Investigating Six Common Pesticides Residues and Dietary Risk Assessment of Romanian Wine Varieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. QuEChERS Extraction
2.3. Equipment and Instrumental Parameters
2.4. Samples
2.5. Risk Assessment
Pesticide Residue Intake Model (PRIMo) Calculations
2.6. Statistical Analyses
3. Results
3.1. Method Validation
3.2. Screening of Pesticide Residues in Wine Samples
3.3. Pesticide Residues in the Wines
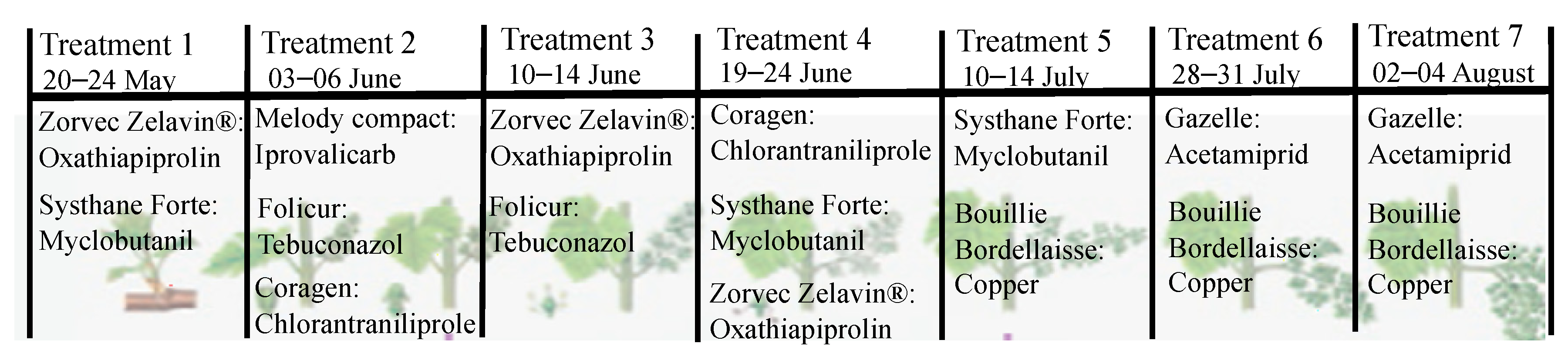
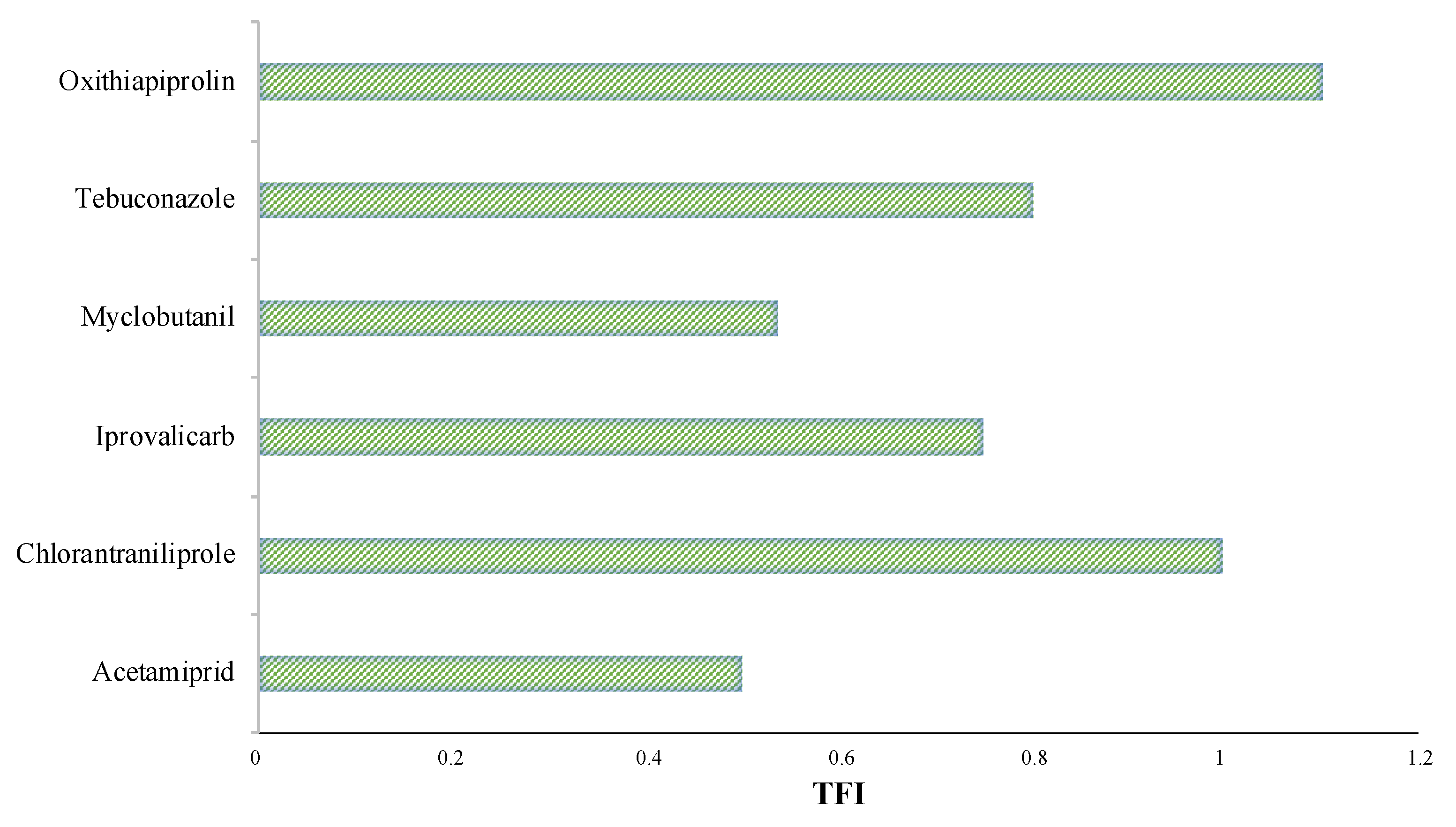
3.4. The Treatment Frequency Index (TFI)
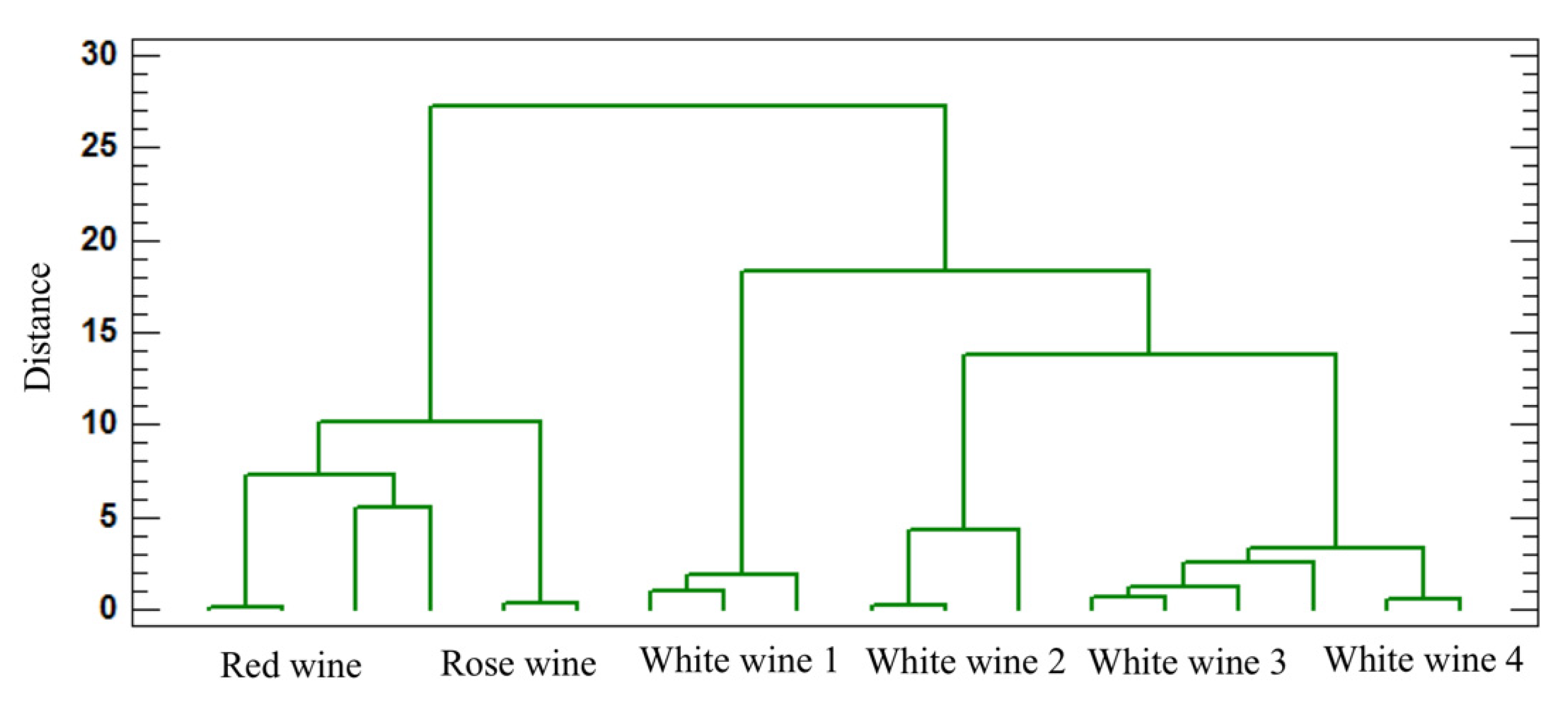
3.5. Statistical Analysis
3.6. Risk Assessment
3.7. Chronic (Long-Term) Risk Assessment
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organisation Internationale de la Vigne et du Vin. 2022. Available online: https://www.oiv.int/js/lib/pdfjs/web/viewer.html?file=/public/medias/8767/acitvityreporteng.pdf (accessed on 24 April 2022).
- Dumitriu, D.; Peinado, R.A.; Peinado, J.; de Lerma, N.L. Grape pomace extract improves the in vitro and in vivo antioxidant properties of wines from sun light dried Pedro Ximénez grapes. J. Funct. Foods 2015, 17, 380–387. [Google Scholar] [CrossRef]
- Fermaud, M.; Smits, N.; Merot, A.; Roudet, J.; Thiery, D.; Wery, J.; Delbac, L. New multipest damage indicator to assess protection strategies in grapevine cropping systems: An indicator of multipest damage in grapevine. Aust. J. Grape Wine Res. 2016, 22, 450–461. [Google Scholar] [CrossRef]
- Simonovici, M. Enquete Pratiques phytosanitaires en viticulture en 2016. Nombre de traitements et indicateurs de fréquence de traitement. Agreste Les Dossiers; 2019; 2. Available online: https://www.agreste.agriculture.gouv.fr/agreste-web/download/publication/publie/Dos1902/Dossier2019-2.pdf (accessed on 20 June 2022). (In French)
- Jermini, M.; Blaise, P.; Gessler, C. Quantitative effect of leaf damage caused by downy mildew (Plasmopara viticola) on growth and yield quality of grapevine “Merlot” (Vitis vinifera). J. Grapevine Res. 2010, 79, 77–85. [Google Scholar] [CrossRef]
- Pons, A.; Mouakka, N.; Deliere, L.; Crachereau, J.C.; Davidou, L.; Sauris, P.; Guilbault, P.; Darriet, P. Impact of Plasmopara viticola infection of Merlot and Cabernet Sauvignon grapes on wine composition and flavor. Food Chem. 2018, 239, 102–110. [Google Scholar] [CrossRef]
- Merot, A.; Smits, N. Does conversion to organic farming impact vineyards yield? A diachronic study in southeastern France. Agronomy 2020, 10, 1626. [Google Scholar] [CrossRef]
- Pizzutti, I.R.; Scholten, J.; Righi, L.W.; Cardoso, C.D.; Rohers, G.N.; da Silva, R.C. Development, optimization and validation of a multimethod for the determination of 36 mycotoxins in wines by liquid chromatography-tandem mass spectrometry. Talanta 2014, 129, 352–363. [Google Scholar] [CrossRef]
- Petit, A.-N.; Fontaine, F.; Clément, C.; Vaillant-Gaveau, N. Photosynthesis Limitations of Grapevine after Treatment with the Fungicide Fludioxonil. J. Agric. Food Chem. 2008, 56, 6761–6767. [Google Scholar] [CrossRef] [PubMed]
- Tsakirakis, A.N.; Kasiotis, K.M.; Charistou, A.N.; Arapaki, N.; Tsatsakis, A.; Tsakalof, A.; Machera, K. Dermal & inhalation exposure of operators during fungicide application in vineyards. Evaluation of coverall performance. Sci. Total Environ. 2014, 470–471, 282–289. [Google Scholar] [CrossRef]
- Raherison, C.; Baldi, I.; Pouquet, M.; Berteaud, E.; Moesch, C.; Bouvier, G.; Canal-Raffin, M. Airborne Pesticide Exposure in Vineyard Rural Areas and Respiratory Health in Children: A pilot study. Environ. Res. 2018, 169, 189–195. [Google Scholar] [CrossRef]
- Thierry, D.; Yengue, J.-L. Vigne et ville. Le paradoxe de l′urbanisme. Rev. Des. Oenogues 2018, 169, 60–61. (In French) [Google Scholar]
- Dumitriu (Gabur), G.-D.; Teodosiu, C.; Cotea, V.V. Management of Pesticides from Vineyard to Wines: Focus on Wine Safety and Pesticides Removal by Emerging Technologies. In Grapes and Wine; Morata, A., Loira, I., González, C., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- EC, European Commission-Farm to Fork Strategy. 2022. Available online: https://ec.europa.eu/food/plants/pesticides/sustainable-use-pesticides/farm-fork-targets-progress_en (accessed on 14 April 2022).
- European Food Safety Authority (EFSA); Brancato, A.; Brocca, D.; Ferreira, L.; Greco, L.; Jarrah, S.; Leuschner, R.; Medina, P.; Miron, I.; Nougadere, A.; et al. Use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA J. 2018, 16, e05147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-González, R.; Garrido Frenich, A.; Martínez Vidal, J.L.; Prestes, O.D.; Grio, S.L. Simultaneous determination of pesticides, biopesticides and mycotoxins in organic products applying a quick, easy, cheap, effective, rugged and safe extraction procedure and ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef]
- Słowik-Borowiec, M.; Szpyrka, E. Multiresidue Analysis of Pesticides in Wine and Grape Using Gas Chromatography with Microelectron Capture and Nitrogen–Phosphorus Detection. Food Anal. Methods 2018, 11, 3516–3530. [Google Scholar] [CrossRef]
- Anastasiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extrac- tion/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Banerjee, K.; Dhumal, K.N.; Adsule, P.G. Optimization of Detection Conditions and Single-Laboratory Validation of a Multiresidue Method for the Determination of 135 Pesticides and 25 Organic Pollutants in Grapes and Wine by Gas Chromatography Time-of-Flight Mass Spectrometry. J. AOAC Int. 2011, 94, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Walorczyk, S.; Drozdzynski, D.; Gnusowski, B. Multiresidue determination of 160 pesticides in wines employing mixed-mode dispersive-solid phase extraction and gas chromatography–tandem mass spectrometry. Talanta 2011, 85, 1856–1870. [Google Scholar] [CrossRef]
- Semla, M.; Schwarcz, P.; Mezey, J.; Binkowski, Ł.J.; Błaszczyk, M.; Formicki, G.; Gren, A.; Stawarz, R.; Massanyi, P. Biogenic and risk elements in wines from the Slovak market with the estimation of consumer exposure. Biol. Trace Elem. Res. 2018, 184, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu (Gabur), G.D.; Teodosiu, C.; Morosanu, I.; Plavan, O.; Gabur, I.; Cotea, V.V. Heavy metals assessment in the major stages of winemaking: Chemometric analysis and impacts on human health and environment. J. Food Compost. Anal. 2021, 100, 103935. [Google Scholar] [CrossRef]
- OIV. Comparison of International Alcohol Drinking Guidelines. 2019. Available online: https://www.oiv.int/public/medias/7169/oiv-report-alcohol-drinking-guidelines-collective-expertise.pdf (accessed on 20 May 2022).
- Health Promotion and Disease Prevention Knowledge Gateway. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/national-low-risk-drinking-recommendations-drinking-guidelines_en (accessed on 20 May 2022).
- Gad Alla, S.A.; Loutfy, N.M.; Shendy, A.H.; Ahmed, M.T. Hazard index, a tool for a long–term risk assessment of pesticide residues in some commodities, a pilot study. Regul. Toxicol. Pharmacol. 2015, 73, 985–991. [Google Scholar] [CrossRef]
- Sharafi, K.; Yunesian, M.; Nodehi, R.N.; Mahvi, A.H.; Pirsaheb, M. A systematic literature review for some toxic metals in widely consumed rice types (domestic and imported) in Iran: Human health risk assessment, uncertainty and sensitivity analysis. Ecotoxicol. Environ. Saf. 2019, 176, 64–75. [Google Scholar] [CrossRef]
- European Commission. Analytical Quality Control and Method Validation for Pesticide Residues Analysis in Food and Feed. 2019. Available online: https://ec.europa.eu/food/system/files/2020-01/pesticides_mrl_guidelines_wrkdoc_2019-12682.pdf (accessed on 20 May 2022).
- Drogue, S.; DeMaria, F. Pesticide residues and trade, the apple of discord. Food Policy 2012, 37, 641–649. [Google Scholar] [CrossRef]
- Schusterova, D.; Hajslova, J.; Kocourek, V.; Pulkrabova, J. Pesticide Residues and Their Metabolites in Grapes and Wines from Conventional and Organic Farming System. Foods 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Cordova, D.; Benner, E.; Sacher, M.; Rauh, J.; Sopa, J.; Lahm, G.; Selby, T.; Stevenson, T.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pest. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Malhat, F.M. Determination of Chlorantraniliprole Residues in Grape by High-Performance Liquid Chromatography. Food Anal. Methods 2012, 5, 1492–1496. [Google Scholar] [CrossRef]
- Caboni, P.; Sarais, G.; Angioni, A.; Vargiu, S.; Pagnozzi, D.; Cabras, P.; Casida, J. Liquid chromatography–tandem mass spectrometric ion- switching determination of chlorantraniliprole and flubendiamide in fruits and vegetables. J. Agric. Food Chem. 2008, 56, 7696–7699. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Dedola, F.; Garau, A.; Sarais, G.; Cabras, P.; Caboni, P. Chlorpyrifos residues levels in fruits and vegetables after field treatment. J. Environ. Sci. Health B 2011, 46, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rodrıguez, R.M.; Cancho-Grande, B.; Simal-Gandara, J. Efficacy of new commercial formulations to control downy mildew and dissipation of their active fungicides in wine after good agricultural practices. J. Sci. Food Agric. 2009, 89, 2625–2635. [Google Scholar] [CrossRef]
- Athanasopoulos, P.E.; Pappas, C.J.; Kyriakidis, N.V. Decomposition of myclobutanil and triadimefon in grapes on the vines and during refrigerated storage. Food Chem. 2003, 82, 367–371. [Google Scholar] [CrossRef]
- Carpinteiro, I.; Ramil, M.; Rodríguez, I.; Cela, R. Determination of fungicides in wine by mixed-mode solid phase extraction and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7484–7492. [Google Scholar] [CrossRef]
- Pelajic, M.; Pecek, G.; Pavlovic, D.M.; Cepo, D.V. Novel multiresidue method for determination of pesticides in red wine using gas chromatography-mass spectrometry and solid phase extraction. Food Chem. 2016, 200, 98–106. [Google Scholar] [CrossRef]
- Strickland, T.C.; Potter, T.L.; Joo, H. Tebuconazole dissipation and metabolism in Tifton loamy sand during laboratory incubation. Pest Manag. Sci. 2004, 60, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Pasteris, R.J.; Hanagan, M.A.; Shapiro, R.J.I. Fungicidal Azocyclic Amides. Patent Application No. WO2008013622, 31 January 2008. [Google Scholar]
- Pasteris, R.J.; Hanagan, M.A.; Bisaha, J.J.; Finkelstein, B.L.; Hoffman, L.E.; Gregory, V.; Andreassi, J.L.; Sweigard, J.A.; Klyashchitsky, B.A.; Henry, Y.T.; et al. Discovery of oxathiapiprolin, a new oomycete fungicide that targets an oxysterol binding protein. Bioorgan. Med. Chem. 2016, 24, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Pingzhong, Y.; Jia, C.; Zhao, E.; Chen, L.; He, H.; Jing, J.; He, M. Determination of oxathiapiprolin concentration and dissipation in grapes and soil by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Sci. Food Agric. 2017, 10, 3294–3299. [Google Scholar] [CrossRef]
- Wu, X.; Xu, J.; Dong, F.; Liu, X.; Li, Y.; Zheng, Y. Simultaneous determination of oxathiapiprolin and two metabolites in fruits, vegetables and cereal using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2014, 1329, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sattler, C.; Kächele, H.; Verch, G. Assessing the intensity of pesticide use in agriculture. Agr. Ecosyst. Environ. 2007, 119, 299–304. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Pesticide residues in table grapes and exposure assessment. J. Agric. Food Chem. 2018, 66, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Predicting Dietary Intake of Pesticide Residues; GEMS/Food WHO: Geneva, Switzerland, 1989. [Google Scholar]

| Time (min) | MPA % | MPB % |
|---|---|---|
| 0.70 | 95 | 5 |
| 1.00 | 50 | 50 |
| 1.50 | 40 | 60 |
| 2.50 | 22 | 78 |
| 4.00 | 12 | 88 |
| 10.00 | 8 | 92 |
| 12.00 | 0 | 100 |
| 24.00 | 0 | 100 |
| 25.00 | 95 | 5 |
| 29.50 | 95 | 5 |
| Pesticides | Q1 (m/z) | Q3 (m/z) | Retention Time | R2 | LOQ (ng/g) | LOD (ng/g) | Regression Equation | Mean Calculated Concentration (ng/mL) | Accuracy (%) | Standard Deviation (ng/mL) | Coefficient of Variation (CV %) | Response Factor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetamiprid | 223.2 | 126.0 | 4.44 | 0.9942 | 0.40 | 0.12 | y = 0.933 × + −0.192 | 2.46 | 99.5 | 0.08 | 3.2 | 2.93 |
| 4.98 | 101.0 | 0.40 | 8.0 | 3.41 | ||||||||
| Confirmation transition: | 9.92 | 100.5 | 0.19 | 1.9 | 2.96 | |||||||
| 19.35 | 98.2 | 1.13 | 5.9 | 3.37 | ||||||||
| 223.200 | 90.0 | 29.76 | 100.5 | 1.68 | 5.6 | 3.22 | ||||||
| 49.41 | 100.2 | 1.39 | 2.8 | 3.46 | ||||||||
| Chlorantraniliprole | 481.9 | 283.9 | 5.26 | 0.9948 | 0.22 | 0.07 | y = 0.906 × + −0.253 | 2.54 | 100.1 | 0.06 | 2.2 | 0.87 |
| 5.07 | 100.0 | 0.52 | 10.2 | 1.04 | ||||||||
| Confirmation transition: | 10.17 | 100.7 | 0.44 | 4.3 | 1.00 | |||||||
| 19.95 | 98.3 | 0.93 | 4.7 | 1.01 | ||||||||
| 481.9 | 450.9 | 30.72 | 101.1 | 1.73 | 5.6 | 1.06 | ||||||
| 50.66 | 99.9 | 1.82 | 3.6 | 1.08 | ||||||||
| Iprovalicarb | 321.2 | 119.1 | 5.07 | 0.9944 | 1.62 | 0.04 | y = 0.113 × + −0.0535 | 2.58 | 102.8 | 0.17 | 6.6 | 3.54 |
| 5.04 | 100.2 | 0.44 | 8.6 | 4.12 | ||||||||
| Confirmation transition: | 10.00 | 99.1 | 0.33 | 3.3 | 3.52 | |||||||
| 19.57 | 97.4 | 1.35 | 6.9 | 4.04 | ||||||||
| 321.2 | 91.1 | 29.72 | 98.7 | 1.48 | 5.0 | 3.95 | ||||||
| 51.23 | 101.8 | 1.85 | 3.6 | 4.14 | ||||||||
| Myclobutanil | 289.1 | 70.1 | 5.41 | 0.9900 | 0.74 | 0.22 | y = 0.298 × + −0.125 | 2.52 | 100.1 | 0.19 | 7.6 | 0.46 |
| 5.03 | 100.1 | 0.39 | 7.8 | 0.44 | ||||||||
| Confirmation transition: | 10.17 | 100.7 | 0.25 | 2.5 | 0.41 | |||||||
| 19.94 | 99.2 | 0.85 | 4.2 | 0.47 | ||||||||
| 289.1 | 125.2 | 30.06 | 99.5 | 1.70 | 5.7 | 0.48 | ||||||
| 50.53 | 100.5 | 1.62 | 3.2 | 0.48 | ||||||||
| Tebuconazole | 308.2 | 70.0 | 5.58 | 0.9930 | 0.59 | 0.17 | y = 0.132 × + −0.0859 | 2.62 | 105.2 | 0.20 | 7.6 | 0.35 |
| 4.91 | 98.4 | 0.39 | 7.9 | 0.4 | ||||||||
| 9.68 | 97.1 | 0.36 | 3.7 | 0.35 | ||||||||
| 19.58 | 98.4 | 0.80 | 4.1 | 0.42 | ||||||||
| 29.76 | 99.5 | 1.79 | 6.0 | 0.39 | ||||||||
| 50.61 | 101.4 | 1.94 | 3.8 | 0.42 | ||||||||
| Oxiathiapiprolin | 540.0 | 500.0 | 6.67 | 0.9924 | 1.58 | 0.47 | y = 0.183 × + −0.144 | 2.63 | 103.9 | 0.16 | 6.1 | 0.24 |
| 4.94 | 97.4 | 0.38 | 7.7 | 0.32 | ||||||||
| Confirmation transition: | 10.11 | 100.1 | 0.32 | 3.1 | 0.3 | |||||||
| 19.73 | 97.2 | 1.31 | 6.7 | 0.34 | ||||||||
| 308.2 | 125.1 | 30.60 | 100.6 | 2.11 | 6.9 | 0.35 | ||||||
| 51.00 | 100.8 | 2.75 | 5.4 | 0.36 | ||||||||
| Pesticides | Red Wine | Rosé Wine | White Wine 1 | White Wine 2 | White Wine 3 | White Wine 4 |
|---|---|---|---|---|---|---|
| Acetamiprid | 0.0697 ± 0.03 ab | 0.0781 ± 0.03 ab | 0.0937 ± 0.02 b | 0.0608 ± 0.02 ab | 0.0556 ± 0.01 a | 0.0504 ± 0.02 a |
| Chlorantraniliprole | 0.5456 ± 0.09 c | 0.4480 ± 0.08 b | 0.2156 ± 0.01 a | 0.4296 ± 0.04 b | 0.1943 ± 0.02 a | 0.2203 ± 0.02 a |
| Iprovalicarb | 0.1216 ± 0.07 a | 0.1901 ± 0.07 b | 0.0729 ± 0.00 a | 0.7509 ± 0.02 e | 0.5164 ± 0.01 c | 0.6871 ± 0.01 d |
| Myclobutanil | 0.1364 ± 0.01 ab | 0.1515 ± 0.00 c | 0.1314 ± 0.00 ab | 0.1309 ± 0.00 a | 0.1379 ± 0.01 b | 0.1379 ± 0.00 b |
| Tebuconazole | 0.1771 ± 0.01 c | 0.1134 ± 0.00 b | nd | 0.0972 ± 0.00 a | nd | nd |
| Oxathiapiprolin | 0.1466 ± 0.00 b | 0.1354 ± 0.00 a | nd | nd | nd | nd |
| Pesticides | Red Wine | Rosé Wine | White Wine 1 | White Wine 2 | White Wine 3 | White Wine 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | |
| Estimated Daily Intake (EDI) (mg/kg bw/day) | ||||||||||||
| Acetamiprid | 1.45 × 10−4 | 2.05 × 10−4 | 1.63 × 10−4 | 2.30 × 10−4 | 1.95 × 10−4 | 2.76 × 10−4 | 1.27 × 10−4 | 1.79 × 10−4 | 1.16 × 10−4 | 1.63 × 10−4 | 1.05 × 10−4 | 1.48 × 10−4 |
| Chlorantraniliprole | 1.14 × 10−3 | 1.60 × 10−3 | 9.33 × 10−4 | 1.32 × 10−3 | 4.49 × 10−4 | 6.34 × 10−4 | 8.95 × 10−4 | 1.26 × 10−3 | 4.05 × 10−4 | 5.71 × 10−4 | 4.59 × 10−4 | 6.48 × 10−4 |
| Iprovalicarb | 2.53 × 10−4 | 3.58 × 10−4 | 3.96 × 10−4 | 5.59 × 10−4 | 1.52 × 10−4 | 2.14 × 10−4 | 1.56 × 10−3 | 2.21 × 10−3 | 1.08 × 10−3 | 1.52 × 10−3 | 1.43 × 10−3 | 2.02 × 10−3 |
| Myclobutanil | 2.84 × 10−4 | 4.01 × 10−4 | 3.16 × 10−4 | 4.46 × 10−4 | 2.74 × 10−4 | 3.86 × 10−4 | 2.73 × 10−4 | 3.85 × 10−4 | 2.87 × 10−4 | 4.06 × 10−4 | 2.87 × 10−4 | 4.06 × 10−4 |
| Tebuconazole | 3.69 × 10−4 | 5.21 × 10−4 | 2.36 × 10−4 | 3.33 × 10−4 | - | - | 2.02 × 10−4 | 2.86 × 10−4 | - | - | - | - |
| Oxathiapiprolin | 3.05 × 10−4 | 4.31 × 10−4 | 2.82 × 10−4 | 3.98 × 10−4 | - | - | - | - | - | - | - | - |
| Red wine | Rosé wine | White wine 1 | White wine 2 | White wine 3 | White wine 4 | |||||||
| Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | Men | |
| Hazard Quotient (HQ) | ||||||||||||
| Acetamiprid | 5.81 × 10−3 | 8.20 × 10−3 | 6.50 × 10−3 | 9.18 × 10−3 | 7.81 × 10−3 | 1.10 × 10−2 | 5.07 × 10−3 | 7.16 × 10−3 | 4.63 × 10−3 | 6.54 × 10−3 | 4.20 × 10−3 | 5.94 × 10−3 |
| Chlorantraniliprole | 7.29 × 10−4 | 1.03 × 10−3 | 5.98 × 10−4 | 8.45 × 10−4 | 2.88 × 10−4 | 4.07 × 10−4 | 5.74 × 10−4 | 8.10 × 10−4 | 2.59 × 10−4 | 3.66 × 10−4 | 2.94 × 10−4 | 4.15 × 10−4 |
| Iprovalicarb | 1.69 × 10−2 | 2.39 × 10−2 | 2.64 × 10−2 | 3.73 × 10−2 | 1.01 × 10−2 | 1.43 × 10−2 | 1.04 × 10−1 | 1.47 × 10−1 | 7.17 × 10−2 | 1.01 × 10−1 | 9.54 × 10−2 | 1.35 × 10−1 |
| Myclobutanil | 1.14 × 10−2 | 1.60 × 10−2 | 1.26 × 10−2 | 1.78 × 10−2 | 1.09 × 10−2 | 1.55 × 10−2 | 1.09 × 10−2 | 1.54 × 10−2 | 1.15 × 10−2 | 1.62 × 10−2 | 1.15 × 10−2 | 1.62 × 10−2 |
| Tebuconazole | 1.23 × 10−2 | 1.74 × 10−2 | 7.87 × 10−3 | 1.11 × 10−2 | - | - | 6.75 × 10−3 | 9.53 × 10−3 | - | - | - | - |
| Oxathiapiprolin | 2.18 × 10−3 | 3.08 × 10−3 | 2.02 × 10−3 | 2.85 × 10−3 | - | - | - | - | - | - | - | - |
| Hazard Index (HI) | 4.93 × 10−2 | 6.96 × 10−2 | 5.60 × 10−2 | 7.91 × 10−2 | 2.92 × 10−2 | 4.12 × 10−2 | 1.28 × 10−1 | 1.80 × 10−1 | 8.81 × 10−2 | 1.24 × 10−1 | 1.11 × 10−1 | 1.57 × 10−1 |
| Pesticide Residues | Family-Activity | Pest Control | Molecular Weight | Log P | MRL Wines (ng/g) | EU Pesticides Data | Hazard Class and Category Code(s) |
|---|---|---|---|---|---|---|---|
| Acetamiprid | Neonicotinoid insecticide | Leafhoppers and other small insect pests | 222.67 | 0.80 | 500 | ADI = 0.025 mg/kg bw/day ARfD = 0.025 mg/kg bw AOEL = 0.025 mg/kg bw/day, Reg. (EU) 2018/113 | H302: Harmful if swallowed Acute Tox. 4—Acute toxicity H412: Harmful to aquatic life with long lasting effects Aquatic Chronic 3—Hazardous to the aquatic environment |
| Chlorantraniliprole | Diamide insecticides | 483.1 | 2.76 | 1000 | ADI = 1.56 mg/kg bw/day ARfD = Not applicable AOEL = 0.36 mg/kg bw/day, EFSA 2013 | No classification | |
| Iprovalicarb | Carabamate fungicide and valinamide fungicide | Downey mildew | 320.4 | 3.2 | 2000 | ADI = 0.015 mg/kg bw/day AOEL = 0.015 mg/kg bw/day, Reg. (EU) 2016/147 | No classification |
| Myclobutanil | Conazole fungicide | Powdery mildew | 288.77 | 2.94 | 100 | ADI = 0.025 mg/kg bw/day ARfD = 0.31 mg/kg bw AOEL = 0.03 mg/kg bw/day, EFSA 10 | H302: Harmful if swallowed Acute Tox. 4—Acute toxicity H361d: Suspected of damaging the unborn child. Repr. 2—Reproductive toxicity H319: Causes serious eye irritation Eye Irrit. 2—Eye irritation H411: Toxic to aquatic life with long lasting effects Aquatic Chronic 2—Hazardous to the aquatic environment |
| Tebuconazole | Conazole fungicide | Powdery mildew | 307.82 | 3.7 | 1000 | ADI = 0.03 mg/kg bw/day ARfD = 0.03 mg/kg bw, EFSA 08 AOEL = 0.03 mg/kg bw/day, Dir 08/125 | H302: Harmful if swallowed H400: Very toxic to aquatic life Aquatic Acute 1—Hazardous to the aquatic environment H361d: Suspected of damaging the unborn child. Repr. 2—Reproductive toxicity H410: Very toxic to aquatic life with long lasting effects Aquatic Chronic 1—Hazardous to the aquatic environment |
| Oxathiapiprolin | Fungicide | Oomycete pathogens | 539.5 | 3.67 | 700 | ADI = 0.14 mg/kg bw/day AOEL = 0.04 mg/kg bw/day, Reg. (EU) 2017/239 | No classification |
| Active Substance | Wine Type | EFSA PRIMo rev.3.1 | Population Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PT General | FR Adult | RO General | IE Adult | UK Adult | DK Adult | DE Women | DE General | UK Vegetarian | NL General | ES Adult | FI Adult | |||
| Acetamiprid | Red wine | ADI (%) | 0.7% | 0.6% | 0.5% | 0.3% | 0.3% | 0.3% | 0.2% | 0.2% | 0.2% | 0.2% | 0.1% | 0.1% |
| Exp (µg/kg bw per day) | 0.17 | 0.16 | 0.12 | 0.09 | 0.08 | 0.07 | 0.06 | 0.06 | 0.06 | 0.04 | 0.03 | 0.02 | ||
| Rosé wine | ADI (%) | 0.8% | 0.7% | 0.5% | 0.4% | 0.3% | 0.3% | 0.3% | 0.3% | 0.3% | 0.2% | 0.1% | 0.1% | |
| Exp (µg/kg bw per day) | 0.19 | 0.18 | 0.13 | 0.10 | 0.08 | 0.07 | 0.07 | 0.06 | 0.06 | 0.05 | 0.03 | 0.02 | ||
| White wine 1 | ADI (%) | 0.9% | 0.9% | 0.6% | 0.5% | 0.4% | 0.4% | 0.3% | 0.3% | 0.3% | 0.2% | 0.2% | 0.1% | |
| Exp (µg/kg bw per day) | 0.23 | 0.22 | 0.16 | 0.12 | 0.10 | 0.09 | 0.08 | 0.08 | 0.08 | 0.06 | 0.04 | 0.03 | ||
| White wine 2 | ADI (%) | 0.6% | 0.6% | 0.4% | 0.3% | 0.3% | 0.2% | 0.2% | 0.2% | 0.2% | 0.1% | 0.1% | 0.1% | |
| Exp (µg/kg bw per day) | 0.15 | 0.14 | 0.10 | 0.08 | 0.07 | 0.06 | 0.05 | 0.05 | 0.05 | 0.04 | 0.03 | 0.02 | ||
| White wine 3 | ADI (%) | 0.6% | 0.5% | 0.4% | 0.3% | 0.2% | 0.2% | 0.2% | 0.2% | 0.2% | 0.1% | 0.1% | 0.1% | |
| Exp (µg/kg bw per day) | 0.14 | 0.13 | 0.09 | 0.07 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.03 | 0.02 | 0.02 | ||
| White wine 4 | ADI (%) | 0.5% | 0.5% | 0.3% | 0.3% | 0.2% | 0.2% | 0.2% | 0.2% | 0.2% | 0.1% | 0.1% | 0.1% | |
| Exp (µg/kg bw per day) | 0.13 | 0.12 | 0.08 | 0.06 | 0.05 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.02 | 0.02 | ||
| Chlorantraniliprole | Red wine | ADI (%) | 0.1% | 0.1% | 0.1% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Exp (µg/kg bw per day) | 1.36 | 1.27 | 0.92 | 0.68 | 0.59 | 0.52 | 0.46 | 0.45 | 0.44 | 0.32 | 0.23 | 0.17 | ||
| Rosé wine | ADI (%) | 0.1% | 0.1% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Exp (µg/kg bw per day) | 1.11 | 1.04 | 0.75 | 0.56 | 0.49 | 0.43 | 0.37 | 0.37 | 0.36 | 0.26 | 0.19 | 0.14 | ||
| White wine 1 | ADI (%) | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Exp (µg/kg bw per day) | 0.54 | 0.50 | 0.36 | 0.27 | 0.23 | 0.21 | 0.18 | 0.18 | 0.18 | 0.13 | 0.09 | 0.07 | ||
| White wine 2 | ADI (%) | 0.1% | 0.1% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Exp (µg/kg bw per day) | 1.07 | 1.00 | 0.72 | 0.54 | 0.47 | 0.41 | 0.36 | 0.36 | 0.35 | 0.25 | 0.18 | 0.13 | ||
| White wine 3 | ADI (%) | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Exp (µg/kg bw per day) | 0.48 | 0.45 | 0.33 | 0.24 | 0.21 | 0.19 | 0.16 | 0.16 | 0.16 | 0.11 | 0.08 | 0.06 | ||
| White wine 4 | ADI (%) | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Exp (µg/kg bw per day) | 0.55 | 0.51 | 0.37 | 0.28 | 0.24 | 0.21 | 0.18 | 0.18 | 0.18 | 0.13 | 0.09 | 0.07 | ||
| Iprovalicarb | Red wine | ADI (%) | 2.0% | 1.9% | 1.4% | 1.0% | 0.9% | 0.8% | 0.7% | 0.7% | 0.7% | 0.5% | 0.3% | 0.2% |
| Exp (µg/kg bw per day) | 0.30 | 0.28 | 0.20 | 0.15 | 0.13 | 0.12 | 0.10 | 0.10 | 0.10 | 0.07 | 0.05 | 0.04 | ||
| Rosé wine | ADI (%) | 3.2% | 2.9% | 2.1% | 1.6% | 1.4% | 1.2% | 1.1% | 1.1% | 1.0% | 0.7% | 0.5% | 0.4% | |
| Exp (µg/kg bw per day) | 0.47 | 0.44 | 0.32 | 0.24 | 0.21 | 0.18 | 0.16 | 0.16 | 0.15 | 0.11 | 0.08 | 0.06 | ||
| White wine 1 | ADI (%) | 1.2% | 1.1% | 0.8% | 0.6% | 0.5% | 0.5% | 0.4% | 0.4% | 0.4% | 0.3% | 0.2% | 0.1% | |
| Exp (µg/kg bw per day) | 0.18 | 0.17 | 0.12 | 0.09 | 0.08 | 0.07 | 0.06 | 0.06 | 0.06 | 0.04 | 0.03 | 0.02 | ||
| White wine 2 | ADI (%) | 12.5% | 11.6% | 8.4% | 6.3% | 5.4% | 4.8% | 4.2% | 4.2% | 4.1% | 2.9% | 2.1% | 1.5% | |
| Exp (µg/kg bw per day) | 1.87 | 1.74 | 1.26 | 0.94 | 0.81 | 0.72 | 0.63 | 0.62 | 0.61 | 0.44 | 0.31 | 0.23 | ||
| White wine 3 | ADI (%) | 8.6% | 8.0% | 5.8% | 4.3% | 3.7% | 3.3% | 2.9% | 2.9% | 2.8% | 2.0% | 1.4% | 1.1% | |
| Exp (µg/kg bw per day) | 1.28 | 1.20 | 0.87 | 0.65 | 0.56 | 0.49 | 0.43 | 0.43 | 0.42 | 0.30 | 0.22 | 0.16 | ||
| White wine 4 | ADI (%) | 11.4% | 10.6% | 7.7% | 5.7% | 5.0% | 4.4% | 3.8% | 3.8% | 3.7% | 2.7% | 1.9% | 1.4% | |
| Exp (µg/kg bw per day) | 1.71 | 1.59 | 1.15 | 0.86 | 0.74 | 0.66 | 0.57 | 0.57 | 0.56 | 0.40 | 0.29 | 0.21 | ||
| Myclobutanil | Red wine | ADI (%) | 1.4% | 1.3% | 0.9% | 0.7% | 0.6% | 0.5% | 0.5% | 0.5% | 0.4% | 0.3% | 0.2% | 0.2% |
| Exp (µg/kg bw per day) | 0.34 | 0.32 | 0.23 | 0.17 | 0.15 | 0.13 | 0.11 | 0.11 | 0.11 | 0.08 | 0.06 | 0.04 | ||
| Rosé wine | ADI (%) | 1.5% | 1.4% | 1.0% | 0.8% | 0.7% | 0.6% | 0.5% | 0.5% | 0.5% | 0.4% | 0.3% | 0.2% | |
| Exp (µg/kg bw per day) | 0.38 | 0.35 | 0.25 | 0.19 | 0.16 | 0.14 | 0.13 | 0.13 | 0.12 | 0.09 | 0.06 | 0.05 | ||
| White wine 1 | ADI (%) | 1.3% | 1.2% | 0.9% | 0.7% | 0.6% | 0.5% | 0.4% | 0.4% | 0.4% | 0.3% | 0.2% | 0.2% | |
| Exp (µg/kg bw per day) | 0.33 | 0.30 | 0.22 | 0.16 | 0.14 | 0.13 | 0.11 | 0.11 | 0.11 | 0.08 | 0.05 | 0.04 | ||
| White wine 2 | ADI (%) | 1.3% | 1.2% | 0.9% | 0.7% | 0.6% | 0.5% | 0.4% | 0.4% | 0.4% | 0.3% | 0.2% | 0.2% | |
| Exp (µg/kg bw per day) | 0.33 | 0.30 | 0.22 | 0.16 | 0.14 | 0.12 | 0.11 | 0.11 | 0.11 | 0.08 | 0.05 | 0.04 | ||
| White wine 3 | ADI (%) | 1.4% | 1.3% | 0.9% | 0.7% | 0.6% | 0.5% | 0.5% | 0.5% | 0.4% | 0.3% | 0.2% | 0.2% | |
| Exp (µg/kg bw per day) | 0.34 | 0.32 | 0.23 | 0.17 | 0.15 | 0.13 | 0.12 | 0.11 | 0.11 | 0.08 | 0.06 | 0.04 | ||
| White wine 4 | ADI (%) | 1.4% | 1.3% | 0.9% | 0.7% | 0.6% | 0.5% | 0.5% | 0.5% | 0.4% | 0.3% | 0.2% | 0.2% | |
| Exp (µg/kg bw per day) | 0.34 | 0.32 | 0.23 | 0.17 | 0.15 | 0.13 | 0.12 | 0.11 | 0.11 | 0.08 | 0.06 | 0.04 | ||
| Tebuconazole | Red wine | ADI (%) | 1.5% | 1.4% | 1.0% | 0.7% | 0.6% | 0.6% | 0.5% | 0.5% | 0.5% | 0.3% | 0.2% | 0.2% |
| Exp (µg/kg bw per day) | 0.44 | 0.41 | 0.30 | 0.22 | 0.19 | 0.17 | 0.15 | 0.15 | 0.14 | 0.10 | 0.07 | 0.05 | ||
| Rosé wine | ADI (%) | 0.9% | 0.9% | 0.6% | 0.5% | 0.4% | 0.4% | 0.3% | 0.3% | 0.3% | 0.2% | 0.2% | 0.1% | |
| Exp (µg/kg bw per day) | 0.28 | 0.26 | 0.19 | 0.14 | 0.12 | 0.11 | 0.09 | 0.09 | 0.09 | 0.07 | 0.05 | 0.03 | ||
| White wine 1 | ADI (%) | - | - | - | - | - | - | - | - | - | - | - | - | |
| Exp (µg/kg bw per day) | - | - | - | - | - | - | - | - | - | - | - | - | ||
| White wine 2 | ADI (%) | 0.8% | 0.8% | 0.5% | 0.4% | 0.4% | 0.3% | 0.3% | 0.3% | 0.3% | 0.2% | 0.1% | 0.1% | |
| Exp (µg/kg bw per day) | 0.24 | 0.23 | 0.16 | 0.12 | 0.11 | 0.09 | 0.08 | 0.08 | 0.08 | 0.06 | 0.04 | 0.03 | ||
| White wine 3 | ADI (%) | - | - | - | - | - | - | - | - | - | - | - | - | |
| Exp (µg/kg bw per day) | - | - | - | - | - | - | - | - | - | - | - | - | ||
| White wine 4 | ADI (%) | - | - | - | - | - | - | - | - | - | - | - | - | |
| Exp (µg/kg bw per day) | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Oxathiapiprolin | Red wine | ADI (%) | 0.3% | 0.2% | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.0% | 0.0% |
| Exp (µg/kg bw per day) | 0.36 | 0.34 | 0.25 | 0.18 | 0.16 | 0.14 | 0.12 | 0.12 | 0.12 | 0.09 | 0.06 | 0.05 | ||
| Rosé wine | ADI (%) | 0.2% | 0.2% | 0.2% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.1% | 0.0% | 0.0% | |
| Exp (µg/kg bw per day) | 0.34 | 0.31 | 0.23 | 0.17 | 0.15 | 0.13 | 0.11 | 0.11 | 0.11 | 0.08 | 0.06 | 0.04 | ||
| White wine 1 | ADI (%) | - | - | - | - | - | - | - | - | - | - | - | - | |
| Exp (µg/kg bw per day) | - | - | - | - | - | - | - | - | - | - | - | - | ||
| White wine 2 | ADI (%) | - | - | - | - | - | - | - | - | - | - | - | - | |
| Exp (µg/kg bw per day) | - | - | - | - | - | - | - | - | - | - | - | - | ||
| White wine 3 | ADI (%) | - | - | - | - | - | - | - | - | - | - | - | - | |
| Exp (µg/kg bw per day) | - | - | - | - | - | - | - | - | - | - | - | - | ||
| White wine 4 | ADI (%) | - | - | - | - | - | - | - | - | - | - | - | - | |
| Exp (µg/kg bw per day) | - | - | - | - | - | - | - | - | - | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitriu Gabur, G.-D.; Gabur, I.; Cucolea, E.I.; Costache, T.; Rambu, D.; Cotea, V.V.; Teodosiu, C. Investigating Six Common Pesticides Residues and Dietary Risk Assessment of Romanian Wine Varieties. Foods 2022, 11, 2225. https://doi.org/10.3390/foods11152225
Dumitriu Gabur G-D, Gabur I, Cucolea EI, Costache T, Rambu D, Cotea VV, Teodosiu C. Investigating Six Common Pesticides Residues and Dietary Risk Assessment of Romanian Wine Varieties. Foods. 2022; 11(15):2225. https://doi.org/10.3390/foods11152225
Chicago/Turabian StyleDumitriu Gabur, Georgiana-Diana, Iulian Gabur, Elena Iulia Cucolea, Teodor Costache, Dan Rambu, Valeriu V. Cotea, and Carmen Teodosiu. 2022. "Investigating Six Common Pesticides Residues and Dietary Risk Assessment of Romanian Wine Varieties" Foods 11, no. 15: 2225. https://doi.org/10.3390/foods11152225
APA StyleDumitriu Gabur, G.-D., Gabur, I., Cucolea, E. I., Costache, T., Rambu, D., Cotea, V. V., & Teodosiu, C. (2022). Investigating Six Common Pesticides Residues and Dietary Risk Assessment of Romanian Wine Varieties. Foods, 11(15), 2225. https://doi.org/10.3390/foods11152225









