Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes
Abstract
:1. Introduction
2. Nutritional and Chemical Composition of Apricot Kernel
3. Extraction of Bioactive Compounds from Apricot Kernel
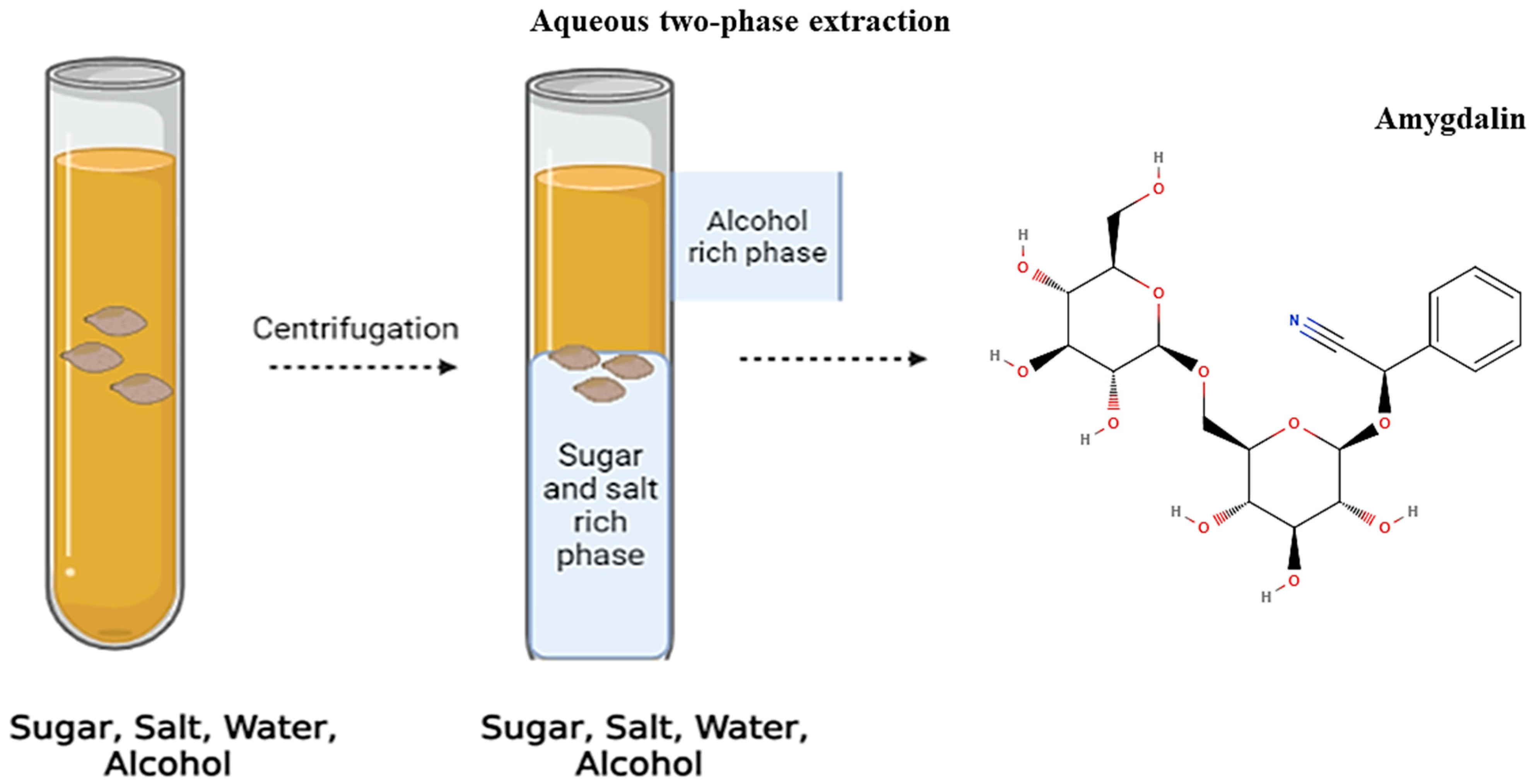
3.1. Two-Phase Extraction Method
3.2. Solvent Extraction Method
3.3. Cold Press Extraction Method
4. Measured Characteristics of Apricot Kernel Using Different Techniques
4.1. Fourier Transform Infrared Spectroscopy
4.2. Scanning Electron Microscopy (SEM)
4.3. High-Performance Liquid Chromatography (HPLC)
4.4. X-ray Diffraction (XRD)
5. Application of Apricot Kernel
5.1. Application of Apricot Kernel Flour in the Food Industry
5.2. Application of Apricot Kernel in Pharmaceuticals:
6. Therapeutical Properties of Apricot Kernel
6.1. Antioxidant Capacity of the Apricot Kernel
6.2. Anti-Cancer
6.3. Cardiovascular Diseases
6.4. Hemostasis
6.5. Contraindication
6.6. Hepatic Steatosis and Kernel as Folk Medicines
7. Market Value of Apricot Kernel
8. Future Research Prospectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gecer, M.K.; Kan, T.; Gundogdu, M.; Ercisli, S.; Ilhan, G.; Sagbas, H.I. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet. Resour. Crop Evol. 2020, 67, 935–945. [Google Scholar] [CrossRef]
- Gençer, A.; Ozgul, U.; Onat, S.M.; Gunduz, G.; Yaman, B.; Yazici, H. Chemical and morphological properties of Apricot wood (Prunus armeniaca L.) and fruit endocarp. Bartın Orman Fakültesi Derg. 2018, 20, 205–209. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer properties of Prunus mume extracts (Chinese plum, Japanese apricot). J. Ethnopharmacol. 2020, 246, 112215. [Google Scholar] [CrossRef] [PubMed]
- Stobdan, T.; Namgial, D.; Chaurasia, O.P.; Wani, M.; Phunchok, T.; Zaffar, M. Apricot (Prunus armeniaca L.) In Trans-Himalayan Ladakh, India: Current Status and Future Directions. J. Food Agric. Res. 2021, 1, 86–105. [Google Scholar]
- Tanwar, B.; Modgil, R.; Goyal, A. Antinutritional factors and hypocholesterolemic effect of wild apricot kernel (Prunus armeniaca L.) as affected by detoxification. Food Funct. 2018, 9, 2121–2135. [Google Scholar] [CrossRef]
- Özarslan, S.; Atelge, M.R.; Kaya, M.; Ünalan, S. A Novel Tea factory waste metal-free catalyst as promising supercapacitor electrode for hydrogen production and energy storage: A dual functional material. Fuel 2021, 305, 121578. [Google Scholar] [CrossRef]
- Jaafar, H.J. Effects of Apricot and Apricot Kernels on Human Health and Nutrition: A Review of Recent Human Research. Tech. Biochem. 2021, 2, 139–162. [Google Scholar] [CrossRef]
- El Shemy, M.A. Effect of Some Essential Oils, Salts and Salicylic Acid on Reducing Decay, Keeping Quality and Prolonging Shelf-Life of Canino Apricot Fruits. Menoufia J. Plant Prod. 2020, 5, 111–128. [Google Scholar] [CrossRef]
- Kiralan, M.; Ketenoglu, O. Apricot (Prunus armeniaca L.) Kernel: A Valuable by-Product. In Mediterranean Fruits Biowastes; Springer: Cham, Switzerland, 2022; pp. 547–558. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.; Farooq, M.A.; Kumari, A.; Murtaza, M.A.; Ahmad, N.; Manzoor, M.F.; Hassan, S.; Ahmad, Z.; Bo-Ru, C.; et al. Effect of pulsed electric fields processing on physiochemical properties and bioactive compounds of apricot juice. J. Food Process Eng. 2020, 43, e13449. [Google Scholar] [CrossRef]
- Zhou, S.; Zhai, X.; Zhang, R.; Wang, W.; Lim, L.-T.; Hou, H. High-Throughput Fabrication of Antibacterial Starch/PBAT/AgNPs@SiO2 Films for Food Packaging. Nanomaterials 2021, 11, 3062. [Google Scholar] [CrossRef]
- Farag, M.A.; Ramadan, N.S.; Shorbagi, M.; Farag, N.; Gad, H.A. Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools. Foods 2022, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Hayta, M.; Alpaslan, M. Apricot Kernel Flour and Its Use in Maintaining Health. In Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 213–221. [Google Scholar] [CrossRef]
- Jaswal, V.; Palanivelu, J.; Ramalingam, C. Effects of the Gut microbiota on amygdalin and its use as an anti-cancer therapy: Substantial review on the key components involved in altering dose efficacy and toxicity. Biochem. Biophys.Rep. 2018, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Wu, L.-J.; Wang, W.-X.; Xie, P.; Chen, Y.-H.; Wang, F. Amygdalin—A pharmacological and toxicological review. J. Ethnopharmacol. 2020, 254, 112717. [Google Scholar] [CrossRef] [PubMed]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Vasudev, S.; Chandran, D.; Sharma, K.; Kumar, M.; Lorenzo, J.M. Chemical Characterization of Apricot Kernel: Nutraceutical Composition, Amino Acid, and Fatty Acid Profile. Food Anal. Methods 2022, 15, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Al-Ghamdi, A.A.; Elshikh, M.S.; Shah, M.H.; Al-Dosary, M.A.; Abbasi, A.M. Phytochemical profiling, antioxidant and HepG2 cancer cells’ antiproliferation potential in the kernels of apricot cultivars. Saudi J. Biol. Sci. 2020, 27, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.; Rawat, N.; Negi, T.; Barthwal, R.; Sharma, S.K. Utilization, valorization and functional properties of wild apricot kernels. J. Pharmacogn. Phytochem. 2021, 10, 119–126. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E. The effect of microwave roasting on bioactive compounds, antioxidant activity and fatty acid composition of apricot kernel and oils. Food Chem. 2018, 243, 414–419. [Google Scholar] [CrossRef]
- Moustafa, K.; Cross, J. Production, pomological and nutraceutical properties of apricot. J. Food Sci. Technol. 2019, 56, 12–23. [Google Scholar] [CrossRef]
- Qin, F.; Yao, L.; Lu, C.; Li, C.; Zhou, Y.; Su, C.; Chen, B.; Shen, Y. Phenolic composition, antioxidant and antibacterial properties, and in vitro anti-HepG2 cell activities of wild apricot (Armeniaca sibirica L. Lam) kernel skins. Food Chem. Toxicol. 2019, 129, 354–364. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Netto, F.M.; Bertoldo-Pacheco, M.T.; Alegría, A.; Cilla, A. Peptide-metal complexes: Obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit. Rev. Food Sci. Nutr. 2021, 61, 1470–1489. [Google Scholar] [CrossRef] [PubMed]
- Janković, B.; Manić, N.; Dodevski, V.; Radović, I.; Pijović, M.; Katnić, Đ.; Tasić, G. Physico-chemical characterization of carbonized apricot kernel shell as precursor for activated carbon preparation in clean technology utilization. J. Clean. Prod. 2019, 236, 117614. [Google Scholar] [CrossRef]
- Sharif, M.N.; Warriach, A.R.; Ali, M.U.; Akram, M.N.; Ashfaq, F.; Raza, A. Proximate Composition of Apricot (Prunus armeniaca) Fruit and Kernel. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 2109–2112. [Google Scholar] [CrossRef]
- Wani, S.; Jan, N.; Wani, T.; Ahmad, M.; Masoodi, F.; Gani, A. Optimization of antioxidant activity and total polyphenols of dried apricot fruit extracts (Prunus armeniaca L.) using response surface methodology. J. Saudi Soc. Agric. Sci. 2017, 16, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Jin, F.; Regenstein, J.M.; Wang, F. Transglutaminase induced gels using bitter apricot kernel protein: Chemical, textural and release properties. Food Biosci. 2018, 26, 15–22. [Google Scholar] [CrossRef]
- Ahmed, E.; Amin, A.; Zoltán, S.; Holb, I.J. Salicylic acid treatment saves quality and enhances antioxidant properties of apricot fruit. Hortic. Sci. 2017, 44, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Faieta, M.; Neri, L.; Di Michele, A.; Di Mattia, C.D.; Pittia, P. High hydrostatic pressure treatment of Arthrospira (Spirulina) platensis extracts and the baroprotective effect of sugars on phycobiliproteins. Innov. Food Sci. Emerg. Technol. 2021, 70, 102693. [Google Scholar] [CrossRef]
- Al-Bachir, M. Compositions and microbial properties of gamma irradiated apricot (Prunus armeniaca L.) kernel. J. Stress Physiol. Biochem. 2021, 17, 79–87. [Google Scholar]
- Matthäus, B.; Özcan, M.M.; AL Juhaimi, F. Fatty acid composition and tocopherol content of the kernel oil from apricot varieties (Hasanbey, Hacihaliloglu, Kabaasi and Soganci) collected at different harvest times. Eur. Food Res. Technol. 2016, 242, 221–226. [Google Scholar] [CrossRef]
- Sharma, A.; Kshetrimayum, C.; Sadhu, H.G.; Kumar, S. Arsenic-induced oxidative stress, cholinesterase activity in the brain of Swiss albino mice, and its amelioration by antioxidants Vitamin E and Coenzyme Q10. Environ. Sci. Pollut. Res. 2018, 25, 23946–23953. [Google Scholar] [CrossRef]
- Çelik, Y.H.; Yalcin, R.; Topkaya, T.; Başaran, E.; Kilickap, E. Characterization of hazelnut, pistachio, and apricot kernel shell particles and analysis of their composite properties. J. Nat. Fibers 2021, 18, 1054–1068. [Google Scholar] [CrossRef]
- Beyer, R.; Melton, L.D. Composition of New Zealand apricot kernels. N. Z. J. Crop Hortic. Sci. 1990, 18, 39–42. [Google Scholar] [CrossRef]
- Shariatifar, N.; Pourfard, I.M.; Khaniki, G.J.; Nabizadeh, R.; Akbarzadeh, A.; Nejad, A.S.M. Mineral Composition, Physico-chemical Properties and Fatty Acids Profile of Prunus armeniaca Apricot Seed Oil. Asian J. Chem. 2017, 29, 2011–2015. [Google Scholar] [CrossRef]
- Chatterjee, S.M. Effect of Avocado on Cancer Cell Immunotherapy and Vitamin-B 17. World J. Pharm. Res. 2021, 10, 2461–2469. [Google Scholar] [CrossRef]
- Anwar, F.; Manzoor, M.; Ashraf, M.; Alkharfy, K.M. Physico-chemical characteristics of seed oils extracted from different apricot (Prunus armeniaca L.) varieties from Pakistan. Grasas Aceites 2012, 63, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Čakarević, J.; Vidović, S.; Vladić, J.; Gavarić, A.; Jokić, S.; Pavlović, N.; Blažić, M.; Popović, L. Production of Bio-Functional Protein through Revalorization of Apricot Kernel Cake. Foods 2019, 8, 318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2013, 136, 1169–1176. [Google Scholar] [CrossRef]
- Yao, J.-L.; Zhang, Q.-A.; Liu, M.-J. Utilization of apricot kernel skins by ultrasonic treatment of the dough to produce a bread with better flavor and good shelf life. LWT 2021, 145, 111545. [Google Scholar] [CrossRef]
- Cheaib, D.; El Darra, N.; Rajha, H.N.; El-Ghazzawi, I.; Mouneimne, Y.; Jammoul, A.; Maroun, R.G.; Louka, N. Study of the selectivity and bioactivity of polyphenols using infrared assisted extraction from apricot pomace compared to conventional methods. Antioxidants 2018, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Rampáčková, E.; Göttingerová, M.; Gála, P.; Kiss, T.; Ercişli, S.; Nečas, T. Evaluation of Protein and Antioxidant Content in Apricot Kernels as a Sustainable Additional Source of Nutrition. Sustainability 2021, 13, 4742. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Rather, M.A.; Anjum, N. To study the effect of apricot kernel flour (by-product) on physico-chemical, sensorial and antioxidant properties of biscuits. J. Curr. Res. Food Sci. 2022, 1, 16–22. [Google Scholar] [CrossRef]
- Huang, C.; Tang, X.; Liu, Z.; Huang, W.; Ye, Y. Enzymes-dependent antioxidant activity of sweet apricot kernel protein hydrolysates. LWT 2022, 154, 112825. [Google Scholar] [CrossRef]
- Chaouali, N.; Gana, I.; Dorra, A.; Khelifi, F.; Nouioui, A.; Masri, W.; Belwaer, I.; Ghorbel, H.; Hedhili, A. Potential Toxic Levels of Cyanide in Almonds (Prunus amygdalus), Apricot Kernels (Prunus armeniaca), and Almond Syrup. ISRN Toxicol. 2013, 2013, 610648. [Google Scholar] [CrossRef] [Green Version]
- Aybastier, Ö. Antioxidants and health. In Research & Reviews in Science and Mathematics-II; Birinci Basım: Aralık, Turkey, 2021; p. 1986. [Google Scholar]
- Fogarasi, M.; Socaciu, M.-I.; Sălăgean, C.-D.; Ranga, F.; Fărcaș, A.C.; Socaci, S.A.; Socaciu, C.; Țibulcă, D.; Fogarasi, S.; Semeniuc, C.A. Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania. Molecules 2021, 26, 7508. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Farcas, A.C.; Tofana, M.; Semeniuc, C.A. Bioactive Compounds and Volatile Profiles of Five Transylvanian Wild Edible Mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef] [Green Version]
- Yau, Y.K.; Ooi, C.W.; Ng, E.-P.; Lan, J.C.-W.; Ling, T.C.; Show, P.L. Current applications of different type of aqueous two-phase systems. Bioresour. Bioprocess. 2015, 2, 49. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef] [Green Version]
- Popa, A.L.; Jurcoane, S.; Dumitriu, B. Camelina sativa oil-a review. Sci. Bull. Ser. F Biotechnol. 2017, 21, 233–238. [Google Scholar]
- Gaya, P.; Peirotén, Á.; Landete, J.M. Transformation of plant isoflavones into bioactive isoflavones by lactic acid bacteria and bifidobacteria. J. Funct. Foods 2017, 39, 198–205. [Google Scholar] [CrossRef]
- Pavlović, N.; Vidović, S.; Vladić, J.; Popović, L.; Moslavac, T.; Jakobović, S.; Jokić, S. Recovery of Tocopherols, Amygdalin, and Fatty Acids From Apricot Kernel Oil: Cold Pressing Versus Supercritical Carbon Dioxide. Eur. J. Lipid Sci. Technol. 2018, 120, 1800043. [Google Scholar] [CrossRef]
- Bhanger, M.I.; Anwar, F.; Memon, N.; Qadir, R. Cold pressed apricot (Prunus armeniaca L.) kernel oil. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 725–730. [Google Scholar] [CrossRef]
- Tareen, A.K.; Panezai, M.A.; Sajjad, A.; Achakzai, J.K.; Kakar, A.M.; Khan, N.Y. Comparative analysis of antioxidant activity, toxicity, and mineral composition of kernel and pomace of apricot (Prunus armeniaca L.) grown in Balochistan, Pakistan. Saudi J. Biol. Sci. 2021, 28, 2830–2839. [Google Scholar] [CrossRef] [PubMed]
- Rombaut, N.; Chave, T.; Nikitenko, S.I.; El Maâtaoui, M.; Fabiano-Tixier, A.S.; Chemat, F. Modification of Olive Leaves’ Surface by Ultrasound Cavitation. Correlation with Polyphenol Extraction Enhancement. Appl. Sci. 2020, 11, 232. [Google Scholar] [CrossRef]
- Karaboğa, I.; Ovalı, M.A.; Yılmaz, A.; Alpaslan, M. Gastroprotective effect of apricot kernel oil in ethanol-induced gastric mucosal injury in rats. Biotech. Histochem. 2018, 93, 601–607. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Klepacka, J.; Bains, A.; Chawla, P.; Kumar, A.; Sharma, M.; Sridhar, K.; Gautam, S.P.; Kaushik, R. A Concise Review on Taro Mucilage: Extraction Techniques, Chemical Composition, Characterization, Applications, and Health Attributes. Polymers 2022, 14, 1163. [Google Scholar] [CrossRef] [PubMed]
- Alatabe, M.J.A.; Hameed, M.A.R.; Al-zobai, K.M.M. Exfoliate apricot kernels, natural low-cost bio-sorbent for rapid and efficient adsorption of CN-ions from aqueous solutions. Isotherm, kinetic and thermodynamic models. Int. J. Appl. Sci. Eng. 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Fangaj, E.; Ceyhan, A.A. Apricot Kernel shell waste treated with phosphoric acid used as a green, metal-free catalyst for hydrogen generation from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2020, 45, 17104–17117. [Google Scholar] [CrossRef]
- Martínez, J.; Rosas, J.; Pérez, J.; Saavedra, Z.; Carranza, V.; Alonso, P. Green approach to the extraction of major capsaicinoids from habanero pepper using near-infrared, microwave, ultrasound and Soxhlet methods, a comparative study. Nat. Prod. Res. 2019, 33, 447–452. [Google Scholar] [CrossRef]
- Ali, A.; Ali, S.; Yu, L.; Liu, H.; Khalid, S.; Hussain, A.; Qayum, M.M.N.; Ying, C. Preparation and characterization of starch-based composite films reinforced by apricot and walnut shells. J. Appl. Polym. Sci. 2019, 136, 47978. [Google Scholar] [CrossRef]
- Hyun, S.W.; Kim, J.; Park, B.; Jo, K.; Lee, T.G.; Kim, J.S.; Kim, C.S. Apricot kernel extract and amygdalin inhibit urban particulate matter-induced keratoconjunctivitis sicca. Molecules 2019, 24, 650. [Google Scholar] [CrossRef] [Green Version]
- Kopčeková, J.; Kolesárová, A.; Schwarzová, M.; Kováčik, A.; Mrázová, J.; Gažarová, M.; Lenártová, P.; Chlebo, P.; Kolesárová, A. Phytonutrients of Bitter Apricot Seeds Modulate Human Lipid Profile and LDL Subfractions in Adults with Elevated Cholesterol Levels. Int. J. Environ. Res. Public Health 2022, 19, 857. [Google Scholar] [CrossRef]
- Zhang, H.S.; Guo, P.H.; Zhang, Q.A.; Wu, D.D.; Zheng, H.R. Effects of saturated hot air pretreatment compared to traditional blanching on the physicochemical properties of Apricot (Prunus armeniaca L.) kernels and its skin during removing skin. LWT 2021, 150, 111947. [Google Scholar] [CrossRef]
- Ferrari, P.; Lamagna, L.; Revello, F.D. Thin Films Characterization and Metrology. In Silicon Sensors and Actuators; Springer: Cham, Switzerland, 2022; pp. 105–132. [Google Scholar] [CrossRef]
- Saadi, S.; Saari, N.; Ariffin, A.A.; Ghazali, H.M.; Hamid, A.A.; Abdulkarim, S.M.; Anwar, F.; Nacer, N.E. Novel emulsifiers and stabilizers from apricot (Prunus armeniaca L.): Their potential therapeutic targets and functional properties. Appl. Food Res. 2022, 2, 100085. [Google Scholar] [CrossRef]
- Stryjecka, M.; Kiełtyka-Dadasiewicz, A.; Michalak, M.; Rachoń, L.; Głowacka, A. Chemical composition and antioxidant properties of oils from the seeds of five apricot (Prunus armeniaca L.) cultivars. J. Oleo Sci. 2019, 68, 729–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhen, N.; Ben Rejeb, I.; Boukhris, H.; Damergi, C.; Gargouri, M. Physicochemical and sensory properties of wheat- Apricot kernels composite bread. LWT 2018, 95, 262–267. [Google Scholar] [CrossRef]
- Raj, V.; Mishra, A.K.; Mishra, A.; Khan, N.A. Hepatoprotective effect of Prunus armeniaca L. (Apricot) leaf extracts on Paracetamol induced liver damage in Wistar rats. Pharmacogn. J. 2016, 8, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Chhajed1, M.; Arora, S.; Thakur, G.; Gupta2, R. Medicinal Value of Apricot: A Review. Indian J. Pharm. Sci. 2018, 80, 790–794. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P. Profile of Phenolic Compounds of Prunus armeniaca L. Leaf Extract Determined by LC-ESI-QTOF-MS/MS and Their Antioxidant, Anti-Diabetic, Anti-Cholinesterase, and Anti-Inflammatory Potency. Antioxidants 2021, 10, 1869. [Google Scholar] [CrossRef]
- Seker, I.T.; Ozboy-Ozbas, O.; Gokbulut, I.; Ozturk, S.; Koksel, H.A.M.İ.T. Utilization of apricot kernel flour as fat replacer in cookies. J. Food Process. Preserv. 2010, 34, 15–26. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 60, 1738–1746. [Google Scholar] [CrossRef]
- Karatas, N. Evaluation of Nutritional Content in Wild Apricot Fruits for Sustainable Apricot Production. Sustainability 2022, 14, 1063. [Google Scholar] [CrossRef]
- Błachucki, W.; Czapla-Masztafiak, J.; Sá, J.; Szlachetko, J. A laboratory-based double X-ray spectrometer for simultaneous X-ray emission and X-ray absorption studies. J. Anal. At. Spectrom. 2019, 34, 1409–1415. [Google Scholar] [CrossRef] [Green Version]
- Chu, A.J. Quarter-Century Explorations of Bioactive Polyphenols: Diverse Health Benefits. Front. Biosci. 2022, 27, 134. [Google Scholar] [CrossRef] [PubMed]
- Saeed, I.; Guo, X.; Azeem, M.; Elshikh, M.S.; Zainab, B.; Ayaz, Z.; You, L.; Alwahibi, M.S.; Abbasi, A.M. Comparative assessment of polyphenolics’ content, free radicals’ scavenging and cellular antioxidant potential in apricot fruit. J. King Saud Univ. Sci. 2021, 33, 101459. [Google Scholar] [CrossRef]
- Górnaś, P.; Radziejewska-Kubzdela, E.; Mišina, I.; Biegańska-Marecik, R.; Grygier, A.; Rudzińska, M. Tocopher-ols, tocotrienols and carotenoids in kernel oils recovered from 15 apricot (Prunus armeniaca L.) geno-types. J. Am. Oil Chem. Soc. 2017, 94, 693–699. [Google Scholar] [CrossRef]
- Townsend, N.; Nichols, M.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe—Epidemiological update 2015. Eur. Heart J. 2015, 36, 2696–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Mele, M.C.; Raoul, P.; Cintoni, M.; Gasbarrini, A. Vitamin D and colorectal cancer: Chemopreventive perspectives through the gut microbiota and the immune system. BioFactors 2021, 48, 285–293. [Google Scholar] [CrossRef]
- Fatima, T.; Bashir, O.; Gani, G.; Bhat, T.; Jan, N. Nutritional and health benefits of apricots. Int. J. Unani Integr. Med. 2018, 2, 5–9. [Google Scholar] [CrossRef]
- Painuli, S.; Semwal, P.; Cruz-Martins, N.; Bachheti, R.K. Medicinal Plants of Himalayan Forests. In Non-Timber Forest Products; Springer: Cham, Switzerland, 2021; pp. 175–212. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Balint, B.; Jepchumba, V.K.; Guéant, J.L.; Guéant-Rodriguez, R.M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef]
- Viorica-Mirela, P.; Nicoleta, R.D.; Camelia, M.; Gabriela, D.D.; Constantin, M.; Alexandra, G. The possibilities of obtaining, characterizing and valorification of almond oil (Prunus amygdalus). J. Agroaliment Process Technol. 2013, 19, 455–458. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldızlı, G.; Coral, G.; Ayaz, F. Biochar as a Biocompatible Mild Anti-Inflammatory Supplement for Animal Feed and Agricultural Fields. Chem. Biodivers. 2021, 18, e2001002. [Google Scholar] [CrossRef] [PubMed]


| Composition | Amount/100 g | Reference |
|---|---|---|
| Protein content | 14.6–27.1 | [23,24] |
| Carbohydrate | 17.5–35.6 | [18,25] |
| Vitamin E | 0.003–0.040 | [26] |
| Vitamin B17 | 0.003–0.0058 | [27] |
| Mineral (Ca, Fe, P, Na, Mg, Cu, & Mn) | 0.0076, 0.0042, 0.0028, 0.0011, 0.003, 0.007, and 0.001 | [7,28] |
| Crude fiber | 11.85–13.6 | [24,29] |
| Crude fat Oleic acid | 2.1–3 54.1–61.91 | [20,30,31] [16] |
| Linolieic acid Palmatic acid Ash content | 25.13–35.81 1.58–2.27 1.3–2.23 | [16] [16] [30,32,33] |
| Moisture content | 27.4–38.8 | [34,35] |
| Hydro cyanide | 0.009–0.012 | [36,37] |
| Anthocyanin | 0.005–0.002 | [20,38] |
| Total phenol content Gallic acid | 0.036–0.072 2.1–4.1 | [39,40] [41] |
| Flavonoid content | 0.012–0.034 | [40,42] |
| Carotenoid content | 0.005–0.012 | [40] |
| Ascorbic acid Caffiec acid | 0.010–0.022 1.01–2.5 | [39] [41] |
| Extraction Method | Bioactive Compound | Yield g/100 g | Applications | References |
|---|---|---|---|---|
| Two-phase Cold pressing (at 40–120 °C) | Amygdalin | 90.37 | Application on recycling of amygdalin | [55] |
| Hot water treatment (at 160 °C) | Amygdalin | 0.129 | Application in Prevention of keratoconjunctivitis sicca diseases | [51] |
| Solvent extraction Acetone, acetyl chloroform, and ethanol. | Tocopherols | 73.4–94.4 | - | [52] |
| Cold pressing and SC-CO2 | Amygdalin and Tocopherols | 5.84–62.73 | Production of oils | [55] |
| Ultrasonic extraction | Phenolic compounds | 40.86–46.01 | Helps in enhancing the yield and maintaining the quality of the oils. | [52] |
| Freeze drying | TPC and TFC | 6–9 | - | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhone, M.A.; Bains, A.; Tosif, M.M.; Chawla, P.; Fogarasi, M.; Fogarasi, S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods 2022, 11, 2184. https://doi.org/10.3390/foods11152184
Akhone MA, Bains A, Tosif MM, Chawla P, Fogarasi M, Fogarasi S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods. 2022; 11(15):2184. https://doi.org/10.3390/foods11152184
Chicago/Turabian StyleAkhone, Mansoor Ali, Aarti Bains, Mansuri M. Tosif, Prince Chawla, Melinda Fogarasi, and Szabolcs Fogarasi. 2022. "Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes" Foods 11, no. 15: 2184. https://doi.org/10.3390/foods11152184






