, R.; Venkatachalam, P.; Vellingiri, P. Physicochemical, thermal, pasting and microstructural characterization of commercial Curcuma angustifolia starch. Food Hydrocoll. 2017, 67, 27–36. [Google Scholar] [CrossRef]
- Cérantola, S.; Kervarec, N.; Pichon, R.; Magné, C.; Bessieres, M.A.; Deslandes, E. NMR characterisation of inulin-type fructooligosaccharides as the major water-soluble carbohydrates from Matricaria maritima (L.). Carbohydr. Res. 2004, 339, 2445–2449. [Google Scholar] [CrossRef]
- Mensink M, A.; Frijlink H, W.; Van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Tako, M.; Tamaki, Y.; Teruya, T.; Takeda, Y. The principles of starch gelatinization and retrogradation. Food Nutr. Sci. 2014, 5, 3. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Huang, J.; Yang, Q.; Pu, H.; Liu, S.; Zhu, Z. Adsorption capacity and cold-water solubility of honeycomb-like potato starch granule. Int. J. Biol. Macromol. 2020, 147, 741–749. [Google Scholar] [CrossRef]
- Lin, L.; Guo, D.; Zhao, L.; Zhang, X.; Wang, J.; Zhang, F.; Wei, C. Comparative structure of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocoll. 2016, 52, 19–28. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Ultrasound-treated starch: SEM and TEM imaging, and functional behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Ayala Valencia, G.; Freitas Moraes, I.C.; Vinicius Lourenço, R.; Barbosa Bittante, A.M.Q.; Do Amaral Sobral, P.J. Physicochemical Properties of Maranta (Maranta arundinaceaL.) Starch. Int. J. Food Prop. 2014, 18, 1990–2001. [Google Scholar] [CrossRef]
- Villas-Boas, F.; Franco, C.M.L. Effect of bacterial β-amylase and fungal α-amylase on the digestibility and structural characteristics of potato and arrowroot starches. Food Hydrocoll. 2016, 52, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, G.F.; Fakhouri, F.M.; De Oliveira, R.A. Extraction and characterization of arrowroot (Maranta arundinaceae L.) starch and its application in edible films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Li, C.; Wu, A.; Yu, W.; Hu, Y.; Li, E.; Zhang, C.; Liu, Q. Parameterizing starch chain-length distributions for structure-property relations. Carbohydr. Polym. 2020, 241, 116390. [Google Scholar] [CrossRef]
- Lourdin, D.; Putaux J, L.; Potocki-Véronèse, G.; Chevigny, C.; Rolland-Sabaté, A.; Buléon, A. Crystalline structure in starch. In Starch: Metabolism and Structure; Springer: Tokyo, Japan, 2015; pp. 61–90. [Google Scholar] [CrossRef]
- Bogracheva, T.Y.; Wang, Y.L.; Hedley, C.L. The effect of water content on the ordered/disordered structures in starches. Biopolymers 2001, 58, 247–259. [Google Scholar] [CrossRef]
- Tan, I.; Flanagan, B.M.; Hally, P.J.; Whittaker, A.K.; Gidley, M.J. A method for estimating the nature and relative proportions of amorphous, single, and double-helical components in starch granules by 13C CP/MAS NMR. Biomacromolecules 2007, 8, 885–891. [Google Scholar] [CrossRef]
- Man, J.; Yang, Y.; Huang, J.; Zhang, C.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q.; Wei, C. Effect of simultaneous inhibition of starch branching enzymes I and IIb on the crystalline structure of rice starches with different amylose contents. J. Agric. Food Chem. 2013, 61, 9930–9937. [Google Scholar] [CrossRef]
- Katoh, E.; Murata, K.; Fujita, N. 13C CP/MAS NMR Can Discriminate Genetic Backgrounds of Rice Starch. ACS Omega 2020, 5, 24592–24600. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.; Zheng, B.; Xie, F.; Chen, L. Understanding the structure and rheological properties of potato starch induced by hot-extrusion 3D printing. Food Hydrocoll. 2020, 105, 10512. [Google Scholar] [CrossRef]
- Maher, Z.E.; Hala, F.N.; Rania Elsayed, M. Chitosan based nanofibers, review. Mater. Sci. Eng. C 2012, 32, 1711–1726. [Google Scholar] [CrossRef]
- Guo, L.; Hu, J.; Zhang, J.; Du, X. The role of entanglement concentration on the hydrodynamic properties of potato and sweet potato starches. Int. J. Biol. Macromol. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L. Understanding the mechanism of starch digestion mitigation by rice protein and its enzymatic hydrolysates. Food Hydrocoll. 2018, 84, 473–480. [Google Scholar] [CrossRef]
- Chen, H.; Xie, F.; Chen, L.; Zheng, B. Effect of rheological properties of potato, rice and corn starches on their hot-extrusion 3D printing behaviors. J. Food Eng. 2019, 244, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of starch–water interactions and their effects on two key functional properties: Starch gelatinization and retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Willett, J.L.; Jasberg, B.K.; Swanson, C.L. Rheology of thermoplastic starch: Effects of temperature, moisture content, and additives on melt viscosity. Polym. Eng. Sci. 1995, 35, 202–210. [Google Scholar] [CrossRef]
- Lii, C.Y.; Shao, Y.Y.; Tseng, K.H. Gelation mechanism and rheological properties of rice starch. Cereal Chem. 1995, 72, 393–400. [Google Scholar]
- Ai, Y.; Jane, J.l. Gelatinization and rheological properties of starch. Starch-Stärke 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, P. Starch gelatinization, retrogradation, and enzyme susceptibility of retrograded starch: Effect of amylopectin internal molecular structure. Food Chem. 2020, 316, 126036. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, M.; Liu, Y. Effect of post-treatment microwave vacuum drying on the quality of 3D-printed mango juice gel. Dry. Technol. 2018, 37, 1757–1765. [Google Scholar] [CrossRef]
- Yang, F.; Guo, C.; Zhang, M.; Bhandari, B. Improving 3D printing process of lemon juice gel based on fluid flow numerical simulation. LWT-Food Sci. Technol. 2018, 102, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Derossi, A.; Paolillo, M.; Caporizzi, R.; Severini, C. Extending the 3D food printing tests at high speed. Material deposition and effect of non-printing movements on the final quality of printed structures. J. Food Eng. 2020, 275, 109865. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, Y.; Tong, Z.; Zou, Q.; Han, S.; Jiang, H. The characteristics of starch gels molded by 3D printing. J. Food Process. Preserv. 2019, 43, e13993. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, H.; Chen, L.; Zheng, B. Insights into the relationship between structure and rheological properties of starch gels in hot-extrusion 3D printing. Food Chem. 2020, 342, 128362. [Google Scholar] [CrossRef]



| Variety | Concentration (%) | G′1 (Pa) | G′3 (Pa) | G′3/G′1 (%) |
|---|---|---|---|---|
| AS | 10 | 1107.6 | 887.6 | 80% |
| 15 | 1336.2 | 927.3 | 69% | |
| 20 | 2527.4 | 1783 | 71% | |
| 25 | 4844.5 | 3090.4 | 64% | |
| 30 | 10,371.9 | 6289.4 | 60% |
| Nozzle Diameter (mm) | Length (mm) | Width (mm) | Height (mm) | Print Shape |
|---|---|---|---|---|
| Model | 40 | 40 | 5 |  |
| 0.41 | 38.7 ± 1.5 b | 39.0 ± 1.0 a | 4.6 ± 0.1 d |  |
| 0.60 | 41.0 ± 1.0 a | 40.5 ± 0.5 a | 5.1 ± 0.1 c |  |
| 0.84 | 41.0 ± 1.0 a | 40.2 ± 0.5 a | 5.9 ± 0.1 a |  |
| 1.20 | 40.5 ± 0.5 b | 38.7 ± 1.5 a | 5.7 ± 0.1 b |  |
| Printing/Extrusion Speed (mm/s) | Length (mm) | Width (mm) | Height (mm) | Print Shape |
|---|---|---|---|---|
| Model | 35 | 35 | 5 |  |
| 80/80 | 32.3 ± 0.8 c | 29.6 ± 1.1 c | 4.6 ± 0.4 ab |  |
| 80/100 | 34.8 ± 0.2 a | 34.8 ± 0.2 b | 4.4 ± 0.1 b |  |
| 100/100 | 34.9 ± 0.1 a | 34.9 ± 0.1 a | 4.9 ± 0.7 a |  |
| 120/100 | 34.4 ± 0.4 a | 35.0 ± 0.2 a | 4.8 ± 0.2 a |  |
| 100/80 | 33.5 ± 0.5 b | 34.2 ± 0.2 a | 3.7 ± 0.1 c |  |
| 100/100 | 34.9 ± 0.1 a | 34.9 ± 0.1 a | 4.9 ± 0.7 a |  |
| 100/120 | 35.0 ± 0.2 a | 34.9 ± 0.7 a | 5.0 ± 0.4 a |  |
| 120/120 | 34.3 ± 0.4 a | 33.3 ± 0.3 b | 4.9 ± 0.1 ab |  |
| Printing Temperature (°C) | Length (mm) | Width (mm) | Height (mm) | Print Shape |
|---|---|---|---|---|
| Model | 28 | 28 | 5 |  |
| 25 | 27.7 ± 0.3 a | 27.9 ± 0.8 a | 5.0 ± 0.1 a |  |
| 37 | 28.0 ± 0.2 a | 28.0 ± 0.3 a | 5.1 ± 0.6 a |  |
| 45 | 28.0 ± 0.8 a | 27.6 ± 0.1 b | 5.1 ± 0.1 a |  |
| 60 | 27.8 ± 0.2 a | 27.9 ± 0.7 a | 5.1 ± 0.6 a |  |
| 75 | 28.0 ± 0.1 a | 28.0 ± 0.1 a | 5.1 ± 0.5 a |  |
| 85 | 27.7 ± 0.2 c | 27.1 ± 0.1 c | 5.3 ± 0.3 a |  |
| AS Concentration (%) | Length (mm) | Width (mm) | Height (mm) | Print Shape |
|---|---|---|---|---|
| Model | 30 | 30 | 20 |  |
| 10 | 26.9 ± 0.6 b | 23.3 ± 1.0 b | 14.8 ± 0.2 b | 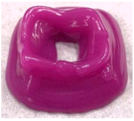 |
| 15 | 30.3 ± 1.0 a | 28.6 ± 1.3 a | 20.2 ± 0.9 a |  |
| 20 | 30.0 ± 1.0 a | 28.7 ± 0.6 a | 20.5 ± 0.4 a |  |
| 25 | 29.4 ± 0.9 a | 29.0 ± 1.0 a | 20.7 ± 0.2 a |  |
| 30 | 28.8 ± 0.6 a | 28.0 ± 1.0 a | 20.3 ± 0.3 a |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Dong, Q.; Huang, G.; Zhang, Y.; Lu, X.; Zhang, J.; Zhang, K.; Huang, Q. Physical and 3D Printing Properties of Arrowroot Starch Gels. Foods 2022, 11, 2140. https://doi.org/10.3390/foods11142140
Xu M, Dong Q, Huang G, Zhang Y, Lu X, Zhang J, Zhang K, Huang Q. Physical and 3D Printing Properties of Arrowroot Starch Gels. Foods. 2022; 11(14):2140. https://doi.org/10.3390/foods11142140
Chicago/Turabian StyleXu, Meiling, Qiaoru Dong, Guiying Huang, Ya Zhang, Xuanxuan Lu, Jiaduo Zhang, Kun Zhang, and Qingrong Huang. 2022. "Physical and 3D Printing Properties of Arrowroot Starch Gels" Foods 11, no. 14: 2140. https://doi.org/10.3390/foods11142140
APA StyleXu, M., Dong, Q., Huang, G., Zhang, Y., Lu, X., Zhang, J., Zhang, K., & Huang, Q. (2022). Physical and 3D Printing Properties of Arrowroot Starch Gels. Foods, 11(14), 2140. https://doi.org/10.3390/foods11142140






