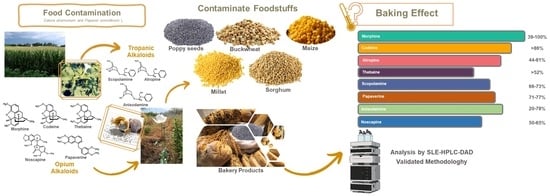
Evaluation of Thermal Degradation of Tropane and Opium Alkaloids in Gluten-Free Corn Breadsticks Samples Contaminated with Stramonium Seeds and Baked with Poppy Seeds under Different Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Poppy Seeds, Corn Flour, and Dough Contamination
2.3. Preparation of Breadsticks Samples
2.4. Analytical Methodology
2.4.1. Chromatographic Conditions
2.4.2. Extraction of TAs and OAs in Breadsticks
2.5. Method Validation
3. Results
3.1. Optimization of HPLC-DAD Analysis
3.2. Optimization of the Extraction of TAs and OAs in Breadsticks
3.3. Method Validation
3.4. Evaluation of Thermal Degradation of TAs and OAs in Baked Breadsticks
3.4.1. Reduction of Alkaloids Naturally Present in the Stramonium and Poppy Seeds
3.4.2. Reduction of Alkaloids in Samples Spiked with Standards
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Therdthai, N.; Hui, Y.H. Introduction to Baking and Bakery Products. In Bakery Products Science and Technology, 2nd ed.; Zhou, W., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA,, 2014; pp. 1–16. [Google Scholar]
- Collin, P.; Kaukinen, K.; Välimäki, M.; Salmi, J. Endocrinological disorders and celiac disease. Endocr. Rev. 2002, 23, 464–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, N.; Underhill, J. Morphologic Features Suggestive of Gluten Sensitivity in Architecturally Normal Duodenal Biopsy Specimens. Am. J. Clin. Pathol. 2001, 116, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, J.; Barriga, K.; Leung, D.; Hoffman, M.; Eisenbarth, G.; Rewers, M.; Norris, J. Timing of Initial Exposure to Cereal Grains and the Risk of Wheat Allergy. Pediatrics 2006, 117, 2175–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods 2022, 11, 407. [Google Scholar] [CrossRef]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review. Sci. Afr. 2020, 10, e00575. [Google Scholar] [CrossRef]
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current uses and future applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Kamal-Eldin, A.; Moazzami, A.; Washi, S. Sesame seed lignans: Potent physiological modulators and possible ingredients in functional foods & nutraceuticals. Recent Pat. Food Nutr. Agric. 2011, 3, 17–29. [Google Scholar] [CrossRef]
- Melo, D.; Machado, T.B.; Oliveira, M.; Beatriz, P.P. Chia seeds: An ancient grain trending in modern human diets. Food Funct. 2019, 10, 3068–3089. [Google Scholar] [CrossRef]
- Ferrús, M.A.; Barat, J.M.; Conchello, M.P.; Guix, S.; Palop, A.; Santos, J.A.; Herrera, A.; Martín de Santos, M.R.; y Martínez, A. Informe del Comité Científico de la Agencia Española de Consumo, Seguridad Alimentaria y Nutrición (AECOSAN) sobre los riesgos microbiológicos asociados al consumo de leche cruda y productos lácteos elaborados a base de leche cruda. Rev. Del Com. Científico De La AECOSAN 2015, 21, 45–78. Available online: http://www.aesan.gob.es/AECOSAN/docs/documentos/publicaciones/revistas_comite_cientifico/RCC_23.pdf (accessed on 3 May 2022).
- González-Gómez, L.; Gañán, J.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Sulfonic Acid-Functionalized SBA-15 as Strong Cation-Exchange Sorbent for Solid-Phase Extraction of Atropine and Scopolamine in Gluten-Free Grains and Flours. Foods 2020, 9, 1854. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain, (CONTAM) Scientific Opinion on Tropane alkaloids in food and feed. EFSA J. 2013, 11, 3386. [CrossRef]
- Mulder, P.P.J.; de Nijs, M.; Castellari, M.; Hortos, M.; MacDonald, S.; Crews, C.; Hajslova, J.; Stranska, M. Occurrence of tropane alkaloids in food. EFSA Supporting Publ. 2016, 13, 1140E. [Google Scholar] [CrossRef]
- Casado-Hidalgo, G.; Pérez-Quintanilla, D.; Morante-Zarcero, S.; Sierra, I. Mesostructured Silica-Coated Magnetic Nanoparticles to Extract Six Opium Alkaloids in Poppy Seeds Prior to Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry Analysis. Foods 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Acevska, J.; Stefkov, G.; Kulevanova, S.; Dimitrovska, A. Chapter 98—Assay for Opium Alkaloids. In Neuropathology of Drug Addictions and Substance Misuse; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 1051–1060. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2021/2142 of 3 December 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Opium Alkaloids in Certain Foodstuffs. Official Journal of the European Union, L 433/8. 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2142&from=EN- (accessed on 14 May 2022).
- Commission Regulation (EU) 2021/1408 of 27 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of tropane alkaloids in certain foodstuffs. Official Journal of the European Union, L 304/1. 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R1408&from=EN (accessed on 14 May 2022).
- Lachenmeier, D.W.; Sproll, C.; Musshoff, F. Poppy Seed Foods and Opiate Drug Testing-Where Are We Today? Ther. Drug Monit. 2010, 32, 11–18. [Google Scholar] [CrossRef]
- Shetge, S.A.; Dzakovich, M.P.; Cooperstone, J.L.; Kleinmeier, D.; Redan, B.W. Concentrations of the Opium Alkaloids Morphine, Codeine, and Thebaine in Poppy Seeds are Reduced after Thermal and Washing Treatments but are Not Affected when Incorporated in a Model Baked Product. J. Agric. Food Chem. 2020, 68, 5241–5248. [Google Scholar] [CrossRef]
- Sproll, C.; Perz, R.C.; Lachenmeier, D.W. Optimized LC/MS/MS Analysis of Morphine and Codeine in Poppy Seed and Evaluation of Their Fate during Food Processing as a Basis for Risk Analysis. J. Agric. Food Chem. 2006, 54, 5292–5298. [Google Scholar] [CrossRef]
- Marín-Sáez, J.; Romero-González, R.; Garrido Frenich, A. Degradation of tropane alkaloids in baked bread samples contaminated with Solanaceae seeds. Food Res. Int. 2019, 122, 585–592. [Google Scholar] [CrossRef]
- Marín-Sáez, J.; Romero-González, R.; Garrido Frenich, A. Effect of tea making and boiling processes on the degradation of tropane alkaloids in tea and pasta samples contaminated with Solanaceae seeds and coca leaf. Food Chem. 2019, 287, 265–272. [Google Scholar] [CrossRef]
- Guidance SANTE 11312/2021—Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed; Accredia: Rome, Italy, 2021; pp. 1–57. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 5 May 2022).
- Acevska, J.; Dimitrovska, A.; Stefkov, G.; Brezovska, K.; Karapandzova, M.; Kulevanova, S. Development and validation of a reversed-phase HPLC method for determination of alkaloids from Papaver somniferum L. (Papaveraceae). J. AOAC. Int. 2012, 95, 399–405. [Google Scholar] [CrossRef]
- Dams, R.; Benijts, T.; Lambert, W.E.; De Leenheer, A.P. Simultaneous determination of in total 17 opium alkaloids and opioids in blood and urine by fast liquid chromatography–diode-array detection–fluorescence detection, after solid-phase extraction. J. Chromatogr. B 2002, 773, 53–61. [Google Scholar] [CrossRef]
- Shen, Y.; van Beek, T.A.; Zuilhof, H.; Chen, B. Hyphenation of optimized microfluidic sample preparation with nano liquid chromatography for faster and greener alkaloid analysis. Anal. Chim. Acta 2013, 797, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Acevska, J.; Stefkov, G.; Petkovska, R.; Kulevanova, S.; Dimitrovska, A. Chemometric approach for development, optimization, and validation of different chromatographic methods for separation of opium alkaloids. Anal. Bioanal. Chem. 2012, 403, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Li, S.; He, C.; Li, K.; Liu, F. Extraction and determination of papaverin in pericarpium papaveris using aqueous two-phase system of poly(ethylene glycol)–(NH4)2SO4 coupled with high-performance liquid chromatography. Anal. Chim. Acta 2007, 590, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, X.; Wang, Y.; Lv, J. ‘Click’ preparation of a novel ‘native-phenylcarbamoylated’ bilayer cyclodextrin stationary phase for enhanced chiral differentiation. J. Chromatogr. A 2015, 1381, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zanolari, B.; Wolfender, J.-L.; Guilet, D.; Marston, A.; Queiroz, E.F.; Paulo, M.Q.; Hostettmann, K. On-line identification of tropane alkaloids from Erythroxylum vacciniifolium by liquid chromatography–UV detection–multiple mass spectrometry and liquid chromatography–nuclear magnetic resonance spectrometry. J. Chromatogr. A 2003, 1020, 75–89. [Google Scholar] [CrossRef]
- Dams, R.; Lambert, W.E.; Clauwaert, K.M.; De Leenheer, A.P. Comparison of phenyl-type columns in the development of a fast liquid chromatographic system for eighteen opiates commonly found in forensic toxicology. J. Chromatogr. A 2000, 896, 311–319. [Google Scholar] [CrossRef]
- Fernández, P.; Lago, M.; Lorenzo, R.A.; Carro, A.M.; Bermejo, A.M.; Tabernero, M.J. Optimization of a rapid microwave-assisted extraction method for the simultaneous determination of opiates, cocaine and their metabolites in human hair. J. Chromatogr. B 2009, 877, 1743–1750. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Bitar, Y.; Ebel, S.; Holzgrabe, U. Analysis of atropine, its degradation products and related substances of natural origin by means of reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2004, 1046, 115–120. [Google Scholar] [CrossRef]
- Bagheri, M.; Taheri, M.; Farhadpour, M.; Rezadoost, H.; Ghassempour, A.; Aboul-Enein, H.Y. Evaluation of hydrophilic interaction liquid chromatography stationary phases for analysis of opium alkaloids. J. Chromatogr. A 2017, 1511, 77–84. [Google Scholar] [CrossRef]
- Ahmadi-Jouibari, T.; Fattahi, N.; Shamsipur, M.; Pirsaheb, M. Dispersive liquid–liquid microextraction followed by high-performance liquid chromatography–ultraviolet detection to determination of opium alkaloids in human plasma. J. Pharm. Biomed. Anal. 2013, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.T.; Conlan, X.A.; Theakstone, A.G.; Purcell, S.D.; Barnett, N.W.; Smith, Z.M. Extraction and Determination of Morphine Present on the Surface of Australian Food Grade Poppy Seeds Using Acidic Potassium Permanganate Chemiluminescence Detection. Food Anal. Methods 2020, 13, 1159–1165. [Google Scholar] [CrossRef]
- Powers, D.; Erickson, S.; Swortwood, M.J. Quantification of Morphine, Codeine, and Thebaine in Home-Brewed Poppy Seed Tea by LC-MS/MS. J. Forensic Sci. 2018, 63, 1229–1235. [Google Scholar] [CrossRef]
- López, P.; Pereboom-de Fauw, D.P.K.H.; Mulder, P.P.J.; Spanjer, M.; de Stoppelaar, J.; Mol, H.G.J.; de Nijs, M. Straightforward analytical method to determine opium alkaloids in poppy seeds and bakery products. Food Chem. 2018, 242, 443–450. [Google Scholar] [CrossRef]
- Carlin, M.G.; Dean, J.R.; Ames, J.M. Opium Alkaloids in Harvested and Thermally Processed Poppy Seeds. Front. Chem. 2020, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Romera-Torres, A.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Simultaneous analysis of tropane alkaloids in teas and herbal teas by liquid chromatography coupled to high-resolution mass spectrometry (Orbitrap). J. Sep. Sci. 2018, 41, 1938–1946. [Google Scholar] [CrossRef]
- Cirlini, M.; Demuth, T.M.; Biancardi, A.; Rychlik, M.; Dall’Asta, C.; Bruni, R. Are tropane alkaloids present in organic foods? Detection of scopolamine and atropine in organic buckwheat (Fagopyron esculentum L.) products by UHPLC–MS/MS. Food Chem. 2018, 239, 141–147. [Google Scholar] [CrossRef]
- Caligiani, A.; Palla, G.; Bonzanini, F.; Bianchi, A.; Bruni, R. A validated GC–MS method for the detection of tropane alkaloids in buckwheat (Fagopyron esculentum L.) fruits, flours and commercial foods. Food Chem. 2011, 127, 204–209. [Google Scholar] [CrossRef]
- Kaltner, F. Fate of Food-Relevant Toxic Plant Alkaloids during Food Processing or Storing and Analytical Strategies to Unveil Potential Transformation Products. J. Agric. Food Chem. 2022, 70, 5975–5981. [Google Scholar] [CrossRef]
- Casado-Hidalgo, G.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Opium alkaloids in food products: Current and future perspectives. Trends Food Sci. Technol. 2021, 108, 92–102. [Google Scholar] [CrossRef]
- Kleinmeier, D.; Pettengill, E.; Redan, B.W. Commentary: Opium Alkaloids in Harvested and Thermally Processed Poppy Seeds. Front. Chem. 2021, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, M.; Golombek, P.; Lachenmeier, D.W. Reduction of Morphine During Baking? Response: Commentary: Opium Alkaloids in Harvested and Thermally Processed Poppy Seeds. Front. Chem. 2021, 9, 692045. [Google Scholar] [CrossRef] [PubMed]



| Samples | Corn Flour Amount (g) | Stramonium Seed Powder (g) | TAs h Added to the Dough (µg) | Poppy Seeds into Dough (g) | Poppy Seed on the Surface (g) | OAs i Added to the Seeds (µg) | OAs i Added to the Dough (µg) |
|---|---|---|---|---|---|---|---|
| Breadsticks 1 a | 100 | 0.1 | - | 5 | - | - | - |
| Breadsticks 2 b | 100 | 0.1 | - | 10 | - | - | - |
| Breadsticks 3 c | 100 | 1 | - | 5 | - | - | - |
| Breadsticks 4 d | 100 | 1 | - | 10 | - | - | - |
| Breadsticks 5 e | 100 | 0.1 | - | - | 5 | 60 | - |
| Breadsticks 6 f | 100 | 0.1 | - | 5 | - | 60 | - |
| Breadsticks 7 g | 100 | 0.1 | 190 | 5 | - | - | 60 |
| Accuracy c | Precision c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Linear Range | Matrix Calibration (mg/L), R2 | MDL a | MQL a | Matrix Effect (%) b | Recovery (% ± SD) | Mean Recovery (% ± SD) | Intra-Day Presicion (RSD%) | Inter-Day Presicion (RSD%) | |||
| mg/L | mg/kg | mg/L | mg/kg | mg/L | mg/kg | |||||||
| Morphine | 0.2–6.0 | 1.6–48 | y = 318.55x − 2.7892 0.999 | 0.05 | 0.40 | 0.17 | 1.36 | 96 | 95 ± 2 a | 101 ± 4 | 2 a | 7 a |
| 102 ± 1 b | 1 b | 1 b | ||||||||||
| 107 ± 11 c | 10 c | 6 c | ||||||||||
| Codeine | 0.2–3.0 | 1.6–24 | y = 388.9x + 0.4358 0.9996 | 0.06 | 0.48 | 0.21 | 1.68 | 95 | 97 ± 5 a | 103 ± 6 | 6 a | 5 a |
| 103 ± 1 b | 1 b | 2 b | ||||||||||
| 109 ± 12 c | 11 c | 7 c | ||||||||||
| Anisodamine-1 | 0.6–6.0 | 4.8–48 | y = 68.925x − 3.7223 0.9999 | 0.18 | 1.44 | 0.60 | 4.80 | 109 | 99 ± 2 a | 98 ± 4 | 2 a | 4 a |
| 95 ± 2 b | 2 b | 3 b | ||||||||||
| 101 ± 8 c | 8 c | 2 c | ||||||||||
| Scopolamine | 0.2–6.0 | 1.6–48 | y = 78.934x − 6.926 0.9999 | 0.07 | 0.56 | 0.20 | 1.60 | 101 | 99 ± 5 a | 104 ± 5 | 5 a | 5 a |
| 113 ± 5 b | 4 b | 5 b | ||||||||||
| 102 ± 7 c | 7 c | 2 c | ||||||||||
| Anisodamine-2 | 1.0–6.0 | 8–48 | y = 45.16x + 2.1179 0.9997 | 0.31 | 2.48 | 1.02 | 8.16 | 98 | 111 ± 2 a | 110 ± 8 | 2 a | 6 a |
| 101 ± 1 b | 1 b | 1 b | ||||||||||
| 118 ± 21 c | 18 c | 11 c | ||||||||||
| Atropine | 1.0–10 | 8–80 | y = 105.07x − 6.4501 0.9991 | 0.32 | 2.56 | 1.00 | 8.00 | 88 | 97 ± 2 a | 100 ± 2 | 2 a | 2 a |
| 105 ± 1 b | 1 b | 3 b | ||||||||||
| 100 ± 4 c | 4 c | 2 c | ||||||||||
| Thebaine | 0.2–6.0 | 1.6–48 | y = 270.86x + 5.9803 (0.9999) | 0.05 | 0.45 | 0.18 | 1.49 | 85 | 87 ± 11 a | 96 ± 5 | 11 a | 10 a |
| 106 ± 3 b | 2 b | 6 b | ||||||||||
| 97 ± 3 c | 3 c | 1 c | ||||||||||
| Papaverine | 0.2–6.0 | 1.6–48 | y = 506.88x − 8.0069 (0.9997) | 0.04 | 0.37 | 0.15 | 1.25 | 80 | 98 ± 14 a | 101 ± 6 | 15 a | 7 a |
| 108 ± 4 b | 4 b | 7 b | ||||||||||
| 98 ± 2 c | 2 c | 2 c | ||||||||||
| Noscapine | 0.2–6.0 | 1.6–48 | y = 634.82x − 68.207 (0.999) | 0.03 | 0.30 | 0.12 | 1.01 | 82 | 90 ± 14 a | 100 ± 7 | 16 a | 16 a |
| 108 ± 1 b | 1 b | 4 b | ||||||||||
| 103 ± 6 c | 6 c | 2 c | ||||||||||
| Analyte | Breadsticks 1 | Breadsticks 2 | Breadsticks 3 | Breadsticks 4 | ||||
|---|---|---|---|---|---|---|---|---|
| mg/100 g d.w. | Degradation (%) | mg/100 g d.w. | Degradation (%) | mg/100 g d.w. | Degradation (%) | mg/100 g d.w. | Degradation (%) | |
| Mor b | 0.012–0.014 | 0–11 | 0.020–0.026 | 0–27 | 0.016–0.036 | 0–27 | 0.025–0.031 | 0–12 |
| Atro c | 0.12 ± 0.01 | 20 ± 6 | 0.14 ± 0.01 | 7 ± 4 | 0.51 ± 0.02 | 64 ± 1 | 0.51 ± 0.02 | 65 ± 1 |
| Analyte | Breadsticks 5 | Breadsticks 6 | Breadsticks 7 | |||
|---|---|---|---|---|---|---|
| mg/100 g d.w. | Degradation (%) | mg/100 g d.w. | Degradation (%) | mg/100 g d.w. | Degradation (%) | |
| Morphine | ND b | 100 | 0.003–0.006 | 0–81 | 0.003–0.007 | 0–77 |
| Codeine | ND | 100 | 0.0001 ± 0.0005 | >88 | 0.004 ± 0.001 | >88 |
| Thebaine | 0.004 ± 0.002 | >58 | 0.004 ± 0.002 | >58 | 0.003 ± 0.001 | >58 |
| Papaverine | 0.020 ± 0.001 | 55 ± 2 | 0.0209 ± 0.0004 | 53 ± 1 | 0.023 ± 0.0004 | 49 ± 1 |
| Noscapine | 0.035 ± 0.001 | 23 ± 2 | 0.034 ± 0.003 | 24 ± 7 | 0.039 ± 0.001 | 14 ± 1 |
| Atropine | 0.093 ± 0.002 | 35 ± 1 | 0.09 ± 0.01 | 34 ± 9 | 0.11 ± 0.02 | 32 ± 4 |
| Scopolamine | NP c | NP | NP | NP | 0.08 ± 0.01 | 45 ± 6 |
| Anisodamine-1 | NP | NP | NP | NP | 0.14 ± 0.05 | 35 ± 20 |
| Anisodamine-2 | NP | NP | NP | NP | 0.17 ± 0.08 | 49 ± 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Baquero, F.L.; Morante-Zarcero, S.; Sierra, I. Evaluation of Thermal Degradation of Tropane and Opium Alkaloids in Gluten-Free Corn Breadsticks Samples Contaminated with Stramonium Seeds and Baked with Poppy Seeds under Different Conditions. Foods 2022, 11, 2196. https://doi.org/10.3390/foods11152196
Vera-Baquero FL, Morante-Zarcero S, Sierra I. Evaluation of Thermal Degradation of Tropane and Opium Alkaloids in Gluten-Free Corn Breadsticks Samples Contaminated with Stramonium Seeds and Baked with Poppy Seeds under Different Conditions. Foods. 2022; 11(15):2196. https://doi.org/10.3390/foods11152196
Chicago/Turabian StyleVera-Baquero, Fernando L., Sonia Morante-Zarcero, and Isabel Sierra. 2022. "Evaluation of Thermal Degradation of Tropane and Opium Alkaloids in Gluten-Free Corn Breadsticks Samples Contaminated with Stramonium Seeds and Baked with Poppy Seeds under Different Conditions" Foods 11, no. 15: 2196. https://doi.org/10.3390/foods11152196