Assessment and Prediction of Fish Freshness Using Mathematical Modelling: A Review
Abstract
:1. Introduction
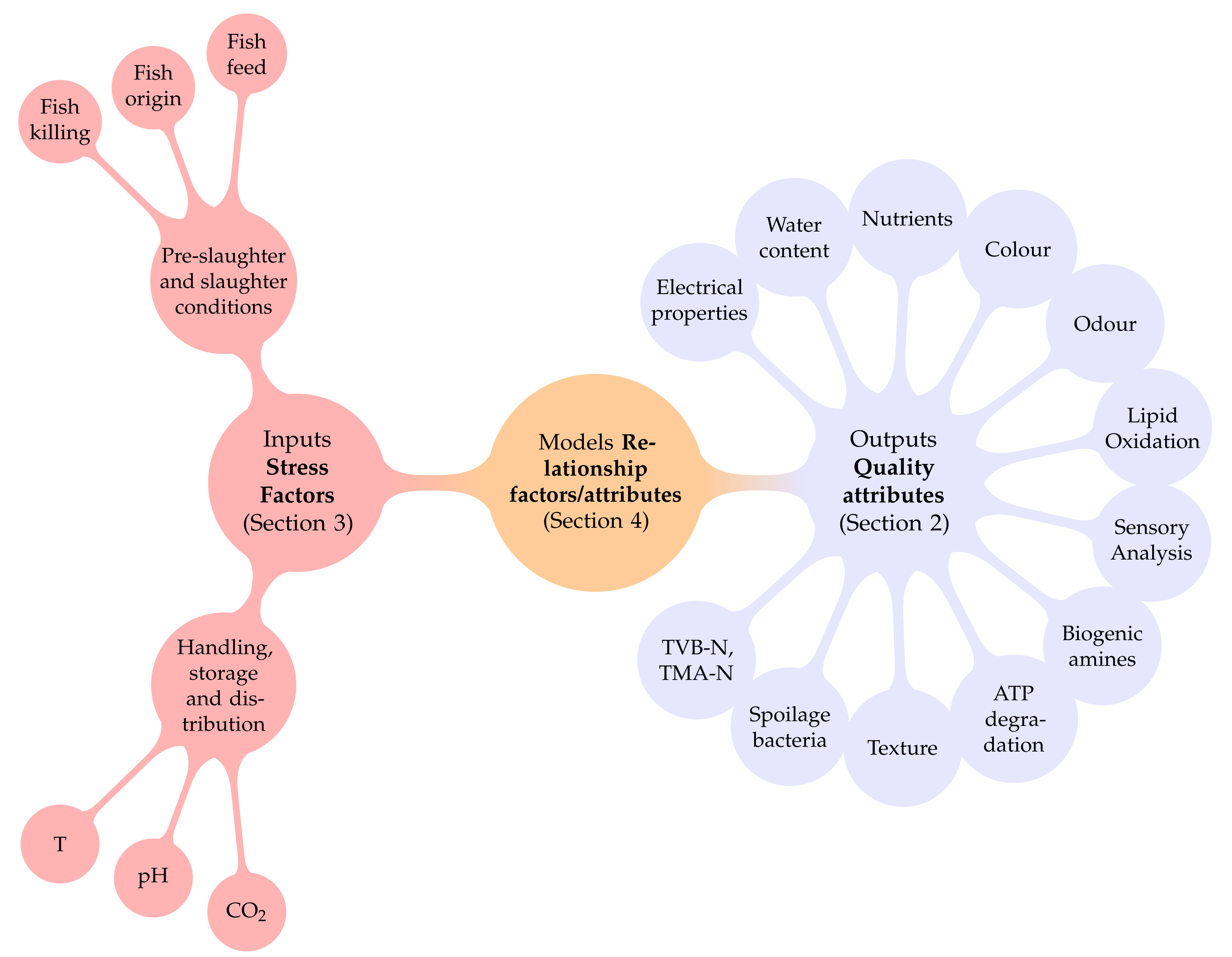
2. Quality Attributes (Model Outputs)
2.1. Lipid Oxidation
2.2. Sensory Analysis
2.3. TVB-N/TMA-N
2.4. Spoilage Bacteria
2.5. Texture Properties
2.6. ATP Degradation
2.7. Biogenic Amines
2.8. Odour
2.9. Colour
2.10. Nutrients
2.11. Water Content/Activity
2.12. Electrical Properties
3. Stress Factors and Their Usual Models (Additionally, Named Secondary Models)
4. Models (Relationship between Model Inputs and Outputs)
- Shelf life soft sensors are models that consider a direct input/output relationship. They consist of empirical functions, denoted by soft (from software) or virtual sensors. Typically, the input and the output are, respectively, temperature and shelf life.
- Quality soft multi-sensors are models considering a general mathematical expression that can be applied to describe more than one attribute.
- Quality ad hoc models are mechanistic-based models with equations specifically derived for one particular quality attribute.
- Sensory or shelf life models are models providing as their output a sensory score or shelf life date. However, they also require the intrinsic modelling of one or several quality indicators (such as spoilage bacterial content). To this purpose, they typically consider a quality ad hoc model. These sensors are also named smart when they are used not only for assessment purposes but for prediction of different degrees of fish quality as well [97].
4.1. Shelf Life Soft Sensors (Input/Output)
4.2. Soft Multi-Sensors
4.3. Quality ad hoc Models
4.3.1. Spoilage Bacteria Models Using Predictive Microbiology
4.3.2. TVB-N and TMA-N Models
4.3.3. Texture Properties
4.3.4. ATP Degradation
4.4. Sensory or Shelf Life Models
5. Modelling Challenges and New Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Systematic Literature Search
| Set | Search | Records |
|---|---|---|
| #1 | (TI OR AB OR AK) = ( (“quality” OR “freshness” OR “shelf life” OR “shelf-life” OR “K-value” OR “KI-value” OR “ATP” OR “adenosine triphosphate” OR “IMP” OR “inosine monophosphate” OR “hypoxanthine” OR “colour” OR “color” OR “chromatism” OR “fatty acid*” OR “lipid oxidation” OR “tba” OR “electrical properties” OR “electrical conductance” OR “electrical conductivity” OR “texture” OR “hardness” OR “firmness” OR “odour” OR “odor” OR “nutrient*” OR “vitamin” OR “biogenic amine*” OR “water content” OR “water activity” OR “tvb-n” OR “tma-n” OR “qim” OR “qsm” OR “sensory analysis” OR “sensory evaluation” OR “sensory method” OR “sso” OR “spoilage bacteria” OR “spoilage microorganism*") NEAR/5 ("fish” OR “fishes” OR “shellfish” OR “seafood*” OR “albacore” OR “amberjack” OR “anchovy” OR “angler” OR “barbel” OR “barracuda*” OR “sea bass” OR “beluga” OR “bigeye” OR “blackfish” OR “bluefish” OR “blue runner” OR “blue shark” OR “branzino” OR “seabream” OR “sea bream” OR “butterfish” OR “carp” OR “catfish” OR “catshark” OR “comber” OR “conger” OR “cutlassfish” OR “danubian wels” OR “dogfish” OR “eel” OR “eels” OR “flounder” OR “flying fish” OR “forkbeard” OR “garfish” OR “garrick” OR “guitarfish” OR “gunard” OR “haddock” OR “hake” OR “halibut” OR “hammerhead” OR “herring” OR “icefish” OR “John dory” OR “lamprey” OR “lanternfish” OR “leerfish” OR “little tunny” OR “mackerel” OR “mahi mahi” OR “marlin” OR “megrim” OR “melva” OR “monkfish” OR “moonfish” OR “needlefish” OR “pandoras” OR “panga” OR “pangasius” OR “parrotfish” OR “parrot fish” OR “perch” OR “pike fish” OR “pilchard” OR “pilotfish” OR “pilot fish” OR “plaice” OR “pollack” OR “pollock” OR “ponyfish” OR “porbeagle” OR “rainbow trout” OR “cownose ray” OR “devilray” OR “butterfly ray” OR “softnose skate” OR “legskate” OR “sawfish” OR “ribbonfish” OR “rockfish” OR “rosefish” OR “sablefish” OR “sailfish” OR “salmon” OR “sardine” OR “sardinella” OR “scabbardfish” OR “scorpionfish” OR “sheatfish” OR “shi drum” OR “sillago” OR “skipjack” OR “smooth hound” OR “smooth-hound” OR “spearfish” OR “St Peter’s fish” OR “stargazer” OR “stingray” OR “sturgeon” OR “surgeon fish” OR “swordfish” OR “tilapia” OR “threadfin” OR “triggerfish” OR “trout” OR “tubefish” OR “tuna” OR “turbot” OR “walleye” OR “whitebait” OR “whiting” OR “yellowtail” OR “octopus” OR “squid*” OR “crab” OR “lobster*” OR “prawn*” OR “shrimp*” OR “cuttlefish*” OR “crayfish*” OR “langoustine*” OR “scampi*” OR “urchin”) ) | 6429 |
| #2 | (TI OR AB OR AK) = ("high pressure” OR “atmospheric cold plasma” OR “modified atmosphere*” OR “sterilization” OR “sterilisation” OR “frozen” OR “thawed” OR “pH-shift processing” OR “cross-processing” OR “fillet*” OR “slice*” OR “fish oil*” OR “quality of water” OR “water quality” OR “frying” OR “garlic” OR “canned fish*” OR “surimi” OR “fish sauce” OR “nugget” OR “chitosan” OR “x-ray*” OR “farming” OR “farm-level” OR “antibacterial” OR “antimicrobial” OR “electronarcosis” OR “immun*” OR “Fluorescence in situ hybridization” OR “cooking” OR “microplastic*” OR “embryo*” OR “rice” OR “drying” OR “dried” OR “nursery” OR “fishmeal” OR “plastic” OR “irradiation” OR “larva*” OR “feed*” OR “coating” OR “diet” OR “dietary” OR “reproductive” OR “ibuprofen” OR “fertilizer*” OR “valorization” OR “catch damage” OR “thermal process*” OR “non-thermal process*” OR “non thermal process*” OR “children” OR “gear design” OR “additive” OR “mimicry” OR “seed quality” OR “sodium alginate” OR “edible film*” OR “TYRP1 gene*” OR “transgene” OR “antioxida* peptide*” OR “antioxida* capacity” OR “antioxida* solution” OR “antioxida* effect*” OR “anti-oxida* activity” OR “antioxida* activity” OR “rearing” OR “algae” OR “collagen expression” OR “genomic*” OR “proteomic*” OR “s-potential*” OR “fish meal” OR “synthesis” OR “egg quality” OR “carotenogenesis” OR “nutrient requirement*” OR “maternal” OR “oily fish” OR “gelatin” OR “polychlorinated” OR “nutrition of salmonoid” OR “fish retina” OR “color picture*” OR “elderly” OR “inheritance of color” OR “short read mapping program” OR “tea polyphenol” OR “fish consumption” OR “inter-specific hybrids” OR “extracellular lipase” OR “pathology” OR “metabolic polymorphisms” OR “transgenic” OR “eco-label” OR “lethality” OR “micro-squid” OR “emulsion*” OR “vegetable production” OR “source of nutrient*” OR “hydrolyzate*” OR “epiphitic” OR “bilirubin” OR “essential oil*” OR “histology” OR “egg-yolk” OR “chd” OR “silver toxicity” OR “biomanipulation” OR “hormonal-control” OR “protein crosslinking” OR “food security” OR “high hydrostatic pressure” OR “red tide*” OR “intake” OR “digestibility” OR “nutrient absorption” OR “nutrition” OR “docking” OR “egg” OR “diet” OR “nutrient recycling” OR “farm effluents” OR “fish behavior” OR “smoked” OR “chromatophore*” OR “hydroponic” OR “recovery of fish” OR “fish recovery” OR “fish-odor syndrome” OR “fish-odour syndrome” OR “water chemistry” OR “natural preservatives” OR “mince” OR “epiphytic” OR “phosphorus” OR “omega3” OR “hemolysate” OR “hemolysis” OR “availability of nutrients” OR “biosynthesis” OR “health” OR “globalization” OR “inhibition of polyphenoloxidase” OR “species identification” OR “virus” OR “norovirus” OR “ferment*” OR “bread” OR “plant extract*” OR “nanoencapsulate*” OR “starch” OR “edible compound*” OR “edible natural compound*” OR “fish burger*” OR “wafer*” OR “bromelain” OR “fish ball*” OR “fish cake*” OR “dehydrat*” OR “grill*” OR “4-hexylresorcinol” OR “freezing” OR “freezing-point” OR “restructure*” OR “squid oil” OR “molecular distilation”) | 332,851 |
| #3 | #1 NOT #2 | 1636 |
| #4 | (TI OR AB OR AK) = (“mathematical model*” OR “predictive model*” OR “dynamic model*” OR “growth model*” OR “predictive microbiology” OR “model”) | 922,980 |
| #5 | #3 AND #4 | 33 |
References
- Raak, N.; Symmank, C.; Zahn, S.; Aschemann-Witzel, J.; Rohm, H. Processing- and product-related causes for food waste and implications for the food supply chain. Waste Manag. 2017, 61, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Corradini, M.G. Shelf Life of Food Products: From Open Labeling to Real-Time Measurements. Annu. Rev. Food Sci. Technol. 2018, 9, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Zöller, S.; Wachtel, M.; Knapp, F.; Steinmetz, R. Going all the way—Detecting and transmitting events with wireless sensor networks in logistics. In Proceedings of the 38th Annual IEEE Conference on Local Computer Networks—Workshops, Sydney, Australia, 21–24 October 2013; pp. 39–47. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. The use of the so-called ‘tubs’ for transporting and storing fresh fishery products. EFSA J. 2020, 18, 1–123. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. The use of the so-called ‘superchilling’ technique for the transport of fresh fishery products. EFSA J. 2021, 19. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Sun, D.W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Venugopal, V. Biosensors in fish production and quality control. Biosens. Bioelectron. 2002, 17, 147–157. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Jonsdottir, R.; Lauzon, H.L.; Luten, J.; Kristbergsson, K. Characterization of volatile compounds in chilled cod (Gadus morhua) fillets by gas chromatography and detection of quality indicators by an electronic nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef]
- Li, J.; Feng, H.; Liu, W.; Gao, Y.; Hui, G. Design of A Portable Electronic Nose system and Application in K Value Prediction for Large Yellow Croaker (Pseudosciaena crocea). Food Anal. Methods 2016, 9, 2943–2951. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W.; He, Y. Novel non-invasive distribution measurement of texture profile analysis (TPA) in salmon fillet by using visible and near infrared hyperspectral imaging. Food Chem. 2014, 145, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.H.; Sun, D.W.; Cheng, J.H.; Pu, H. Mapping moisture contents in grass carp (Ctenopharyngodon idella) slices under different freeze drying periods by Vis-NIR hyperspectral imaging. LWT 2017, 75, 529–536. [Google Scholar] [CrossRef]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, L.; Berardinelli, A.; Dabbou, S.; Ragni, L.; Tartagni, M. Sensing technology for fish freshness and safety: A review. Sensors 2021, 21, 1373. [Google Scholar] [CrossRef] [PubMed]
- Chantarachoti, J.; Oliveira, A.C.; Himelbloom, B.H.; Crapo, C.A.; McLachlan, D.G. Portable electronic nose for detection of spoiling alaska pink salmon (Oncorhynchus gorbuscha). J. Food Sci. 2006, 71, 414–421. [Google Scholar] [CrossRef]
- Ying, X.; Zinnai, A.; Venturi, F.; Sanmartin, C.; Deng, S. Freshness evaluation of grass carp (Ctenopharyngodon idellus) by electronic nose. J. Food Meas. Charact. 2017, 11, 1026–1034. [Google Scholar] [CrossRef]
- Calanche, J.; Pedrós, S.; Roncalés, P.; Beltrán, J.A. Design of predictive tools to estimate freshness index in farmed sea bream (Sparus aurata) stored in ice. Foods 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, R.C.; Lopes, V.V.; Vicente, A.A.; Teixeira, J.A. Computational shelf-life dating: Complex systems approaches to food quality and safety. Food Bioprocess Technol. 2008, 1, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Férez-Rubio, J.; García, M.R.; Vilas, C. A systematic review on fish freshness and quality indicators. Zenodo 2022, 6414360. [Google Scholar] [CrossRef]
- Richards, M.P.; Hultin, H.O. Contributions of blood and blood components to lipid oxidation in fish muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Grigorakis, K.; Alexis, M.N.; Anthony Taylor, K.D.; Hole, M. Comparison of wild and cultured gilthead sea bream (Sparus aurata); composition, appearance and seasonal variations. Int. J. Food Sci. Technol. 2002, 37, 477–484. [Google Scholar] [CrossRef]
- Ólafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.M.; Henehan, G.; Nielsen, J.; Nilsen, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Al Bulushi, I.; Poole, S.; Deeth, H.C.; Dykes, G.A. Biogenic amines in fish: Roles in intoxication, spoilage, and nitrosamine formation—A review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, G.; Nesvadba, P.; Di Natale, C.; Careche, M.; Oehlenschläger, J.; Tryggvadóttir, S.V.; Schubring, R.; Kroeger, M.; Heia, K.; Esaiassen, M.; et al. Multisensor for fish quality determination. Trends Food Sci. Technol. 2004, 15, 86–93. [Google Scholar] [CrossRef]
- Pacquit, A.; Frisby, J.; Diamond, D.; Lau, K.T.; Farrell, A.; Quilty, B.; Diamond, D. Development of a smart packaging for the monitoring of fish spoilage. Food Chem. 2007, 102, 466–470. [Google Scholar] [CrossRef]
- Papadopoulos, V.; Chouliara, I.; Badeka, A.; Savvaidis, I.N.; Kontominas, M.G. Effect of gutting on microbiological, chemical, and sensory properties of aquacultured sea bass (Dicentrarchus labrax) stored in ice. Food Microbiol. 2003, 20, 411–420. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Sensory and biochemical aspects of quality of whole bigeye tuna (Thunnus obesus) during bulk storage in controlled atmospheres. Food Chem. 2005, 89, 347–354. [Google Scholar] [CrossRef]
- Gram, L.; Trolle, G.; Huss, H.H. Detection of specific spoilage bacteria from fish stored at low (0 °C) and high (20 °C) temperatures. Int. J. Food Microbiol. 1987, 4, 65–72. [Google Scholar] [CrossRef]
- Dalgaard, P. Modelling of microbial activity and prediction of shelf life for packed fresh fish. Int. J. Food Microbiol. 1995, 26, 305–317. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.; Öksüz, A.; Garthwaite, T.; Alexis, M.N.; Grigorakis, K. Freshness assessment of cultured sea bream (Sparus aurata) by chemical, physical and sensory methods. Food Chem. 2001, 72, 33–40. [Google Scholar] [CrossRef]
- Veciana-Nogués, M.T.; Mariné-Font, A.; Vidal-Carou, M.C. Biogenic Amines as Hygienic Quality Indicators of Tuna. Relationships with Microbial Counts, ATP-Related Compounds, Volatile Amines, and Organoleptic Changes. J. Agric. Food Chem. 1997, 45, 2036–2041. [Google Scholar] [CrossRef]
- Jones, N.R.; Murray, J.; Livingston, E.I.; Murray, C.K. Rapid estimations of hypoxanthine concentrations as indices of the freshness of chill-stored fish. J. Sci. Food Agric. 1964, 15, 763–774. [Google Scholar] [CrossRef]
- Kim, M.K.; Mah, J.H.; Hwang, H.J. Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chem. 2009, 116, 87–95. [Google Scholar] [CrossRef]
- Ramanathan, L.; Das, N.P. Studies on the Control of Lipid Oxidation in Ground Fish by Some Polyphenolic Natural Products. J. Agric. Food Chem. 1992, 40, 17–21. [Google Scholar] [CrossRef]
- Kawai, T. Fish Flavor. Crit. Rev. Food Sci. Nutr. 1996, 36, 257–298. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Huang, X.; Xin, J.; Zhao, J. A novel technique for rapid evaluation of fish freshness using colorimetric sensor array. J. Food Eng. 2011, 105, 632–637. [Google Scholar] [CrossRef]
- Chakraborty, K.; Raj, R.P. An extra-cellular alkaline metallolipase from Bacillus licheniformisMTCC 6824: Purification and biochemical characterization. Food Chem. 2008, 109, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Moreda-Piñeiro, J.; Alonso-Rodríguez, E.; Romarís-Hortas, V.; Moreda-Piñeiro, A.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Bermejo-Barrera, P. Assessment of the bioavailability of toxic and non-toxic arsenic species in seafood samples. Food Chem. 2012, 130, 552–560. [Google Scholar] [CrossRef]
- Palaniappan, P.R.; Vijayasundaram, V. Fourier transform infrared study of protein secondary structural changes in the muscle of Labeo rohita due to arsenic intoxication. Food Chem. Toxicol. 2008, 46, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Cakli, S.; Kilinc, B.; Cadun, A.; Dincer, T.; Tolasa, S. Quality differences of whole ungutted sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) while stored in ice. Food Control 2007, 18, 391–397. [Google Scholar] [CrossRef]
- Morzel, M.; Sohier, D.; Van De Vis, H. Evaluation of slaughtering methods for turbot with respect to animal welfare and flesh quality. J. Sci. Food Agric. 2003, 83, 19–28. [Google Scholar] [CrossRef]
- Raju, C.V.; Shamasundar, B.A.; Udupa, K.S. The use of nisin as a preservative in fish sausage stored at ambient (28 ± 2 °C) and refrigerated (6 ± 2 °C) temperatures. Int. J. Food Sci. Technol. 2003, 38, 171–185. [Google Scholar] [CrossRef]
- Vaz-Pires, P.; Seixas, P.; Mota, M.; Lapa-Guimarães, J.; Pickova, J.; Lindo, A.; Silva, T. Sensory, microbiological, physical and chemical properties of cuttlefish (Sepia officinalis) and broadtail shortfin squid (Illex coindetii) stored in ice. LWT - Food Sci. Technol. 2008, 41, 1655–1664. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Luo, Y.; Sun, Y.; Shen, H. Establishment of kinetic models based on electrical conductivity and freshness indictors for the forecasting of crucian carp (Carassius carassius) freshness. J. Food Eng. 2011, 107, 147–151. [Google Scholar] [CrossRef]
- Ryder, J.M. Determination of Adenosine Triphosphate and Its Breakdown Products in Fish Muscle by High-Performance Liquid Chromatography. J. Agric. Food Chem. 1985, 33, 678–680. [Google Scholar] [CrossRef]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. A review. Ital. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- (EC) Regulation Council. Laying down common marketing standards foe certain fishery products. Off. J. Eur. Communities 1996, 334, L-334/2. [Google Scholar]
- Luten, J.B.; Martinsdottir, E. QIM: A European tool for fish freshness evaluation in the fishery chain. In Methods to Determine the Freshness of Fish in Research and Industry, Proceedings of the Final Meeting of the Concerted Action ‘Evaluation of Fish Freshness’ AIR3CT94 2283, Nantes, France, 12–14 November l997; Elsevier: Amsterdam, The Netherlands, 1997; pp. 287–296. [Google Scholar]
- Hong, H.; Luo, Y.; Zhu, S.; Shen, H. Application of the general stability index method to predict quality deterioration in bighead carp (Aristichthys nobilis) heads during storage at different temperatures. J. Food Eng. 2012, 113, 554–558. [Google Scholar] [CrossRef]
- Howgate, P. A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage. Int. J. Food Sci. Technol. 2006, 41, 341–353. [Google Scholar] [CrossRef]
- Dai, Q.; Cheng, J.H.; Sun, D.W.; Zhu, Z.; Pu, H. Prediction of total volatile basic nitrogen contents using wavelet features from visible/near-infrared hyperspectral images of prawn (Metapenaeus ensis). Food Chem. 2016, 197, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological spoilage and investigation of volatile profile during storage of sea bream fillets under various conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.A.; Nicholson, F.J.; Roger, A.; Stroud, G.D. Freezing and Refrigerated Storage in Fisheries; Food & Agriculture Org.: Rome, Italy, 1994. [Google Scholar]
- Taoukis, P.S.; Koutsoumanis, K.; Nychas, G.J. Use of time-temperature integrators and predictive modelling for shelf life control of chilled fish under dynamic storage conditions. Int. J. Food Microbiol. 1999, 53, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Koutsoumanis, K.; Nychas, G.J.E. Application of a systematic experimental procedure to develop a microbial model for rapid fish shelf life predictions. Int. J. Food Microbiol. 2000, 60, 171–184. [Google Scholar] [CrossRef]
- Correia Peres Costa, J.C.; Floriano, B.; Bascón Villegas, I.M.; Rodríguez-Ruiz, J.P.; Posada-Izquierdo, G.D.; Zurera, G.; Pérez-Rodríguez, F. Study of the microbiological quality, prevalence of foodborne pathogens and product shelf-life of Gilthead sea bream (Sparus aurata) and Sea bass (Dicentrarchus labrax) from aquaculture in estuarine ecosystems of Andalusia (Spain). Food Microbiol. 2020, 90, 103498. [Google Scholar] [CrossRef] [PubMed]
- Dabadé, D.S.; Azokpota, P.; Nout, M.J.; Hounhouigan, D.J.; Zwietering, M.H.; Den Besten, H.M. Prediction of spoilage of tropical shrimp (Penaeus notialis) under dynamic temperature regimes. Int. J. Food Microbiol. 2015, 210, 121–130. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, T.A.; Ross, T.; Olley, J. Application of predictive microbiology to assure the quality and safety of fish and fish products. Int. J. Food Microbiol. 1992, 15, 13–32. [Google Scholar] [CrossRef]
- Davies, A.R. Modified-atmosphere packaging of fish and fish products. Fish Process. Technol. 1997, 33, 200–223. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Dunajski, E. Texture of Fish Muscle. J. Texture Stud. 1980, 10, 301–318. [Google Scholar] [CrossRef]
- Oehlenschläger, J. Seafood Quality Assessment. In Seafood Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; Chapter 14; pp. 359–386. [Google Scholar] [CrossRef]
- Huidobro, A.; Pastor, A.; Tejada, M. Quality index method developed for raw gilthead seabream (Sparus aurata). J. Food Sci. 2000, 65, 1202–1205. [Google Scholar] [CrossRef]
- Lakshmanam, P.T.; Gopakumar, K. K-value, an index for estimating fish freshness and quality. Curr. Sci. 1999, 76, 400–404. [Google Scholar]
- Howgate, P. Kinetics of degradation of adenosine triphosphate in chill-stored rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Technol. 2005, 40, 579–588. [Google Scholar] [CrossRef]
- Vilas, C.; Alonso, A.A.; Herrera, J.R.; Bernárdez, M.; García, M.R. A mathematical model to predict early quality attributes in hake during storage at low temperature. J. Food Eng. 2018, 222, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Kuda, T.; Fujita, M.; Goto, H.; Yano, T. Effects of retort conditions on ATP-related compounds in pouched fish muscle. LWT - Food Sci. Technol. 2008, 41, 469–473. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Haard, N. The role of enzymes in determining seafood color, flavour and texture. In Safety and Quality Issues in Fish Processing; Bremmer, H.A., Ed.; Woodhead Publishing Limited: Sawston, UK, 2002; pp. 220–253. [Google Scholar] [CrossRef]
- Li, K.; Luo, Y.; Shen, H. Postmortem Changes of Crucian Carp (Carassius auratus) During Storage in Ice. Int. J. Food Prop. 2015, 18, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; ichi Arai, K.; Matsuyoshi, M. A New Method for Estimating the Freshness of Fish. Nippon Suisan Gakkaishi 1959, 24, 749–750. [Google Scholar] [CrossRef]
- Karube, I.; Matsuoka, H.; Suzuki, S.; Watanabe, E.; Toyama, K. Determination of Fish Freshness with an Enzyme Sensor System. J. Agric. Food Chem. 1984, 32, 314–319. [Google Scholar] [CrossRef]
- Surette, M.E.; Gill, T.A.; LeBlanc, P.J. Biochemical basis of postmortem nucleotide catabolism in cod (Gadus morhua) and its relationship to spoilage. J. Agric. Food Chem. 1988, 36, 19–22. [Google Scholar] [CrossRef]
- Zaragozá, P.; Fuentes, A.; Fernández-Segovia, I.; Vivancos, J.L.; Rizo, A.; Ros-Lis, J.V.; Barat, J.M.; Martínez-Máñez, R. Evaluation of sea bream (Sparus aurata) shelf life using an optoelectronic nose. Food Chem. 2013, 138, 1374–1380. [Google Scholar] [CrossRef]
- Yamanaka, H.; Shiomi, K.; Kikuchi, T. Cadaverine as a Potential Index for Decomposition of Salmonoid Fishes. Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1989, 30, 170. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Mabuchi, R.; Adachi, M.; Ishimaru, A.; Zhao, H.; Kikutani, H.; Tanimoto, S. Changes in Metabolic Profiles of Yellowtail (Seriola quinqueradiata) Muscle during Cold Storage as a Freshness Evaluation Tool Based on GC-MS Metabolomics. Foods 2019, 8, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhakar, P.K.; Srivastav, P.P.; Pathak, S.S.; Das, K. Mathematical Modeling of Total Volatile Basic Nitrogen and Microbial Biomass in Stored Rohu (Labeo rohita) Fish. Front. Sustain. Food Syst. 2021, 5, 1–14. [Google Scholar] [CrossRef]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Enomoto, T.; Miyamoto, E.; Nakatsuka, T.; Nakano, Y. Characterization of vitamin B 12 compounds from edible shellfish, clam, oyster, and mussel. Int. J. Food Sci. Nutr. 2001, 52, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.O.; Achinewhu, S.C. Effects of shucking method on opening, meat yield and selected quality parameters of West African clam, Galatea paradoxa (Born). J. Food Process. Preserv. 2000, 24, 365–377. [Google Scholar] [CrossRef]
- Antunes-Rohling, A.; Artaiz, Á.; Calero, S.; Halaihel, N.; Guillén, S.; Raso, J.; Álvarez, I.; Cebrián, G. Modelling microbial growth in modified-atmosphere-packed hake (Merluccius merluccius) fillets stored at different temperatures. Food Res. Int. 2019, 122, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, A. Den Einfluss der Temperatur auf die Verdampfung. Z. Anal. Chem. 1889, 28, 329. [Google Scholar] [CrossRef] [Green Version]
- Ratkowsky, D.A.; Olley, J.; McMeekin, T.A.; Ball, A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 1982, 149, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMeekin, T.A.; Chandler, R.E.; Doe, P.E.; Garland, C.D.; Olley, J.; Putro, S.; Ratkowsky, D.A. Model for combined effect of temperature and salt concentration/water activity on the growth rate of Staphylococcus xylosus. J. Appl. Bacteriol. 1987, 62, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Wijtzes, T.; De Wit, J.C.; Riet, K.V.T. A decision support system for prediction of the microbial spoilage in foods. J. Food Prot. 1992, 55, 973–979. [Google Scholar] [CrossRef]
- Dalgaard, P.; Buch, P.; Silberg, S. Seafood Spoilage Predictor—Development and distribution of a product specific application software. Int. J. Food Microbiol. 2002, 73, 343–349. [Google Scholar] [CrossRef]
- Bro, R.; Van den Berg, F.; Thybo, A.; Andersen, C.M.; Jørgensen, B.M.; Andersen, H. Multivariate data analysis as a tool in advanced quality monitoring in the food production chain. Trends Food Sci. Technol. 2002, 13, 235–244. [Google Scholar] [CrossRef]
- Limbo, S.; Sinelli, N.; Torri, L.; Riva, M. Freshness decay and shelf life predictive modelling of European sea bass (Dicentrarchus labrax) applying chemical methods and electronic nose. LWT - Food Sci. Technol. 2009, 42, 977–984. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Lu, W.; Shen, H.; Luo, Y. Quality predictive models of grass carp (Ctenopharyngodon idellus) at different temperatures during storage. Food Control 2011, 22, 1197–1202. [Google Scholar] [CrossRef]
- Giuffrida, A.; Valenti, D.; Giarratana, F.; Ziino, G.; Panebianco, A. A new approach to modelling the shelf life of Gilthead seabream (Sparus aurata). Int. J. Food Sci. Technol. 2013, 48, 1235–1242. [Google Scholar] [CrossRef] [Green Version]
- Dai, Q.; Cheng, J.H.; Sun, D.W.; Zeng, X.A. Potential of hyperspectral imaging for non-invasive determination of mechanical properties of prawn (Metapenaeus ensis). J. Food Eng. 2014, 136, 64–72. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, Y.; Feng, L.; Bao, Y. Establishment of kinetic models based on electrical conductivity and global stability index for predicting the quality of allogynogenetic crucian carps (Carassius auratus gibelio) during chilling storage. J. Food Process. Preserv. 2015, 39, 167–174. [Google Scholar] [CrossRef]
- García, M.R.; Vilas, C.; Herrera, J.R.; Bernárdez, M.; Balsa-Canto, E.; Alonso, A.A. Quality and shelf-life prediction for retail fresh hake (Merluccius merluccius). Int. J. Food Microbiol. 2015, 208, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Vilas, C.; Alonso, A.A.; Herrera, J.R.; García-Blanco, A.; García, M.R. A model for the biochemical degradation of inosine monophosphate in hake (Merluccius merluccius). J. Food Eng. 2017, 200, 95–101. [Google Scholar] [CrossRef] [Green Version]
- García, M.R.; Cabo, M.L.; Herrera, J.R.; Ramilo-Fernández, G.; Alonso, A.A.; Balsa-Canto, E. Smart sensor to predict retail fresh fish quality under ice storage. J. Food Eng. 2017, 197, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Li, B.; Jiang, C. Shelf life prediction and Bacterial flora for the fresh and lightly salted Pseudosciaena crocea stored at different temperatures. Emir. J. Food Agric. 2018, 30, 39–48. [Google Scholar] [CrossRef]
- Genç, I.Y.; Diler, A. Development of Shelf Life Prediction Model in Rainbow Trout Stored at Different Temperatures. J. Aquat. Food Prod. Technol. 2019, 28, 1027–1036. [Google Scholar] [CrossRef]
- Shorten, P.R.; Soboleva, T.K.; Pleasants, A.B.; Membré, J.M. A risk assessment approach applied to the growth of Erwinia carotovora in vegetable juice for variable temperature conditions. Int. J. Food Microbiol. 2006, 109, 60–70. [Google Scholar] [CrossRef]
- García, M.R.; Vázquez, J.A.; Teixeira, I.G.; Alonso, A.A. Stochastic individual-based modeling of bacterial growth and division using flow cytometry. Front. Microbiol. 2018, 8, 2626. [Google Scholar] [CrossRef] [Green Version]
- Garre, A.; Egea, J.A.; Esnoz, A.; Palop, A.; Fernandez, P.S. Tail or artefact? Illustration of the impact that uncertainty of the serial dilution and cell enumeration methods has on microbial inactivation. Food Res. Int. 2019, 119, 76–83. [Google Scholar] [CrossRef]
- Balsa-Canto, E.; Alonso, A.A.; Arias-Méndez, A.; García, M.R.; López-Núñez, A.; Mosquera-Fernández, M.; Vázquez, C.; Vilas, C. Modeling and Optimization Techniques with Applications in Food Processes, Bio-Processes and Bio-Systems; Springer: Berlin/Heidelberg, Germany, 2016; Volume 9, pp. 187–216. [Google Scholar] [CrossRef]
- Vilas, C.; Arias-Méndez, A.; García, M.R.; Alonso, A.A.; Balsa-Canto, E. Toward predictive food process models: A protocol for parameter estimation. Crit. Rev. Food Sci. Nutr. 2018, 58, 436–449. [Google Scholar] [CrossRef]
- Dalgaard, P. Predictive microbiological modelling and seafood quality. Seaf. Prod. Consum. Integr. Approach Qual. 1997, 38, 431–443. [Google Scholar]
- Liu, X.; Jiang, Y.; Shen, S.; Luo, Y.; Gao, L. Comparison of Arrhenius model and artificial neuronal network for the quality prediction of rainbow trout (Oncorhynchus mykiss) fillets during storage at different temperatures. LWT - Food Sci. Technol. 2015, 60, 142–147. [Google Scholar] [CrossRef]
- Emborg, J.; Dalgaard, P. Modelling the effect of temperature, carbon dioxide, water activity and pH on growth and histamine formation by Morganella psychrotolerans. Int. J. Food Microbiol. 2008, 128, 226–233. [Google Scholar] [CrossRef]
- Banga, J.R.; Alonso, A.A.; Gallardo, J.M.; Pérez-Martín, R.I. Kinetics of thermal degradation of thiamine and surface colour in canned tuna. Z. Fur Lebensm.-Unters. Und Forsch. 1993, 197, 131–137. [Google Scholar] [CrossRef]
- Kong, F.; Tang, J.; Rasco, B.; Crapo, C. Kinetics of salmon quality changes during thermal processing. J. Food Eng. 2007, 83, 510–520. [Google Scholar] [CrossRef]
- Scherer, E.; Sandoval, A.; Barreiro, J.A. Kinetics of heat-induced color change of a tuna-vegetable mixture. Interciencia 2009, 34, 888–892. [Google Scholar]
- Van Impe, J.F.; Poschet, F.; Geeraerd, A.H.; Vereecken, K.M. Towards a novel class of predictive microbial growth models. Int. J. Food Microbiol. 2005, 100, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Va not Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- McKellar, R.C.; Lu, X. Primary models. Model. Microb. Responses Food 2003, 21–62. [Google Scholar] [CrossRef]
- Jain, D.; Pathare, P.B.; Manikantan, M.R. Evaluation of texture parameters of Rohu fish (Labeo rohita) during iced storage. J. Food Eng. 2007, 81, 336–340. [Google Scholar] [CrossRef]
- Ross, T.; McMeekin, T.A. Predictive microbiology. Int. J. Food Microbiol. 1994, 23, 241–264. [Google Scholar] [CrossRef]
- Koutsoumanis, K. Predictive Modeling of the Shelf Life of Fish under Nonisothermal Conditions. Appl. Environ. Microbiol. 2001, 67, 1821–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsoumanis, K.; Giannakourou, M.C.; Taoukis, P.S.; Nychas, G.J. Application of shelf life decision system (SLDS) to marine cultured fish quality. Int. J. Food Microbiol. 2002, 73, 375–382. [Google Scholar] [CrossRef]
- Wilson, P.D.; Brocklehurst, T.F.; Arino, S.; Thuault, D.; Jakobsen, M.; Lange, M.; Farkas, J.; Wimpenny, J.W.; Van Impe, J.F. Modelling microbial growth in structured foods: Towards a unified approach. Int. J. Food Microbiol. 2002, 73, 275–289. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Koutsoumanis, K.; Nychas, G.J.; Taoukis, P.S. Field evaluation of the application of time temperature integrators for monitoring fish quality in the chill chain. Int. J. Food Microbiol. 2005, 102, 323–336. [Google Scholar] [CrossRef]
- Nuin, M.; Alfaro, B.; Cruz, Z.; Argarate, N.; George, S.; Le Marc, Y.; Olley, J.; Pin, C. Modelling spoilage of fresh turbot and evaluation of a time-temperature integrator (TTI) label under fluctuating temperature. Int. J. Food Microbiol. 2008, 127, 193–199. [Google Scholar] [CrossRef]
- Possas, A.; Valero, A.; Pérez-Rodríguez, F. New software solutions for microbiological food safety assessment and management. Curr. Opin. Food Sci. 2022, 44, 100814. [Google Scholar] [CrossRef]
- de Prada, C.; Galán-Casado, S.; Pitarch, J.L.; Sarabia, D.; Galán, A.; Gutiérrez, G. Gemelos Digitales en la Industria de Procesos. Rev. Iberoam. Autom. Inform. Ind. 2022; in press. [Google Scholar] [CrossRef]
- Vilas, C.; Mauricio-Iglesias, M.; García, M.R. Model-based design of smart active packaging systems with antimicrobial activity. Food Packag. Shelf Life 2020, 24, 100446. [Google Scholar] [CrossRef]

| Quality Attribute | Citation Counts | No. Works | Avg. Citations per Work | Most Cited Works |
|---|---|---|---|---|
| Lipid oxidation | 10,875 | 474 | 22.9 | Herrero [12], Richards and Hultin [20], Grigorakis et al. [21] |
| Sensory analysis | 5295 | 209 | 25.3 | Ólafsdóttir et al. [22], Al Bulushi et al. [23], Olafsdottir et al. [24] |
| TVB-N, TMA-N | 4386 | 185 | 23.7 | Pacquit et al. [25], Papadopoulos et al. [26], Ruiz-Capillas and Moral [27] |
| Spoilage bacteria | 3876 | 155 | 25.0 | Al Bulushi et al. [23], Gram et al. [28], Dalgaard [29] |
| Texture | 3587 | 176 | 20.4 | Herrero [12], Olafsdottir et al. [24], Alasalvar et al. [30] |
| ATP degradation | 3509 | 124 | 28.3 | Ólafsdóttir et al. [22], Veciana-Nogués et al. [31], Jones et al. [32] |
| Biogenic amines | 3282 | 112 | 29.3 | Al Bulushi et al. [23], Veciana-Nogués et al. [31], Kim et al. [33] |
| Odour | 3066 | 119 | 25.8 | Papadopoulos et al. [26], Ramanathan and Das [34], Kawai [35] |
| Colour | 2830 | 149 | 19.0 | Pacquit et al. [25], Kuswandi et al. [36], Huang et al. [37] |
| Nutrients | 704 | 62 | 11.3 | Chakraborty and Raj [38], Moreda-Piñeiro et al. [39], Palaniappan and Vijayasundaram [40] |
| Water content/activity | 573 | 32 | 17.9 | Cakli et al. [41], Morzel et al. [42], Raju et al. [43] |
| Electrical properties | 381 | 11 | 34.6 | Olafsdottir et al. [24], Vaz-Pires et al. [44], Yao et al. [45] |
| Output | Matrix | Model | References |
|---|---|---|---|
| Shelf life | Bogue | Arrhenius emp. | Taoukis et al. [56] |
| Shelf life | European sea bass | Exponential emp. | Limbo et al. [90] |
| Shelf life | Large yellow croaker | Exponential emp. & school-field | Quanyou et al. [98] |
| Output | Matrix | Secondary Model | Primary Model | References |
|---|---|---|---|---|
| TVB-N, TAC, K-value | Grass carp | Arrhenius | Exponential model | Zhang et al. [91] |
| TVB-N, TAC, K-value, EC | Crucian carp | Arrhenius | Exponential model | Yao et al. [45] |
| GSI (Sensory Score, TAC, TVB-N, K-value) | Bighead carp | Arrhenius | Linear model | Hong et al. [51] |
| GSI (sensory score, K-value, TAC and TVB-N), EC | Crucian carp | Arrhenius | Linear model | Zhu et al. [94] |
| SL, SFI | Gilt-head seabream | (pH, TM, IT, ST, TVB-N) | Weighted regression coefficients. | Calanche et al. [17] |
| Output | Matrix | Secondary Model | Primary Model | References |
|---|---|---|---|---|
| Pseudomonas & Shewanella | Bogue | Arrhenius & Ratkowsky | Baranyi’s model | Taoukis et al. [56] |
| Pseudomonas & Shewanella | Gilt-head seabream | & Arrhenius & Ratkowsky | Mod. logistic model | Koutsoumanis and Nychas [57] |
| Sulphide producers & non-producers | Gilt-head seabream | (not clearly defined) | Baranyi’s model | Giuffrida et al. [92] |
| Pseudomonas & Carnobacterium | Tropical shrimp | Arrhenius & Ratkowsky | Baranyi’s model Rep. Gompertz Model | Dabadé et al. [59] |
| Pseudomonas & Shewanella | Hake | Ratkowsky | Baranyi’s model | García et al. [95] |
| TVC | Grass carp | – | Rep. Gompertz Model | Ying et al. [16] |
| Psychrotrophic counts | Cod | – | Baranyi’s Model | García et al. [97] |
| Pseudomonas, Enterobacteriaceae, TMAB, TPAB & LAB | Rainbox trout | Ratkowsky | Mod. Logistic Model | Genç and Diler [99] |
| Pseudomonas | Gilt-head seabream | – | Mod. Logistic Model | Correia Peres Costa et al. [58] |
| Biomass | Rohu fish | – | Mod. Logistic Model Gompertz Model | Prabhakar et al. [80] |
| Output | Matrix | Secondary Model | Primary Model | References |
|---|---|---|---|---|
| IMP, Ino, Hx | Rainbow trout | Arrhenius | Exponential model, Bacterial catalysis | Howgate [67] |
| IMP, Ino, Hx | Forty-five species | Arrhenius | Exponential model, Bacterial catalysis, leaching | Howgate [52] |
| IMP, Ino, Hx | Hake | Arrhenius | First-order reaction model | Vilas et al. [96] |
| IMP, Ino, Hx | Hake | Arrhenius | First-order reaction model, Bacterial catalysis, leaching | Vilas et al. [68] |
| Output | Matrix | Secondary Model | Primary Model | References |
|---|---|---|---|---|
| (15 levels) | Gilt-head seabream | Not clearly defined | Giuffrida et al. [92] | |
| Council Regulation(EC) No 2406/96 (1996) Standard method (4 levels) | Hake | Ratkowsky | García et al. [95] | |
| SC/T 3108-1986 Standard method (3 levels) | Cod | – | Ying et al. [16] | |
| (23 levels) | Cod | – | García et al. [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.R.; Ferez-Rubio, J.A.; Vilas, C. Assessment and Prediction of Fish Freshness Using Mathematical Modelling: A Review. Foods 2022, 11, 2312. https://doi.org/10.3390/foods11152312
García MR, Ferez-Rubio JA, Vilas C. Assessment and Prediction of Fish Freshness Using Mathematical Modelling: A Review. Foods. 2022; 11(15):2312. https://doi.org/10.3390/foods11152312
Chicago/Turabian StyleGarcía, Míriam R., Jose Antonio Ferez-Rubio, and Carlos Vilas. 2022. "Assessment and Prediction of Fish Freshness Using Mathematical Modelling: A Review" Foods 11, no. 15: 2312. https://doi.org/10.3390/foods11152312
APA StyleGarcía, M. R., Ferez-Rubio, J. A., & Vilas, C. (2022). Assessment and Prediction of Fish Freshness Using Mathematical Modelling: A Review. Foods, 11(15), 2312. https://doi.org/10.3390/foods11152312






