Characterization of Pectin from Grape Pomace: A Comparison of Conventional and Pulsed Ultrasound-Assisted Extraction Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Pectin from Grape Pomace Using Conventional Extraction (CE)
2.2.2. Extraction of Pectin from Grape Pomace Using Pulsed Ultrasound-Assisted Extraction (PUAE)
2.2.3. Pectin Precipitation and Purification
2.2.4. Pectin Yield
2.2.5. Galacturonic Acid Content
2.2.6. Degree of Esterification
2.2.7. Molecular Weight
2.2.8. Color
2.2.9. FT-IR Analysis
2.2.10. Rheological Characterization of Pectin Solutions
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Model Fitting and Statistical Analysis
3.2. Effect on Process Variables
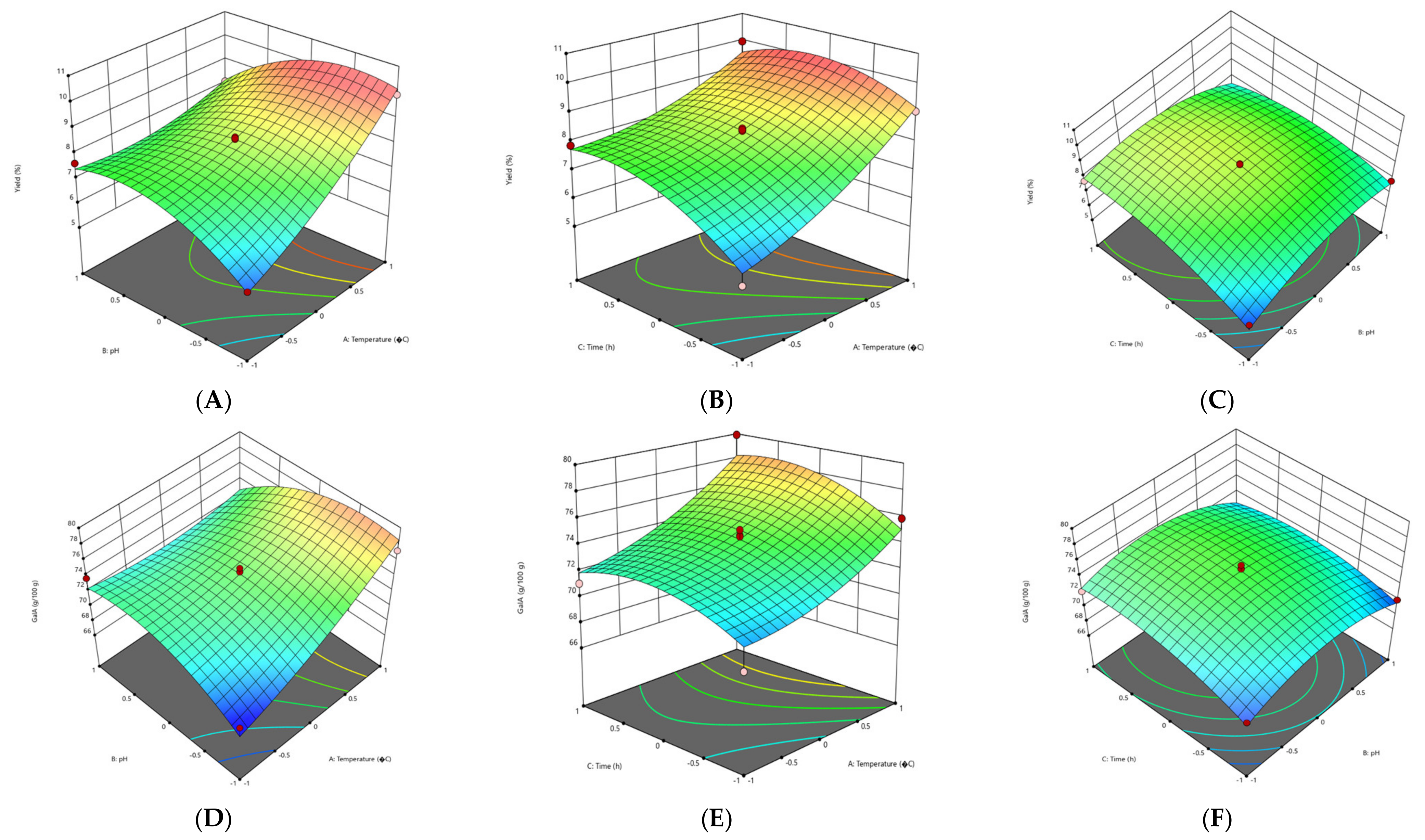
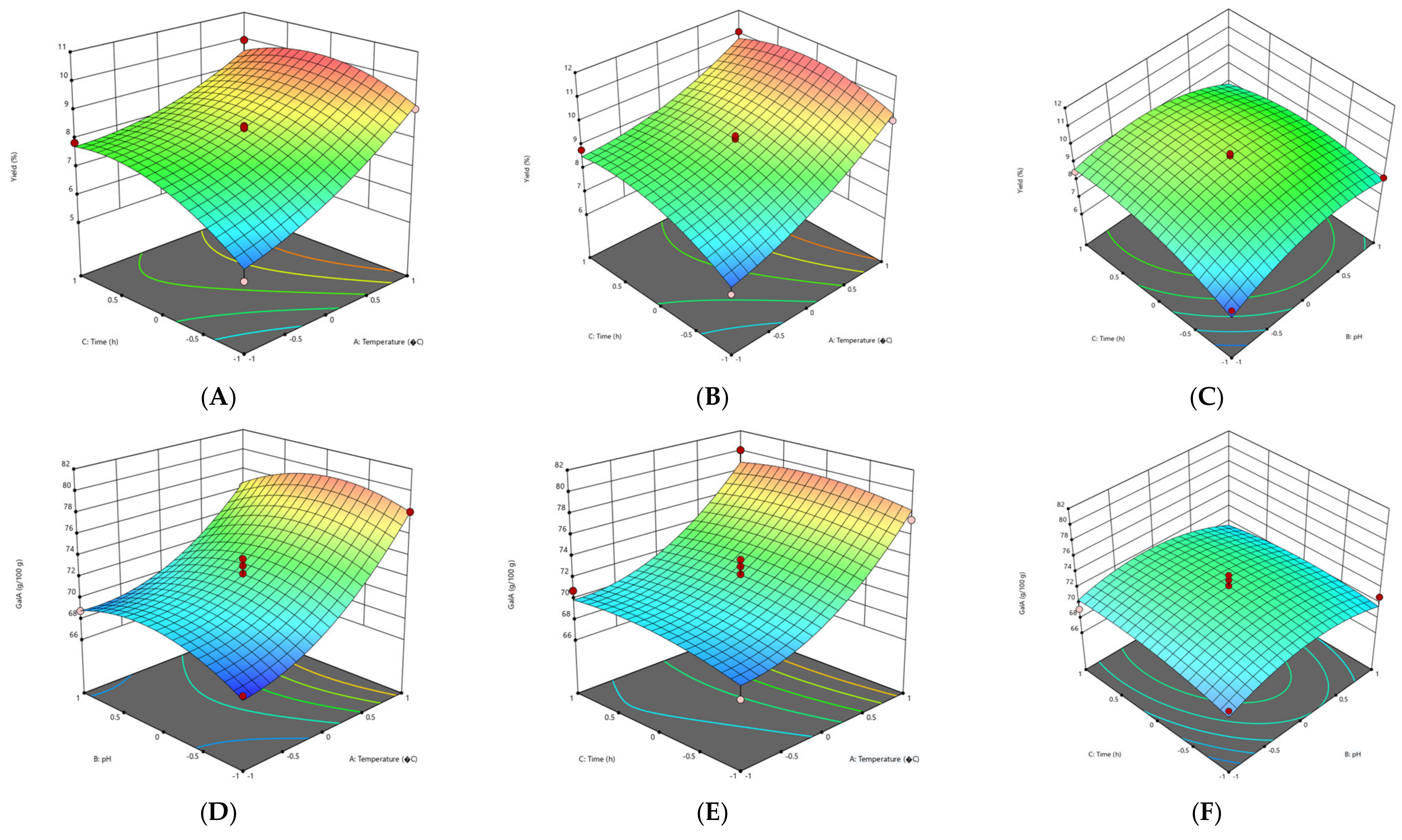
3.2.1. Effect of Extraction Parameters on Pectin Yield
3.2.2. Effect of Extraction Parameters on Galacturonic Acid Content
3.2.3. Effect of Extraction Parameters on Degree of Esterification
3.2.4. Effect of Extraction Parameters on Molecular Weight
3.3. Optimization and Validation of Extraction Conditions
3.4. Color
3.5. FT-IR Analysis
3.6. Rheological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| FN-CE | |||||||||||
| Run | Independent Variables | Measured Response | Predicted Response | ||||||||
| Temperature (°C) | pH | Time (h) | Y (%) | GalA (g/100 g) | DE (%) | Mw (g/mol) | Y (%) | GalA (g/100 g) | DE (%) | Mw (g/mol) | |
| 1 | 80 | 2 | 2 | 8.26 | 73.18 | 77.49 | 5.29 × 104 | 8.38 | 74.34 | 77.51 | 5.33 × 104 |
| 2 | 80 | 3 | 1 | 6.82 | 70.02 | 72.72 | 5.13 × 104 | 6.64 | 69.60 | 73.08 | 5.13 × 104 |
| 3 | 80 | 2 | 2 | 8.47 | 74.23 | 78.16 | 5.32 × 104 | 8.38 | 74.34 | 77.51 | 5.33 × 104 |
| 4 | 90 | 2 | 1 | 9.05 | 75.86 | 80.25 | 5.42 × 104 | 9.18 | 75.03 | 80.10 | 5.40 × 104 |
| 5 | 80 | 3 | 3 | 6.21 | 70.19 | 73.06 | 5.07 × 104 | 6.57 | 70.66 | 72.97 | 5.09 × 104 |
| 6 | 80 | 2 | 2 | 8.38 | 74.65 | 77.22 | 5.34 × 104 | 8.38 | 74.34 | 77.51 | 5.33 × 104 |
| 7 | 80 | 2 | 2 | 8.42 | 75.07 | 78.65 | 5.35 × 104 | 8.38 | 74.34 | 77.51 | 5.33 × 104 |
| 8 | 70 | 2 | 1 | 5.43 | 68.70 | 72.08 | 4.97 × 104 | 5.83 | 70.41 | 72.77 | 5.00 × 104 |
| 9 | 70 | 1 | 2 | 5.75 | 69.13 | 71.68 | 4.99 × 104 | 5.70 | 67.88 | 70.89 | 4.97 × 104 |
| 10 | 90 | 3 | 2 | 8.09 | 71.51 | 75.17 | 5.30 × 104 | 8.14 | 72.76 | 75.95 | 5.31 × 104 |
| 11 | 70 | 2 | 3 | 7.87 | 71.03 | 75.63 | 5.21 × 104 | 7.74 | 71.86 | 75.78 | 5.22 × 104 |
| 12 | 80 | 2 | 2 | 8.38 | 74.56 | 76.01 | 5.37 × 104 | 8.38 | 74.34 | 77.51 | 5.33 × 104 |
| 13 | 80 | 1 | 1 | 5.94 | 69.42 | 72.14 | 4.94 × 104 | 5.58 | 68.95 | 72.23 | 4.92 × 104 |
| 14 | 90 | 2 | 3 | 9.96 | 79.91 | 81.28 | 5.52 × 104 | 9.56 | 78.20 | 80.59 | 5.48 × 104 |
| 15 | 80 | 1 | 3 | 7.75 | 72.10 | 75.20 | 5.27 × 104 | 7.93 | 72.52 | 75.84 | 5.26 × 104 |
| 16 | 90 | 1 | 2 | 9.92 | 77.24 | 81.09 | 5.46 × 104 | 10.14 | 78.54 | 71.15 | 5.49 × 104 |
| 17 | 70 | 3 | 2 | 7.63 | 73.75 | 74.13 | 5.23 × 104 | 7.41 | 74.25 | 74.07 | 5.19 × 104 |
| RN-CE | |||||||||||
| 1 | 80 | 2 | 2 | 9.21 | 73.76 | 71.30 | 5.13 × 104 | 9.35 | 72.32 | 72.50 | 5.07 × 104 |
| 2 | 80 | 3 | 1 | 8.06 | 70.87 | 66.33 | 4.78 × 104 | 7.92 | 69.62 | 66.76 | 4.78 × 104 |
| 3 | 80 | 2 | 2 | 9.45 | 71.28 | 74.27 | 5.01 × 104 | 9.35 | 72.32 | 72.50 | 5.01 × 104 |
| 4 | 90 | 2 | 1 | 10.21 | 77.46 | 77.16 | 5.46 × 104 | 10.48 | 78.33 | 76.34 | 5.46 × 104 |
| 5 | 80 | 3 | 3 | 7.48 | 68.64 | 65.19 | 4.67 × 104 | 7.91 | 69.49 | 66.65 | 4.67 × 104 |
| 6 | 80 | 2 | 2 | 9.37 | 73.11 | 72.74 | 5.02 × 104 | 9.35 | 72.32 | 72.50 | 5.07 × 104 |
| 7 | 80 | 2 | 2 | 9.18 | 72.37 | 70.07 | 5.08 × 104 | 9.35 | 72.32 | 72.50 | 5.07 × 104 |
| 8 | 70 | 2 | 1 | 6.61 | 67.10 | 63.39 | 4.53 × 104 | 6.91 | 68.33 | 65.24 | 4.66 × 104 |
| 9 | 70 | 1 | 2 | 6.82 | 67.39 | 63.60 | 4.65 × 104 | 6.96 | 67.01 | 63.22 | 4.58 × 104 |
| 10 | 90 | 3 | 2 | 10.24 | 76.25 | 76.69 | 5.17 × 104 | 10.10 | 76.63 | 77.07 | 5.23 × 104 |
| 11 | 70 | 2 | 3 | 8.83 | 70.79 | 65.02 | 4.92 × 104 | 8.56 | 69.96 | 65.83 | 4.94 × 104 |
| 12 | 80 | 2 | 2 | 9.52 | 71.09 | 74.13 | 5.13 × 104 | 9.35 | 72.32 | 72.50 | 5.07× 104 |
| 13 | 80 | 1 | 1 | 7.13 | 69.15 | 64.77 | 4.64 × 104 | 6.70 | 68.30 | 63.31 | 4.57 × 104 |
| 14 | 90 | 2 | 3 | 11.08 | 80.05 | 80.86 | 5.59 × 104 | 10.78 | 78.82 | 79.01 | 5.45 × 104 |
| 15 | 80 | 1 | 3 | 8.52 | 69.24 | 67.11 | 4.85 × 104 | 8.66 | 70.49 | 66.68 | 4.88 × 104 |
| 16 | 90 | 1 | 2 | 10.49 | 78.12 | 74.15 | 5.31 × 104 | 10.65 | 78.10 | 76.43 | 5.40 × 104 |
| 17 | 70 | 3 | 2 | 8.15 | 68.79 | 68.28 | 4.86 × 104 | 7.99 | 68.81 | 66.00 | 4.76 × 104 |
| FN-PUAE | |||||||||||
| Run | Independent Variables | Measured Response | Predicted Response | ||||||||
| Amplitude (%) | pH | Time (min) | Y (%) | GalA (g/100 g) | DE (%) | Mw (g/mol) | Y (%) | GalA (g/100 g) | DE (%) | Mw (g/mol) | |
| 1 | 60 | 2 | 40 | 7.08 | 72.46 | 75.20 | 3.78 × 104 | 7.16 | 69.71 | 74.79 | 3.79 × 104 |
| 2 | 100 | 3 | 40 | 8.11 | 76.33 | 78.12 | 4.01 × 104 | 7.73 | 74.20 | 76.76 | 3.88 × 104 |
| 3 | 100 | 2 | 60 | 8.83 | 80.24 | 81.07 | 4.19 × 104 | 9.14 | 81.94 | 83.33 | 4.24 × 104 |
| 4 | 20 | 1 | 40 | 6.71 | 69.91 | 72.91 | 3.62 × 104 | 7.10 | 72.04 | 74.27 | 3.75 × 104 |
| 5 | 60 | 2 | 40 | 7.53 | 70.19 | 76.55 | 3.96 × 104 | 7.16 | 69.71 | 74.79 | 3.79 × 104 |
| 6 | 60 | 3 | 60 | 6.24 | 57.09 | 69.75 | 3.59 × 104 | 6.31 | 57.52 | 68.84 | 3.66 × 104 |
| 7 | 20 | 2 | 60 | 6.35 | 64.32 | 71.14 | 3.51 × 104 | 6.16 | 62.97 | 70.49 | 3.47 × 104 |
| 8 | 60 | 1 | 20 | 5.12 | 53.17 | 62.11 | 3.32 × 104 | 5.05 | 52.74 | 63.02 | 3.24 × 104 |
| 9 | 100 | 1 | 40 | 8.02 | 74.19 | 78.56 | 3.82 × 104 | 7.90 | 73.27 | 77.00 | 3.86 × 104 |
| 10 | 60 | 1 | 60 | 8.42 | 78.15 | 80.23 | 4.05 × 104 | 8.23 | 77.37 | 79.53 | 3.95 × 104 |
| 11 | 20 | 2 | 20 | 5.69 | 62.07 | 68.64 | 3.27 × 104 | 5.38 | 60.37 | 66.38 | 3.21 × 104 |
| 12 | 60 | 2 | 40 | 7.25 | 72.62 | 75.03 | 3.73 × 104 | 7.16 | 69.71 | 74.79 | 3.79 × 104 |
| 13 | 20 | 3 | 40 | 5.28 | 55.55 | 64.31 | 3.29 × 104 | 5.40 | 56.47 | 65.87 | 3.25 × 104 |
| 14 | 60 | 2 | 40 | 7.36 | 71.10 | 75.19 | 3.84 × 104 | 7.16 | 69.71 | 74.79 | 3.79 × 104 |
| 15 | 60 | 2 | 40 | 6.59 | 62.18 | 71.99 | 3.67 × 104 | 7.16 | 69.71 | 74.79 | 3.79 × 104 |
| 16 | 100 | 2 | 20 | 5.33 | 58.99 | 66.50 | 3.15 × 104 | 5.52 | 60.34 | 67.16 | 3.18 × 104 |
| 17 | 60 | 3 | 20 | 4.90 | 57.16 | 64.37 | 2.96 × 104 | 5.09 | 57.94 | 65.07 | 3.05 × 104 |
| RN-PUAE | |||||||||||
| 1 | 60 | 2 | 40 | 7.14 | 70.56 | 72.39 | 3.81 × 104 | 7.47 | 72.53 | 74.52 | 3.96 × 104 |
| 2 | 100 | 3 | 40 | 8.26 | 76.82 | 78.09 | 4.14 × 104 | 7.97 | 75.58 | 76.46 | 3.93 × 104 |
| 3 | 100 | 2 | 60 | 8.94 | 78.64 | 80.04 | 4.23 × 104 | 9.08 | 79.52 | 81.66 | 4.37 × 104 |
| 4 | 20 | 1 | 40 | 6.75 | 69.11 | 71.38 | 3.41 × 104 | 7.04 | 70.35 | 73.01 | 3.62 × 104 |
| 5 | 60 | 2 | 40 | 7.29 | 71.42 | 73.57 | 3.87 × 104 | 7.47 | 72.53 | 74.52 | 3.96 × 104 |
| 6 | 60 | 3 | 60 | 7.37 | 72.68 | 75.12 | 3.95 × 104 | 7.51 | 73.04 | 75.13 | 4.02 × 104 |
| 7 | 20 | 2 | 60 | 6.44 | 68.27 | 73.11 | 3.42 × 104 | 6.04 | 67.45 | 72.14 | 3.39 × 104 |
| 8 | 60 | 1 | 20 | 6.23 | 67.02 | 71.44 | 3.29 × 104 | 6.09 | 66.66 | 71.43 | 3.22 × 104 |
| 9 | 100 | 1 | 40 | 8.11 | 76.94 | 77.56 | 3.98 × 104 | 7.86 | 76.48 | 76.60 | 4.02 × 104 |
| 10 | 60 | 1 | 60 | 8.52 | 77.16 | 78.29 | 4.19 × 104 | 8.63 | 76.74 | 77.63 | 4.01 × 104 |
| 11 | 20 | 2 | 20 | 5.07 | 62.12 | 68.06 | 3.00 × 104 | 4.93 | 61.24 | 66.44 | 2.86 × 104 |
| 12 | 60 | 2 | 40 | 7.73 | 73.09 | 76.03 | 4.08 × 104 | 7.47 | 72.53 | 74.52 | 3.96 × 104 |
| 13 | 20 | 3 | 40 | 4.88 | 64.13 | 64.13 | 3.35 × 104 | 5.13 | 64.59 | 65.09 | 3.31 × 104 |
| 14 | 60 | 2 | 40 | 7.68 | 73.25 | 75.68 | 4.02 × 104 | 7.47 | 72.53 | 74.52 | 3.96 × 104 |
| 15 | 60 | 2 | 40 | 7.51 | 74.33 | 74.92 | 4.06 × 104 | 1.47 | 72.53 | 74.52 | 3.96 × 104 |
| 16 | 100 | 2 | 20 | 5.16 | 65.49 | 70.90 | 2.87 × 104 | 5.56 | 66.31 | 71.87 | 2.90 × 104 |
| 17 | 60 | 3 | 20 | 5.52 | 63.28 | 65.09 | 2.63 × 104 | 5.41 | 63.70 | 65.85 | 2.81 × 104 |
Appendix B
| FN-CE | RN-CE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | Degree of Freedom | Mean Square | F -Value | Source | Sum of Squares | Degree of Freedom | Mean Square | F -Value |
| (A) Pectin Yield (%) | |||||||||
| Model | 28.49 | 9 | 3.17 | 27.32 *** | Model | 27.04 | 9 | 3.00 | 22.97 ** |
| T | 13.36 | 1 | 13.36 | 115.34 *** | T | 16.85 | 1 | 16.85 | 128.82 *** |
| pH | 0.05 | 1 | 0.05 | 0.40 ns | pH | 0.11 | 1 | 0.11 | 0.90 ns |
| t | 2.59 | 1 | 2.56 | 22.33 ** | t | 1.90 | 1 | 1.90 | 14.54 * |
| T × pH | 3.44 | 1 | 3.44 | 29.70 *** | T × pH | 0.62 | 1 | 0.62 | 4.77 ns |
| T × t | 0.58 | 1 | 0.58 | 5.05 ns | T × t | 0.45 | 1 | 0.45 | 3.48 ns |
| pH × t | 1.46 | 1 | 1.46 | 12.64 * | pH × t | 0.97 | 1 | 0.97 | 7.42 * |
| R2 = 0.972 | R2 = 0.967 | ||||||||
| (B) GalA (g/100 g) | |||||||||
| Model | 138.89 | 9 | 15.43 | 6.54 * | Model | 226.43 | 9 | 25.16 | 11.96 * |
| T | 60.01 | 1 | 60.01 | 25.42 ** | T | 178.70 | 1 | 178.70 | 84.93 *** |
| pH | 0.73 | 1 | 0.73 | 0.31 ns | pH | 0.05 | 1 | 0.05 | 0.02 ns |
| t | 10.65 | 1 | 10.65 | 4.51 ns | t | 2.14 | 1 | 2.14 | 1.02 ns |
| T × pH | 26.78 | 1 | 26.78 | 11.34 * | T × pH | 2.67 | 1 | 2.67 | 1.27 ns |
| T × t | 0.74 | 1 | 0.74 | 0.31 ns | T × t | 0.30 | 1 | 0.30 | 0.14 ns |
| pH × t | 1.58 | 1 | 1.58 | 0.66 ns | pH × t | 1.35 | 1 | 1.35 | 0.64 ns |
| R2 = 0.893 | R2 = 0.938 | ||||||||
| (C) DE (%) | |||||||||
| Model | 147.80 | 9 | 16.42 | 16.13 * | Model | 422.21 | 9 | 46.91 | 8.95 * |
| T | 73.63 | 1 | 73.63 | 72.30 *** | T | 294.88 | 1 | 294.88 | 56.27 *** |
| pH | 2.03 | 1 | 2.03 | 1.99 ns | pH | 5.88 | 1 | 5.88 | 1.12 ns |
| t | 6.09 | 1 | 6.09 | 5.98 ns | t | 5.33 | 1 | 5.33 | 1.02 ns |
| T × pH | 17.51 | 1 | 17.51 | 17.20 * | T × pH | 1.14 | 1 | 1.14 | 0.21 ns |
| T × t | 1.59 | 1 | 1.59 | 1.56 ns | T × t | 1.07 | 1 | 1.07 | 0.20 ns |
| pH × t | 3.46 | 1 | 3.46 | 3.40 ns | pH × t | 3.03 | 1 | 3.03 | 0.57 ns |
| R2 = 0.954 | R2 = 0.920 | ||||||||
| (D)Mw (g/mol) | |||||||||
| Model | 4.67 × 107 | 9 | 5.19× 106 | 31.88 *** | Model | 1.13 × 108 | 9 | 8.25 × 107 | 11.83 * |
| T | 2.11 × 107 | 1 | 2.11 × 107 | 129.77 *** | T | 8.25 × 107 | 1 | 0.11 × 105 | 66.82 *** |
| pH | 0.61 × 105 | 1 | 0.61 × 105 | 0.37 ns | pH | 0.11 × 105 | 1 | 4.80 × 106 | 0.01 ns |
| t | 4.65 × 106 | 1 | 4.65 × 106 | 28.57 *** | t | 4.80 × 106 | 1 | 3.06 × 106 | 3.89 ns |
| T × pH | 4.00 × 106 | 1 | 4.00 × 106 | 24.57 ** | T × pH | 3.06 × 106 | 1 | 1.69 × 106 | 2.48 ns |
| T × t | 4.90 × 106 | 1 | 4.90 × 106 | 3.01 ns | T × t | 1.69 × 106 | 1 | 2.56 × 106 | 1.37 ns |
| pH × t | 3.80 × 105 | 1 | 3.80 × 105 | 23.36 ** | pH × t | 2.56 × 106 | 1 | 2.56 × 106 | 2.07 ns |
| R2 = 0.976 | R2 = 0.938 | ||||||||
| FN-PUAE | RN-PUAE | ||||||||
| (A) Pectin Yield (%) | |||||||||
| Model | 22.55 | 9 | 2.51 | 14.67* | Model | 24.18 | 9 | 2.69 | 19.54** |
| A | 4.90 | 1 | 4.90 | 28.68*** | A | 6.72 | 1 | 6.72 | 48.85*** |
| pH | 1.75 | 1 | 1.75 | 10.24* | pH | 1.60 | 1 | 1.60 | 11.65* |
| t | 9.68 | 1 | 9.68 | 56.68*** | t | 10.79 | 1 | 10.79 | 78.47*** |
| A × pH | 0.57 | 1 | 0.57 | 3.38 ns | A × pH | 1.02 | 1 | 1.02 | 7.42* |
| A × t | 2.02 | 1 | 2.02 | 11.81* | A × t | 1.45 | 1 | 1.45 | 10.56* |
| pH × t | 0.96 | 1 | 0.96 | 5.62 ns | pH × t | 0.05 | 1 | 0.05 | 0.35 ns |
| R2 = 0.949 | R2 = 0.961 | ||||||||
| (B) GalA (g/100 g) | |||||||||
| Model | 1074.12 | 9 | 119.35 | 8.63 * | Model | 415.48 | 9 | 46.16 | 20.00 ** |
| A | 179.55 | 1 | 179.55 | 12.99 * | A | 146.72 | 1 | 146.72 | 63.57 *** |
| pH | 107.24 | 1 | 107.24 | 7.76 * | pH | 22.18 | 1 | 22.18 | 9.61 * |
| t | 292.94 | 1 | 292.94 | 21.19 ** | t | 188.57 | 1 | 188.57 | 81.71 *** |
| A × pH | 68.06 | 1 | 68.06 | 4.92 ns | A × pH | 5.90 | 1 | 5.90 | 2.56 ns |
| A × t | 90.25 | 1 | 90.25 | 6.53 * | A × t | 12.25 | 1 | 12.25 | 5.31 ns |
| pH × t | 156.88 | 1 | 156.88 | 11.35 * | pH × t | 0.14 | 1 | 0.14 | 0.06 ns |
| R2 = 0.917 | R2 = 0.963 | ||||||||
| (C) DE (%) | |||||||||
| Model | 507.06 | 9 | 56.34 | 11.74 * | Model | 297.22 | 9 | 33.02 | 9.47 * |
| A | 92.82 | 1 | 92.82 | 19.34 ** | A | 111.83 | 1 | 111.83 | 32.06 *** |
| pH | 37.24 | 1 | 37.24 | 7.76 * | pH | 32.56 | 1 | 32.56 | 9.34 * |
| t | 205.74 | 1 | 205.74 | 42.86 *** | t | 119.89 | 1 | 119.89 | 34.37 *** |
| A × pH | 16.65 | 1 | 16.65 | 3.47 ns | A × pH | 15.13 | 1 | 15.13 | 4.34 ns |
| A × t | 36.42 | 1 | 36.42 | 7.59 * | A × t | 4.18 | 1 | 4.18 | 1.20 ns |
| pH × t | 40.58 | 1 | 40.58 | 8.45 * | pH × t | 2.37 | 1 | 2.37 | 0.68 ns |
| R2 = 0.937 | R2 = 0.924 | ||||||||
| (D)Mw (g/mol) | |||||||||
| Model | 1.77 × 108 | 9 | 1.96 × 107 | 11.09 * | Model | 3.76 × 108 | 9 | 4.18 × 107 | 11.03 * |
| A | 2.73 × 107 | 1 | 2.73 × 107 | 15.44 * | A | 5.20 × 107 | 1 | 5.20 × 107 | 13.73 * |
| pH | 1.15 × 107 | 1 | 1.15 × 107 | 6.50 * | pH | 8.00 × 106 | 1 | 8.00 × 106 | 2.11 ns |
| t | 8.71 × 107 | 1 | 8.71 × 107 | 49.13 *** | t | 2.00 × 108 | 1 | 2.00 × 108 | 52.77 *** |
| A × pH | 6.76 × 106 | 1 | 6.76 × 106 | 3.81 ns | A × pH | 1.21 × 106 | 1 | 1.21 × 106 | 0.32 ns |
| A × t | 1.60 × 107 | 1 | 1.60 × 107 | 9.02 * | A × t | 2.21 × 107 | 1 | 2.21 × 107 | 5.83 ns |
| pH × t | 2.50 × 105 | 1 | 2.50 × 105 | 0.14 ns | pH × t | 4.41 × 106 | 1 | 4.41 × 106 | 1.16 ns |
| R2 = 0.934 | R2 = 0.934 | ||||||||
References
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A sustainable material for food and medical applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 2020, 38, 282–312. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Susanti, S.; Legowo, A.M.; Nurwantoro, N.; Silviana, S.; Arifan, F. Comparing the Chemical Characteristics of Pectin Isolated from Various Indonesian Fruit Peels. Indones. J. Chem. 2021, 21, 1057. [Google Scholar] [CrossRef]
- Rivadeneira, J.P.; Wu, T.; Ybanez, Q.; Dorado, A.A.; Migo, V.P.; Nayve, F.R.P.; Castillo-Israel, K.A.T. Microwave-assisted extraction of pectin from “Saba” banana peel waste: Optimization, characterization, and rheology study. Int. J. Food Sci. 2020, 2020, 8879425. [Google Scholar] [CrossRef]
- Jiang, L.N.; Shang, J.J.; He, L.B.; Dan, J.M. Comparisons of microwave-assisted and conventional heating extraction of pectin from seed watermelon peel. Adv. Mater. Res. 2012, 550–553, 1801–1806. [Google Scholar] [CrossRef]
- Sucheta; Misra, N.N.; Yadav, S.K. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: Kinetics, characterization and process economics. Food Hydrocoll. 2020, 102, 105592. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The influence of extraction conditions on the yield and physico-chemical parameters of pectin from grape pomace. Polymers 2022, 14, 1378. [Google Scholar] [CrossRef]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing green methods for pectin extraction from waste orange peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical properties of pectin from Malus domestica ‘Fălticeni’ apple pomace as affected by non-conventional extraction techniques. Food Hydrocoll. 2020, 100, 105383. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Melton, L.D.; Smith, B.G. Determination of the uronic acid content of plant cell walls using a colorimetric assay. Curr. Protoc. Food Anal. Chem. 2001, E3.3.1–E3.3.4. [Google Scholar] [CrossRef]
- Miceli-Garcia, L.G. Pectin from Apple Pomace: Extraction, Characterization, and Utilization in Encapsulating Alpha-Tocopherol Acetate. Master’s Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2014. [Google Scholar]
- Dranca, F.; Oroian, M. Ultrasound-assisted extraction of pectin from Malus domestica ‘Fălticeni’ apple pomace. Processes 2019, 7, 488. [Google Scholar] [CrossRef] [Green Version]
- Franchi, M.L. Evaluation of enzymatic pectin extraction by a recombinant polygalacturonase (PGI) from apples and pears pomace of argentinean production and characterization of the extracted pectin. J. Food Process. Technol. 2014, 5, 352. [Google Scholar] [CrossRef] [Green Version]
- Wai, W.W.; Alkarkhi, A.F.M.; Easa, A.M. Effect of extraction conditions on yield and degree of esterification of durian rind pectin: An experimental design. Food Bioprod. Process. 2010, 88, 209–214. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Tran, T.T.B.; Saifullah, M.; Nguyen, N.H.; Nguyen, M.H.; Vuong, Q.V. Comparison of ultrasound-assisted and conventional extraction for recovery of pectin from Gac (Momordica cochinchinensis) pulp. Future Foods 2021, 4, 100074. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Pectin extraction from common fig skin by different methods: The physicochemical, rheological, functional, and structural evaluations. Int. J. Biol. Macromol. 2019, 136, 275–283. [Google Scholar] [CrossRef]
- Nguyen, B.M.N.; Pirak, T. Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric. 2019, 5, 1633076. [Google Scholar] [CrossRef]
- Yousuf, O.; Singh, A.; Shahi, N.C.; Kumar, A.; Verma, A.K. Ultrasound assisted extraction of pectin from orange peel. Bull. Environ. Pharmacol. Life Sci. 2018, 7, 48–54. [Google Scholar]
- Patience, N.A.; Schieppati, D.; Boffito, D.C. Continuous and pulsed ultrasound pectin extraction from navel orange peels. Ultrason. Sonochem. 2021, 73, 105480. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef]
- Ke, J.; Jiang, G.; Shen, G.; Wu, H.; Liu, Y.; Zhang, Z. Optimization, characterization and rheological behavior study of pectin extracted from chayote (Sechium edule) using ultrasound assisted method. Int. J. Biol. Macromol. 2020, 147, 688–698. [Google Scholar] [CrossRef]
- Sabater, C.; Sabater, V.; Olano, A.; Montilla, A.; Corzo, N. Ultrasound-assisted extraction of pectin from artichoke by-products. An artificial neural network approach to pectin characterisation. Food Hydrocoll. 2020, 98, 105238. [Google Scholar] [CrossRef]
- Jafari, F.; Khodaiyan, F.; Kiani, H.; Hosseini, S.S. Pectin from carrot pomace: Optimization of extraction and physicochemical properties. Carbohydr. Polym. 2017, 157, 1315–1322. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
- Kute, A.B.; Mohapatra, D.; Kotwaliwale, N.; Giri, S.K.; Sawant, B.P. Characterization of pectin extracted from orange peel powder using microwave-assisted and acid extraction methods. Agric. Res. 2020, 9, 241–248. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Khedmat, L.; Izadi, A.; Mofid, V.; Mojtahedi, S.Y. Recent advances in extracting pectin by single and combined ultrasound techniques: A review of techno-functional and bioactive health-promoting aspects. Carbohydr. Polym. 2020, 229, 115474. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh-Moghaddam, M.; Shaddel, R.; Peighambardoust, S.H. Sugar beet pectin extracted by ultrasound or conventional heating: A comparison. J. Food Sci. Technol. 2021, 58, 2567–2578. [Google Scholar] [CrossRef]
- Matei, P.M.; Barbulescu, I.D.; Drăgotoiu, D.; Tudor, V.; Begea, M.; Teodorescu, R.I. Characterization of phytochemicals present in winery by-products with nutritional potential. Sci. Pap. Ser. D Anim. Sci. 2021, 64, 182–190. [Google Scholar]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Go, E.-J.; Song, K. Bin Development and characterization of citrus Junos pomace pectin films Incorporated with rambutan (Nephelium Lappaceum) peel extract. Coatings 2020, 10, 714. [Google Scholar] [CrossRef]
- Hundie, K.B. Optimization and characterization of ultrasound-assisted pectin extracted from orange waste. Pak. J. Anal. Environ. Chem. 2021, 22, 344–357. [Google Scholar] [CrossRef]
- Kacuráková, M. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Kalapathy, U.; Proctor, A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem. 2001, 73, 393–396. [Google Scholar] [CrossRef]
- Barbulescu, M.S.; Drăgotoiu, D.; Teodorescu, R.F.; Tudor, V.; Mihaela, P.; Matei, R.I.T. Nutritive importance of by-products from winemaking process for feed industry interest. Rom. Biotechnol. Lett. 2020, 25, 1759–1767. [Google Scholar]
- Almohammed, F.; Koubaa, M.; Khelfa, A.; Nakaya, M.; Mhemdi, H.; Vorobiev, E. Pectin recovery from sugar beet pulp enhanced by high-voltage electrical discharges. Food Bioprod. Process. 2017, 103, 95–103. [Google Scholar] [CrossRef]
- Ponmurugan, K.; Al-Dhabi, N.A.; Maran, J.P.; Karthikeyan, K.; Moothy, I.G.; Sivarajasekar, N.; Manoj, J.J.B. Ultrasound assisted pectic polysaccharide extraction and its characterization from waste heads of Helianthus annus. Carbohydr. Polym. 2017, 173, 707–713. [Google Scholar] [CrossRef]
- Chen, X.; Qi, Y.; Zhu, C.; Wang, Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int. J. Biol. Macromol. 2019, 131, 273–281. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.-H. Ultrasonic degradation of sweet potato pectin and its antioxidant activity. Ultrason. Sonochem. 2017, 38, 726–734. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Abboud, K.Y.; Iacomini, M.; Simas, F.F.; Cordeiro, L.M.C. High methoxyl pectin from the soluble dietary fiber of passion fruit peel forms weak gel without the requirement of sugar addition. Carbohydr. Polym. 2020, 246, 116616. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of microwave-assisted extraction and structural characterization of pectin from sweet lemon peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef]
- Yu, M.; Xia, Y.; Zhou, M.; Guo, Y.; Zheng, J.; Zhang, Y. Effects of different extraction methods on structural and physicochemical properties of pectins from finger citron pomace. Carbohydr. Polym. 2021, 258, 117662. [Google Scholar] [CrossRef]
- Sousa, A.G.; Nielsen, H.L.; Armagan, I.; Larsen, J.; Sørensen, S.O. The impact of rhamnogalacturonan-I side chain monosaccharides on the rheological properties of citrus pectin. Food Hydrocoll. 2015, 47, 130–139. [Google Scholar] [CrossRef]
- Vriesmann, L.C.; Teófilo, R.F.; de Oliveira Petkowicz, C.L. Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. LWT 2012, 49, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wang, F.; Wang, M.-M.; Tang, M.-T.; Zhou, T.; Gu, Q. Physicochemical, structural and rheological properties of pectin isolated from citrus canning processing water. Int. J. Biol. Macromol. 2022, 195, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.F.; da Costa Amaral, S.; Ruthes, A.C.; de Oliveira Petkowicz, C.L.; Kerkhoven, N.C.; da Silva, E.R.A.; Silveira, J.L.M. Pectins from the pulp of gabiroba (Campomanesia xanthocarpa Berg): Structural characterization and rheological behavior. Carbohydr. Polym. 2019, 214, 250–258. [Google Scholar] [CrossRef]











| Extraction Technique | Variables | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| CE | Temperature (°C) | 70 | 80 | 90 |
| Time (h) | 1 | 2 | 3 | |
| pH | 1 | 2 | 3 | |
| PUAE | Amplitude (%) | 20 | 60 | 100 |
| Time (min) | 20 | 40 | 60 | |
| pH | 1 | 2 | 3 | |
| Sample | Color Characteristics | Creep and Recovery Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | C*ab | h*ab | Je (1/Pa) | Jr (1/Pa) | (1/s) | η (Pa·s) | ))/d(log(t))·(1/s) | |
| FN-CE | 78.84 (0.16) c | 11.04 (0.21) b | 74.96 (0.28) d | 4.21 (0.08) a | 0.671 (0.31) b | 0.115 (0.03) a | 7.058 (0.28) b | 0.785 (0.22) b |
| RN-CE | 80.20 (0.24) c | 11.57 (0.18) a | 77.28 (0.36) c | 0.981 (0.26) b | 0.205 (0.24) c | 0.048 (0.08) c | 15.28 (0.17) b | 0.845 (0.08) a |
| FN-PUAE | 90.87 (0.19) a | 9.88 (0.29) c | 93.96 (0.14) a | 4.226 (0.18) a | 0.753 (0.18) a | 0.088 (0.12) b | 10.27 (0.23) b | 0.762 (0.18) c |
| RN-PUAE | 89.15 (0.11) b | 11.51 (0.27) a | 87.78 (0.22) b | 0.032 (0.05) c | 0.052 (0.06) d | 0.001 (0.02) d | 1450 (0.58) a | 0.643 (0.12) d |
| F-value | 198.36 *** | 57.25 *** | 900.26 *** | 3249.34 *** | 1.79 × 104 *** | 6836.50 *** | 1268.04 *** | 1.80 × 105 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinei, M.; Oroian, M. Characterization of Pectin from Grape Pomace: A Comparison of Conventional and Pulsed Ultrasound-Assisted Extraction Techniques. Foods 2022, 11, 2274. https://doi.org/10.3390/foods11152274
Spinei M, Oroian M. Characterization of Pectin from Grape Pomace: A Comparison of Conventional and Pulsed Ultrasound-Assisted Extraction Techniques. Foods. 2022; 11(15):2274. https://doi.org/10.3390/foods11152274
Chicago/Turabian StyleSpinei, Mariana, and Mircea Oroian. 2022. "Characterization of Pectin from Grape Pomace: A Comparison of Conventional and Pulsed Ultrasound-Assisted Extraction Techniques" Foods 11, no. 15: 2274. https://doi.org/10.3390/foods11152274
APA StyleSpinei, M., & Oroian, M. (2022). Characterization of Pectin from Grape Pomace: A Comparison of Conventional and Pulsed Ultrasound-Assisted Extraction Techniques. Foods, 11(15), 2274. https://doi.org/10.3390/foods11152274






