Antioxidant Activity and Anti-Nutritional Factors of Selected Wild Edible Plants Collected from Northeastern Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
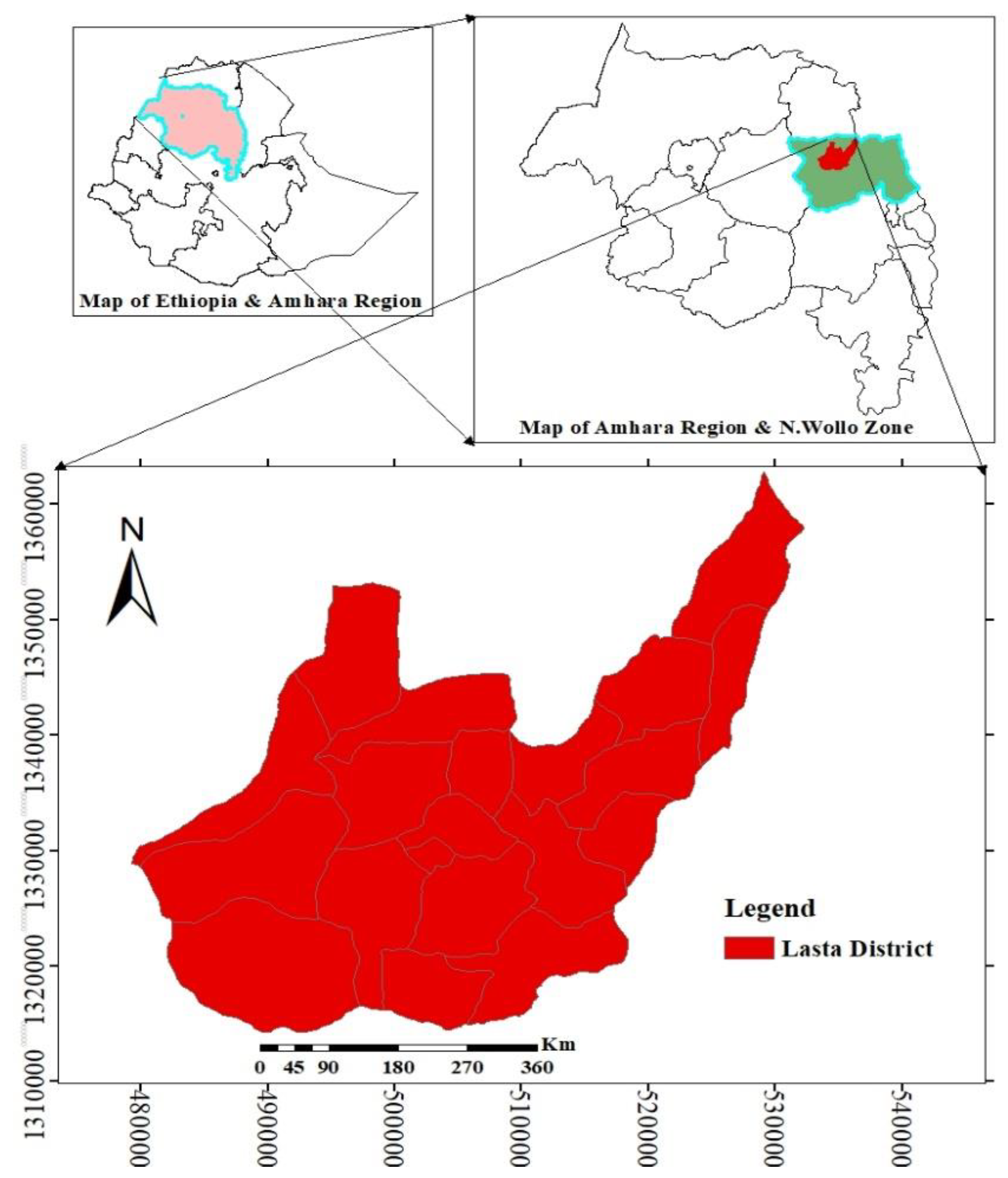
2.1. Description of the Study Area
2.2. Plant Sample Collection for Determination of Antioxidant Activity and Anti-Nutritional Factors
2.3. Determination of Antioxidant Activities
2.4. Determination of Anti-Nutritional Factors
2.5. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolics and Flavonoids
3.2. Radical Scavenging Activity Using DPPH
3.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.4. Anti-Nutritional Factors of WEPs
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Raghavendra, H.; Prashithkekuda, T.; Chetan, D. Phytochemical analysis and in vitro antioxidant activity of Rubus apetalus Poir (Rosaceae). Pharmacol. OnLine 2018, 1, 187–195. [Google Scholar] [CrossRef]
- Rout, P.; Basak, U.C. Screening of antinutritional factors of nine underutilized wild edible fruits of Odisha. Ann. Biol. Sci. 2015, 3, 21–27. [Google Scholar]
- Mekonnen, A.; Desta, W. Comparative study of the antioxidant and antibacterial activities of Rumex abyssinicus with commercially available Zingiber officinale and Curcuma longa in Bahir Dar city, Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 2. [Google Scholar] [CrossRef]
- Getachew, G.A.; Asfaw, Z.; Singh, V.; Woldu, Z.; Baidu-Forson, J.J.; Bhattacharya, S. Dietary values of wild and semi-wild edible plants in Southern Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2013, 13, 7485–7503. [Google Scholar] [CrossRef]
- Reda, T.H.; Atsbha, M.K. Nutritional composition, antinutritional factors, antioxidant activities, functional properties, and sensory evaluation of cactus pear (Opuntia ficus-indica) seeds grown in tigray region, Ethiopia. Int. J. Food Sci. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tekle, A.; Belay, A.; Kelem, K.; Wodajo, B.; Tesfaye, Y. Nutritional Profile of Moringa stenopetala Species Samples Collected from Different Places in Ethiopia. Eur. J. Nutr. Food Saf. 2013, 5, 1100–1101. [Google Scholar] [CrossRef]
- Nuraga, G.W.; Feyissa, T.; Demissew, S.; Tesfaye, K.; Woldegiorgis, A.Z. Comparison of proximate, mineral and phytochemical composition of enset (Ensete ventricosum (Welw.) Cheesman) landraces used for a different purpose. Afr. J. Agric. Res. 2019, 14, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Hailu, A.A.; Addis, G. The content and bioavailability of mineral nutrients of selected wild and traditional edible plants as affected by household preparation methods practiced by local community in Benishangul Gumuz Regional State, Ethiopia. Int. J. Food Sci. 2016, 2016, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nayak, J.; Basak, U.C. Analysis of some nutritional properties in eight wild edible fruits of Odisha, India. Int. J. Curr. Sci. 2015, 14, 55–62. [Google Scholar]
- Alemayehu, G.; Asfaw, Z.; Kelbessa, E. Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora District, North Shewa Zone of Amhara Region, Ethiopia. J. Med. Plants Stud. 2015, 3, 1–11. [Google Scholar]
- Lulekal, E.; Asfaw, Z.; Kelbessa, E.; Van Damme, P. Wild edible plants in Ethiopia: A review on their potential to combat food insecurity. Afr. Focus 2011, 24, 71–122. [Google Scholar] [CrossRef]
- CSA (Central Statistics Agency). Summary and Statistical Report of the 2007 Population and Housing Census Results; Federal Democratic Republic of Ethiopia Population Census Commission: Addis Ababa, Ethiopia, 2007.
- LDARDO (Lasta District Agriculture and Rural Development Office). Annual Report of Lasta District Agriculture and Rural Development Office; Lasta District Agriculture and Rural Development Office: Lalibela, Ethiopia, 2021.
- Friis, I.; Demissew, S.; van Breugel, P. Atlas of the Potential Vegetation of Ethiopia; The Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 2011. [Google Scholar]
- Adamu, E. Plant-Human Interaction in Lasta District, North Wollo Zone, Ethiopia with Emphasis on Abune Yosef Mountain and Its Environs. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2022. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Shukla, R.K.; Painuly, D.; Porval, A.; Shukla, A. Proximate analysis, nutritional value, phytochemical evaluation, and biological activity of Litchi chinensis Sonn. leaves. J. Herbs Spices Med. Plants 2014, 20, 196–208. [Google Scholar] [CrossRef]
- Benzie, I.F.; Szeto, Y. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Ukpabi, U.; Ejidoh, J. Effect of Deep Oil Frying on Oxalates Contents and Degree of Itching of Cocoyam; Computer Edge Publishers: London, UK, 1989; pp. 84–88. [Google Scholar]
- Latta, M.; Eskin, M. A simple and rapid colorimetric method for phytate determination. J. Agric. Food Chem. 1980, 28, 1313–1315. [Google Scholar] [CrossRef]
- Maxson, E.; Rooney, L. Evaluation of methods for tannin analysis in sorghum grain. Cereal Chem. 1972, 49, 719–728. [Google Scholar]
- Maina, S.; Ryu, D.H.; Bakari, G.; Misinzo, G.; Nho, C.W.; Kim, H.-Y. Variation in Phenolic Compounds and Antioxidant Activity of Various Organs of African Cabbage (Cleome gynandra L.) Accessions at Different Growth Stages. Antioxidants 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Kifle, E.A. Nutritional Qualities, Health Benefits and the Potential for Complementary Food Formulation of Three Types of Amaranthus Caudatus Grain Cultivated in Ethiopia as Affected by Processing. Ph.D. Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Song, Y.; Wei, X.-Q.; Li, M.-Y.; Duan, X.-W.; Sun, Y.-M.; Yang, R.-L.; Su, X.-D.; Huang, R.-M.; Wang, H. Nutritional composition and antioxidant properties of the fruits of a Chinese wild Passiflora foetida. Molecules 2018, 23, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Kwinana-Mandindi, T. Phytochemical and antioxidant composition of selected local wild plants in south africa: Consideration of alternative nutrients for health promotion. In Proceedings of the 5th International Conference on Biomedical Engineering and Technology, Seoul, Korea, 10–11 March 2015; pp. 96–102. [Google Scholar]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Mahadkar, S.; Jadhav, V.; Deshmukh, S. Pelagia Research Library. Chem. Sin. 2013, 4, 165–169. [Google Scholar]
- Rabeta, M.; Faraniza, R.N. Total phenolic content and ferric reducing antioxidant power of the leaves and fruits of Garcinia atrovirdis and Cynometra cauliflora. Int. Food Res. J. 2013, 20, 1691–1696. [Google Scholar]
- Adane, T.; Shimelis, A.; Negussie, R.; Tilahun, B.; Haki, G.D. Effect of processing method on the Proximate composition, mineral content and antinutritional factors of Taro (Colocasia esculenta, L.) growth in Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2013, 13, 7383–7396. [Google Scholar]
- Haile, A.; Retta, N.; Abuye, C. Evaluation of methods for roots processing on removal of anti-nutritional Factors of selected cultivars of cassava (Manihot esculenta C.) grown in Ethiopia. J. Sci. Dev. 2014, 2, 87–100. [Google Scholar]
- Unuofin, J.O.; Otunola, G.A.; Afolayan, A.J. Nutritional evaluation of Kedrostis africana (L.) Cogn: An edible wild plant of South Africa. Asian Pac. J. Trop. Biomed. 2017, 7, 443–449. [Google Scholar] [CrossRef]
- Fekadu, H.; Beyene, F.; Desse, G. Effect of traditional processing methods on nutritional composition and anti-nutritional factors of anchote (Coccinia Abyssinica (lam.) Cogn) tubers grown in Western Ethiopia. J. Food Process. Technol. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schjønning, P.; Elmholt, S.; Christensen, B.T. Managing Soil Quality: Challenges in Modern Agriculture; CABI: Wallingford, UK, 2004. [Google Scholar]
- Oke, O.; Redhead, J.; Hussain, M. Roots, Tubers, Plantains and Bananas in Human Nutrition; FAO Food and Nutrition Series; FAO: Rome, Italy, 1990; Volume 24. [Google Scholar]
- Ekop, A.; Eddy, N. Comparative Studies of The Level of Toxicant in the Seeds of Terminalia catappa (india almond) and Coula edulis (African walnut). Chem. Cl. J. 2005, 2, 74–76. [Google Scholar]
- Kumari, M.; Jain, S. Screening of potential sources of tannin and its therapeutic application. Int. J. Nutr. Food Sci. 2015, 4, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Ayalew, D.; Keber, T.; Ayenew, M. Evaluation of anti-nutritional factor reduction techniques for triticale improved utilization system in Amhara region. J. Food Process. Technol. 2017, 8, 1–5. [Google Scholar] [CrossRef]


| No. | Species | Plant Part | Total Phenols (mg GAE/100 g) | Total Flavonoids (mg QE/100 g) |
|---|---|---|---|---|
| 1 | Amaranthus hybridus | Grain | 10.00 ± 0.01 c | 5.97 ± 0.43 cd |
| Leaf | 13.13 ± 0.05 f | 7.12 ± 0.10 d | ||
| 2 | Erucastrum abyssinicum | Leaf | 12.69 ± 0.00 e | 6.23 ± 0.16 cd |
| 3 | Erucastrum arabicum | Leaf | 8.62 ± 0.02 b | 3.57 ± 0.29 b |
| 4 | Haplocarpha rueppelii | Leaf | 17.02 ± 0.03 h | 5.43 ± 0.12 c |
| 5 | Haplocarpha schimperi | Leaf | 11.69 ± 0.03 d | 5.74 ± 0.14 c |
| 6 | Rumex nervosus | Young shoot | 16.28 ± 0.03 g | 2.27 ± 0.16 a |
| 7 | Urtica simensis | Leaf | 0.79 ± 0.02 a | 5.02 ± 0.40 c |
| Species | Concentration (µg/mL) | % of Inhibition |
|---|---|---|
| Amaranthus hybridus (Grain) | 20 | 2.74 ± 0.10 |
| 40 | 12.92 ± 0.24 | |
| 80 | 14.25 ± 0.15 | |
| 120 | 24.63 ± 0.12 | |
| 160 | 33.59 ± 0.22 | |
| 200 | 52.31 ± 0.34 | |
| Amaranthus hybridus (Leaf) | 20 | 29.03 ± 0.16 |
| 40 | 55.82 ± 0.34 | |
| 80 | 89.00 ± 0.26 | |
| 120 | 89.11 ± 0.12 | |
| 160 | 91.94 ± 0.11 | |
| 200 | 92.89 ± 0.22 | |
| Erucastrum abyssinicum (Leaf) | 20 | 14.10 ± 0.27 |
| 40 | 18.76 ± 0.13 | |
| 80 | 36.78 ± 0.25 | |
| 120 | 69.78 ± 0.11 | |
| 160 | 82.97± 0.18 | |
| 200 | 84.99 ± 0.21 | |
| Erucastrum arabicum (Leaf) | 20 | 1.61 ± 0.23 |
| 40 | 5.65 ± 0.14 | |
| 80 | 29.10 ± 0.23 | |
| 120 | 36.63 ± 0.42 | |
| 160 | 51.11 ± 0.31 | |
| 200 | 83.02 ± 0.20 | |
| Haplocarpha rueppelii (Leaf) | 20 | 40.05 ± 0.16 |
| 40 | 42.27 ± 0.29 | |
| 80 | 70.25 ± 0.14 | |
| 120 | 85.12 ± 0.10 | |
| 160 | 89.00 ± 0.22 | |
| 200 | 92.96 ± 0.13 | |
| Haplocarpha schimperi (Leaf) | 20 | 25.93 ± 0.21 |
| 40 | 44.26 ± 0.24 | |
| 80 | 53.05 ± 0.38 | |
| 120 | 83.70 ± 0.33 | |
| 160 | 88.37 ± 0.11 | |
| 200 | 88.69 ± 0.43 | |
| Rumex nervosus (Young shoot) | 20 | 39.50 ± 0.28 |
| 40 | 42.30 ± 0.12 | |
| 80 | 69.40 ± 0.36 | |
| 120 | 73.45 ± 0.10 | |
| 160 | 81.19 ± 0.16 | |
| 200 | 97.30 ± 0.13 | |
| Urtica simensis (Leaf) | 20 | 15.65 ± 0.22 |
| 40 | 19.06 ± 0.13 | |
| 80 | 29.46 ± 0.43 | |
| 120 | 35.80 ± 0.15 | |
| 160 | 45.40 ± 0.22 | |
| 200 | 62.25 ± 0.17 | |
| Ascorbic acid standard | 20 | 25.98 ± 0.11 |
| 40 | 45.40 ± 0.20 | |
| 80 | 89.22 ± 0.16 | |
| 120 | 96.87 ± 0.13 | |
| 160 | 97.64 ± 0.23 | |
| 200 | 97.68 ± 0.29 |
| Species | Concentration (µg/mL) | FRAP Assay (mM) |
|---|---|---|
| Amaranthus hybridus (Grain) | 50 | 65.75 ± 0.02 |
| 175 | 71.79 ± 0.04 | |
| 300 | 139.25 ± 0.01 | |
| Amaranthus hybridus (Leaf) | 50 | 172.51 ± 0.11 |
| 175 | 180.08 ± 0.24 | |
| 300 | 485.45 ± 0.02 | |
| Erucastrum abyssinicum | 50 | 74.80 ± 0.02 |
| 175 | 111.27 ± 0.02 | |
| 300 | 410.90 ± 0.04 | |
| Erucastrum arabicum | 50 | 80.31 ± 0.11 |
| 175 | 162.82 ± 0.13 | |
| 300 | 432.39 ± 0.04 | |
| Haplocarpha rueppelii | 50 | 183.71 ± 0.06 |
| 175 | 312.53 ± 0.11 | |
| 300 | 471.39 ± 0.23 | |
| Haplocarpha schimperi | 50 | 134.43 ± 0.02 |
| 175 | 186.43 ± 0.05 | |
| 300 | 529.82 ± 0.08 | |
| Rumex nervosus | 50 | 169.67 ± 0.12 |
| 175 | 371.07 ± 0.06 | |
| 300 | 455.93 ± 0.14 | |
| Urtica simensis | 50 | 88.13 ± 0.22 |
| 175 | 130.44 ± 0.17 | |
| 300 | 421.79 ± 0.04 | |
| Ascorbic acid | 50 | 45.37 ± 0.15 |
| 175 | 170.76 ± 0.23 | |
| 300 | 304.04 ± 0.22 | |
| FeSO4 standard | 50 | 53.93 ± 0.16 |
| 175 | 167.55 ± 0.28 | |
| 300 | 304.29 ± 0.32 |
| No. | Species | Plant Part | Oxalate (mg/100 g) | Phytate (µg/100 g) | Tannins (mg/100 g) |
|---|---|---|---|---|---|
| 1 | Amaranthus hybridus | Grain | 3.37 ± 0.25 a | 165.88 ± 0.01 ef | 1.71 ± 0.10 b |
| Leaf | 5.13 ± 0.25 bc | 82.81 ± 0.00 c | 3.35 ± 0.10 d | ||
| 2 | Erucastrum abyssinicum | Leaf | 11.73 ± 0.25 e | 33.16 ± 0.02 b | 3.73 ± 0.25 e |
| 3 | Erucastrum arabicum | Leaf | 5.13 ± 0.25 bc | 165.95 ± 0.01 g | 5.49 ± 0.16 f |
| 4 | Haplocarpha rueppelii | Leaf | 9.09 ± 0.25 d | 165.86 ± 0.01 e | 1.38 ± 0.12 a |
| 5 | Haplocarpha schimperi | Leaf | 5.57 ± 0.25 c | 165.92 ± 0.00 fg | 3.60 ± 0.12 de |
| 6 | Rumex nervosus | Young shoot | 4.99 ± 0.25 b | 16.31 ± 0.00 a | 5.44 ± 0.20 f |
| 7 | Urtica simensis | Leaf | 5.13 ± 0.25 bc | 82.99 ± 0.01 d | 2.21 ± 0.13 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamu, E.; Asfaw, Z.; Demissew, S.; Baye, K. Antioxidant Activity and Anti-Nutritional Factors of Selected Wild Edible Plants Collected from Northeastern Ethiopia. Foods 2022, 11, 2291. https://doi.org/10.3390/foods11152291
Adamu E, Asfaw Z, Demissew S, Baye K. Antioxidant Activity and Anti-Nutritional Factors of Selected Wild Edible Plants Collected from Northeastern Ethiopia. Foods. 2022; 11(15):2291. https://doi.org/10.3390/foods11152291
Chicago/Turabian StyleAdamu, Endale, Zemede Asfaw, Sebsebe Demissew, and Kaleab Baye. 2022. "Antioxidant Activity and Anti-Nutritional Factors of Selected Wild Edible Plants Collected from Northeastern Ethiopia" Foods 11, no. 15: 2291. https://doi.org/10.3390/foods11152291
APA StyleAdamu, E., Asfaw, Z., Demissew, S., & Baye, K. (2022). Antioxidant Activity and Anti-Nutritional Factors of Selected Wild Edible Plants Collected from Northeastern Ethiopia. Foods, 11(15), 2291. https://doi.org/10.3390/foods11152291






