Optimization of Corn Resistant Starch Preparation by Dual Enzymatic Modification Using Response Surface Methodology and Its Physicochemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of RS
2.2.2. Determination of RS
2.2.3. Single-Factor Experiment
2.2.4. Experimental Design and Statistical Analysis
2.2.5. Detection of the Average Degree of Polymerization (DP) of Starch
2.2.6. Observation of Particle Morphology
2.2.7. Determination of Thermal Characteristics
2.3. Statistical Analysis
3. Results
3.1. Effect of Modification Parameters
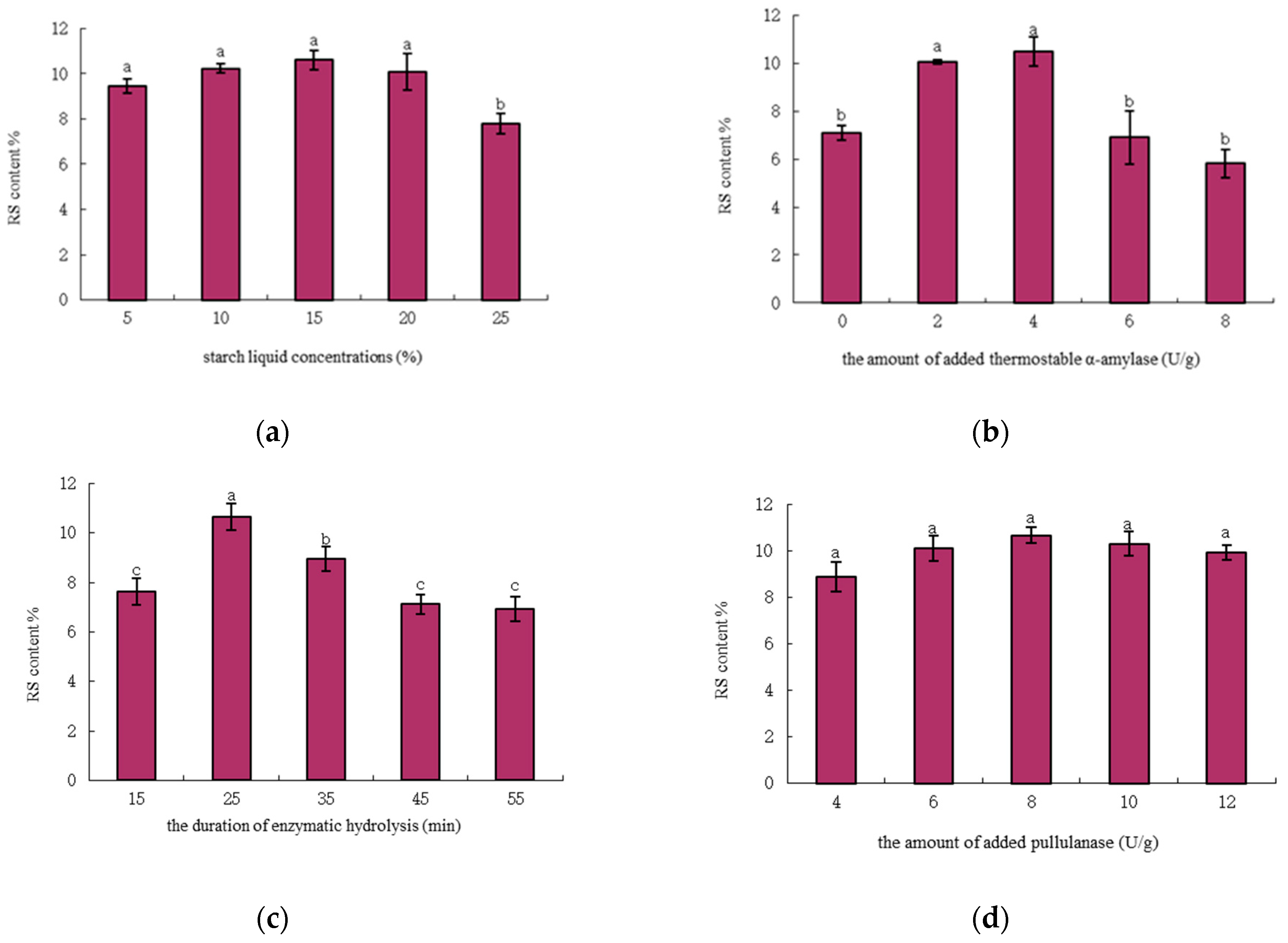
3.1.1. Effect of Starch Liquid Concentration
3.1.2. Effect of the Amount of Added Thermostable α-Amylase
3.1.3. Effect of the duration of Enzymatic (Thermostable α-Amylase) Hydrolysis
3.1.4. Effect of the Amount of Added Pullulanase
3.2. Response Surface Analysis
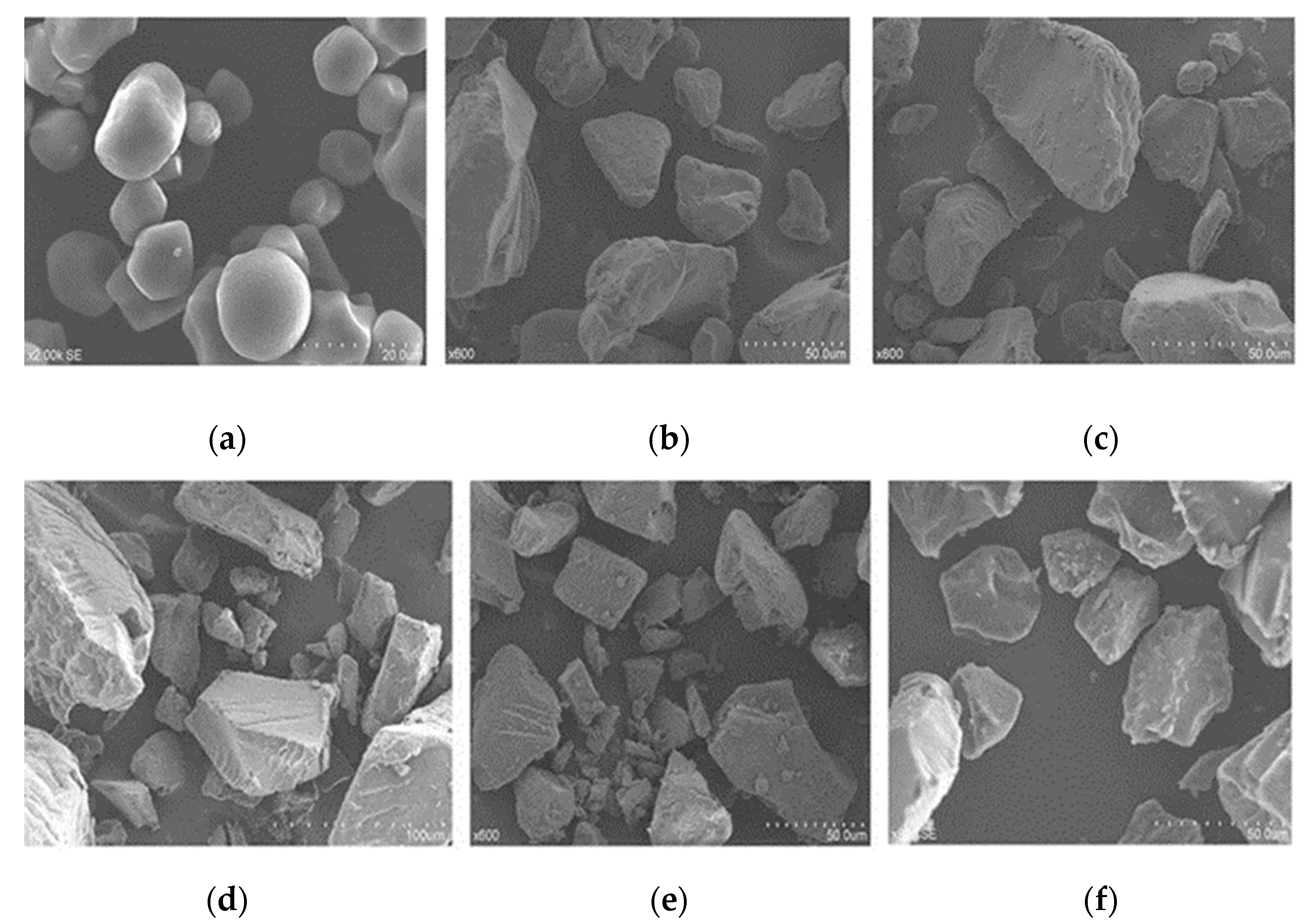
3.3. Morphological Properties
3.4. Thermal Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, T.M.; Hasnain, A. Morphological, physicochemical, and pasting properties of modified white sorghum (Sorghum bicolor) starch. Int. J. Food Prop. 2014, 17, 523–535. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sanchez-Zapata, E.; Perez-Alvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- von Borries-Medrano, E.; Jaime-Fonseca, M.R.; Aguilar-Méndez, M.A.; García-Cruz, H.I. Addition of galactomannans and citric acid in corn starch processed by extrusion: Retrogradation and resistant starch studies. Food Hydrocoll. 2018, 83, 485–496. [Google Scholar] [CrossRef]
- Sjoo, M.; Nilsson, L. Starch in Food-Structure, Function and Applications, 4th ed.; Woodhead Publishing: Sawston, UK, 2017; Chapter 11. [Google Scholar]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2014, 95, 1968–1978. [Google Scholar] [CrossRef]
- Raatz, S.K.; Idso, L.; Johnson, L.K.; Jackson, M.I.; Combs, G.F. Resistant starch analysis of commonly consumed potatoes: Content varies by cooking method and service temperature but not by variety. Food Chem. 2016, 208, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Miao, M.; Jiang, H.; Xue, J.; Jiang, B.; Zhang, T.; Gao, Y.; Jia, Y. Partial branching enzyme treatment increases the low glycaemic property and α-1,6 branching ratio of maize starch. Food Chem. 2014, 164, 502–509. [Google Scholar] [CrossRef]
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of treatment methods on the formation of resistant starch in purple sweet potato. Food Chem. 2021, 367, 130580. [Google Scholar] [CrossRef]
- Reddy, C.K.; Suriya, M.; Haripriya, S. Physico-chemical and functional properties of Resistant starch prepared from red kidney beans (Phaseolus vulgaris L.) starch by enzymatic method. Carbohydr. Polym. 2013, 95, 220–226. [Google Scholar] [CrossRef]
- Das, M.; Rajan, N.; Biswas, P.; Banerjee, R. Dual enzyme treatment strategy for enhancing resistant starch content of green banana flour and in vitro evaluation of prebiotic effect. LWT 2022, 160, 113267. [Google Scholar] [CrossRef]
- Simons, C.W.; Hall, C.; Vatansever, S. Production of resistant starch (RS3) from edible bean starches. J. Food Process. Preserv. 2018, 42, e13587. [Google Scholar] [CrossRef]
- Zou, W.; Schulz, B.L.; Tan, X.; Sissons, M.; Warren, F.J.; Gidley, M.J.; Gilbert, R.G. The role of thermostable proteinaceous α-amylase inhibitors in slowing starch digestion in pasta. Food Hydrocoll. 2019, 90, 241–247. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, H.I.; Moon, T.W.; Choi, S.J. Branch chain elongation by amylosucrase: Production of waxy corn starch with a slow digestion property. Food Chem. 2014, 152, 113–120. [Google Scholar] [CrossRef]
- Liu, G.; Hong, Y.; Gu, Z.; Li, Z.; Cheng, L. Pullulanase hydrolysis behaviors and hydrogel properties of debranched starches from different sources. Food Hydrocoll. 2015, 45, 351–360. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Y.; Yu, S.; Gao, Q. Preparation and properties of RS III from waxy maize starch with pullulanase. Food Hydrocoll. 2013, 33, 19–25. [Google Scholar] [CrossRef]
- Ao, Z.; Simsek, S.; Zhang, G.; Venkatachalam, M.; Reuhs, B.L.; Hamaker, B.R. Starch with a Slow Digestion Property Produced by Altering Its Chain Length, Branch Density, and Crystalline Structure. J. Agric. Food Chem. 2007, 55, 4540–4547. [Google Scholar] [CrossRef]
- Jo, A.R.; Kim, H.R.; Choi, S.J.; Lee, J.S.; Chung, M.N.; Han, S.K.; Park, C.-S.; Moon, T.W. Preparation of slowly digestible sweet potato Daeyumi starch by dual enzyme modification. Carbohydr. Polym. 2016, 143, 164–171. [Google Scholar] [CrossRef]
- Cai, L.; Shi, Y.-C. Preparation, structure, and digestibility of crystalline A- and B-type aggregates from debranched waxy starches. Carbohydr. Polym. 2014, 105, 341–350. [Google Scholar] [CrossRef]
- Arns, B.; Bartz, J.; Radunz, M.; do Evangelho, J.A.; Pinto, V.Z.; da Rosa Zavareze, E.; Dias, A.R.G. Impact of heat-moisture treatment on rice starch, applied directly in grain paddy rice or in isolated starch. LWT 2015, 60, 708–713. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lan, T.; Lei, Y.; Suo, J.; Zhao, Q.; Wang, H.; Lei, J.; Sun, X.; Ma, T. Optimization of ultrasonic-assisted enzymatic extraction of kiwi starch and evaluation of its structural, physicochemical, and functional characteristics. Ultrason. Sonochem. 2021, 81, 105866. [Google Scholar] [CrossRef]
- Kaur, B.; Venkatrao, K.B.; Panesar, P.S.; Chopra, H.K.; Anal, A.K. Optimization of ultrasound-assisted enzymatic extraction of resistant starch from green banana peels and its structural characterization. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Kahraman, K.; Koksel, H.; Ng, P.K. Optimisation of the reaction conditions for the production of cross-linked starch with high resistant starch content. Food Chem. 2015, 174, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, X.; Jin, Z. Fermentation characteristics of resistant starch from maize prepared by the enzymatic method in vitro. Int. J. Biol. Macromol. 2012, 51, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- AACC. Approved methods of the American Association of Cereal Chemists, 10th ed.; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Anderson-Cook, C.M. Responce surface methodology: Process and product optimization using designed experiments by Raymond H. Myers; Douglas C. Montgomery. J. Am. Stat. Assoc. 2002, 97, 293–300. [Google Scholar] [CrossRef]
- Lu, C.-H.; Engelmann, N.J.; Lila, M.A.; Erdman, J.J.W. Optimization of Lycopene Extraction from Tomato Cell Suspension Culture by Response Surface Methodology. J. Agric. Food Chem. 2008, 56, 7710–7714. [Google Scholar] [CrossRef] [Green Version]
- Hizukuri, S.; Takeda, Y.; Yasuda, M.; Suzuki, A. Multi-branched nature of amylose and the action of debranching enzymes. Carbohydr. Res. 1981, 94, 205–213. [Google Scholar] [CrossRef]
- Gong, B.; Xu, M.; Li, B.; Wu, H.; Liu, Y.; Zhang, G.; Ouyang, S.; Li, W. Repeated heat-moisture treatment exhibits superiorities in modification of structural, physicochemical and digestibility properties of red adzuki bean starch compared to continuous heat-moisture way. Food Res. Int. 2017, 102, 776–784. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Yang, Y.; Bian, S.; Zhou, X.; Zhu, K.; Xu, L.; Jin, Z.; Jiao, A. Effects of extrusion and enzymatic debranching on the structural characteristics and digestibility of corn and potato starches. Food Biosci. 2022, 47, 101679. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Z. Preparation of products rich in resistant starch from maize starch by an enzymatic method. Carbohydr. Polym. 2011, 86, 1610–1614. [Google Scholar] [CrossRef]
- Doblado-Maldonado, A.F.; Janssen, F.; Gomand, S.V.; De Ketelaere, B.; Goderis, B.; Delcour, J.A. A response surface analysis of the aqueous leaching of amylose from maize starch. Food Hydrocoll. 2017, 63, 265–272. [Google Scholar] [CrossRef]
- Miao, M.; Xiong, S.; Jiang, B.; Jiang, H.; Cui, S.W.; Zhang, T. Dual-enzymatic modification of maize starch for increasing slow digestion property. Food Hydrocoll. 2014, 38, 180–185. [Google Scholar] [CrossRef]
- Shi, J.; Sweedman, M.C.; Shi, Y.-C. Structural changes and digestibility of waxy maize starch debranched by different levels of pullulanase. Carbohydr. Polym. 2018, 194, 350–356. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, W.; Yu, Y.; Zhang, G.; Guo, W. Preparation and structure characterization of linear long-chain dextrin obtained from pullulanase debranching of cassava starch. Starch-Starke 2015, 67, 884–891. [Google Scholar] [CrossRef]
- Bartz, J.; Zavareze, E.D.R.; Dias, A.R.G. Study of heat-moisture treatment of potato starch granules by chemical surface gelatinization. J. Sci. Food Agric. 2017, 97, 3114–3123. [Google Scholar] [CrossRef]
- Liu, W.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C. In structure and in - vitro digestibility of waxy corn starch debranched by pullulanase. Food Hydrocoll. 2017, 67, 104–110. [Google Scholar] [CrossRef]
- Tan, X.; Li, X.; Chen, L.; Xie, F.; Li, L.; Huang, J. Effect of heat-moisture treatment on multi-scale structures and physicochemical properties of breadfruit starch. Carbohydr. Polym. 2017, 161, 286–294. [Google Scholar] [CrossRef]
- Mutungi, C.; Rost, F.; Onyango, C.; Jaros, D.; Rohm, H. Crystallinity, thermal and morphological characteristics of resistant starch type III produced by hydrothermal treatment of debranched cassava starch. Starch-Starke 2009, 61, 634–645. [Google Scholar] [CrossRef]
- Doan, H.X.N.; Song, Y.; Lee, S.; Lee, B.-H.; Yoo, S.-H. Characterization of rice starch gels reinforced with enzymatically-produced resistant starch. Food Hydrocoll. 2019, 91, 76–82. [Google Scholar] [CrossRef]
| Amount of Added Thermostable α-Amylase U/g | DP | RS Content % |
|---|---|---|
| native corn starch | 298 ± 5.56 a | 1.14 ± 0.09 d |
| 2 | 157 ± 7.58 b | 10.06 ± 0.10 a |
| 4 | 90 ± 5.90 c | 10.52 ± 0.31 a |
| 6 | 56 ± 7.07 d | 6.91 ± 0.89 b |
| 8 | 24 ± 4.61 e | 5.82 ± 0.48 c |
| No. | Concentration of Starch Liquid (%)X1 | Amount of Added Thermostable α-Amylase (U/g) X2 | Duration of ENZYMATIC hydrolysis (min) X3 | Amount of Added Pullulanase (U/g) X4 | RS Content % |
|---|---|---|---|---|---|
| 1 | 5 | 2 | 15 | 4 | 7.42 |
| 2 | 5 | 4 | 15 | 4 | 6.52 |
| 3 | 25 | 2 | 15 | 4 | 6.06 |
| 4 | 25 | 4 | 15 | 4 | 5.51 |
| 5 | 5 | 2 | 55 | 4 | 6.92 |
| 6 | 5 | 4 | 55 | 4 | 6.67 |
| 7 | 25 | 2 | 55 | 4 | 5.97 |
| 8 | 25 | 4 | 55 | 4 | 5.02 |
| 9 | 5 | 2 | 15 | 12 | 7.77 |
| 10 | 5 | 4 | 15 | 12 | 7.24 |
| 11 | 25 | 2 | 15 | 12 | 6.64 |
| 12 | 25 | 4 | 15 | 12 | 6.07 |
| 13 | 5 | 2 | 55 | 12 | 6.97 |
| 14 | 5 | 4 | 55 | 12 | 7.02 |
| 15 | 25 | 2 | 55 | 12 | 6.07 |
| 16 | 25 | 4 | 55 | 12 | 6.67 |
| 17 | 15 | 1 | 35 | 8 | 7.89 |
| 18 | 15 | 5 | 35 | 8 | 6.71 |
| 19 | 0 | 3 | 35 | 8 | 8.22 |
| 20 | 35 | 3 | 35 | 8 | 6.78 |
| 21 | 15 | 3 | 75 | 8 | 5.53 |
| 22 | 15 | 3 | 35 | 0 | 7.84 |
| 23 | 15 | 3 | 35 | 16 | 9.66 |
| 24 | 15 | 3 | 35 | 8 | 10.62 |
| 25 | 15 | 3 | 35 | 8 | 10.75 |
| 26 | 15 | 3 | 35 | 8 | 10.51 |
| 27 | 15 | 3 | 35 | 8 | 10.63 |
| 28 | 15 | 3 | 35 | 8 | 10.42 |
| 29 | 15 | 3 | 35 | 8 | 10.68 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value prob > F |
|---|---|---|---|---|---|
| Model | 86.5004 | 14 | 6.1786 | 21.18 | <0.001 |
| X1 | 3.6038 | 1 | 3.6038 | 12.36 | 0.003 |
| X2 | 11.0703 | 1 | 11.0703 | 37.95 | <0.001 |
| X3 | 10.2196 | 1 | 10.2196 | 35.04 | <0.001 |
| X4 | 2.4574 | 1 | 2.4574 | 8.42 | 0.012 |
| X12 | 19.1035 | 1 | 19.1035 | 65.49 | <0.001 |
| X22 | 21.7944 | 1 | 21.7944 | 74.72 | <0.001 |
| X32 | 31.4824 | 1 | 31.4824 | 107.93 | <0.001 |
| X42 | 7.7561 | 1 | 7.7561 | 26.59 | <0.001 |
| X1X2 | 0.0016 | 1 | 0.0016 | 0.01 | 0.942 |
| X1X3 | 0.0420 | 1 | 0.0420 | 0.14 | 0.710 |
| X1X4 | 0.1260 | 1 | 0.1260 | 0.43 | 0.522 |
| X2X3 | 0.2500 | 1 | 0.2500 | 0.86 | 0.370 |
| X2X4 | 0.3025 | 1 | 0.3025 | 1.04 | 0.326 |
| X3X4 | 0.0002 | 1 | 0.0002 | 0.00 | 0.978 |
| Residual | 4.0836 | 14 | 0.2917 | ||
| Cor Total | 90.5840 | 28 |
| Treatment | RS Content (%) | T0 (°C) | TP (°C) | TC (°C) | △H (J/g) |
|---|---|---|---|---|---|
| Native corn starch | 1.14 ± 0.09 d | 57.18 ± 0.17 e | 61.55 ± 0.15 e | 66.60 ± 0.11 e | 5.22 ± 0.09 e |
| Gelatinized corn starch #1 | 4.38 ± 0.11 c | 63.59 ± 0.11 d | 68.36 ± 0.21 d | 75.53 ± 0.21 d | 6.71 ± 0.10 d |
| Dual enzyme-treated #2 | 7.66 ± 0.53 b | 102.63 ± 0.21 b | 104.43 0.17 b | 106.09 0.25 b | 14.68 ± 0.08 c |
| Dual enzyme-treated #3 | 10.52 ± 0.31 a | 135.66 ± 0.24 a | 135.76 ± 0.24 a | 143.01 ± 0.19 a | 19.11 ± 0.11 a |
| Pullulanase-treated #4 | 7.18 ± 0.31 b | 76.3 ± 0.21 c | 91.01 ± 0.32 c | 100.57 ± 0.26 c | 15.81 ± 0.21 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, F.; Du, C.; Li, M.; Leng, Z.; Yu, X.; Du, S.-K. Optimization of Corn Resistant Starch Preparation by Dual Enzymatic Modification Using Response Surface Methodology and Its Physicochemical Characterization. Foods 2022, 11, 2223. https://doi.org/10.3390/foods11152223
Liu Y, Jiang F, Du C, Li M, Leng Z, Yu X, Du S-K. Optimization of Corn Resistant Starch Preparation by Dual Enzymatic Modification Using Response Surface Methodology and Its Physicochemical Characterization. Foods. 2022; 11(15):2223. https://doi.org/10.3390/foods11152223
Chicago/Turabian StyleLiu, Yangjin, Fan Jiang, Chunwei Du, Mengqing Li, Zhifu Leng, Xiuzhu Yu, and Shuang-Kui Du. 2022. "Optimization of Corn Resistant Starch Preparation by Dual Enzymatic Modification Using Response Surface Methodology and Its Physicochemical Characterization" Foods 11, no. 15: 2223. https://doi.org/10.3390/foods11152223
APA StyleLiu, Y., Jiang, F., Du, C., Li, M., Leng, Z., Yu, X., & Du, S.-K. (2022). Optimization of Corn Resistant Starch Preparation by Dual Enzymatic Modification Using Response Surface Methodology and Its Physicochemical Characterization. Foods, 11(15), 2223. https://doi.org/10.3390/foods11152223






