Effects of Frozen Storage Temperature on Water-Holding Capacity and Physicochemical Properties of Muscles in Different Parts of Bluefin Tuna
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Main Test Equipment
2.3. Experimental Method
2.4. Thawing Method
2.5. Water-Holding Capacity
2.6. LF-NMR Measurements
2.7. MDA
2.8. Salt-Soluble Protein Assay
2.9. FAA
2.10. Endogenous Fluorescent Protein
2.11. Color Difference
2.12. Data Analysis
3. Results
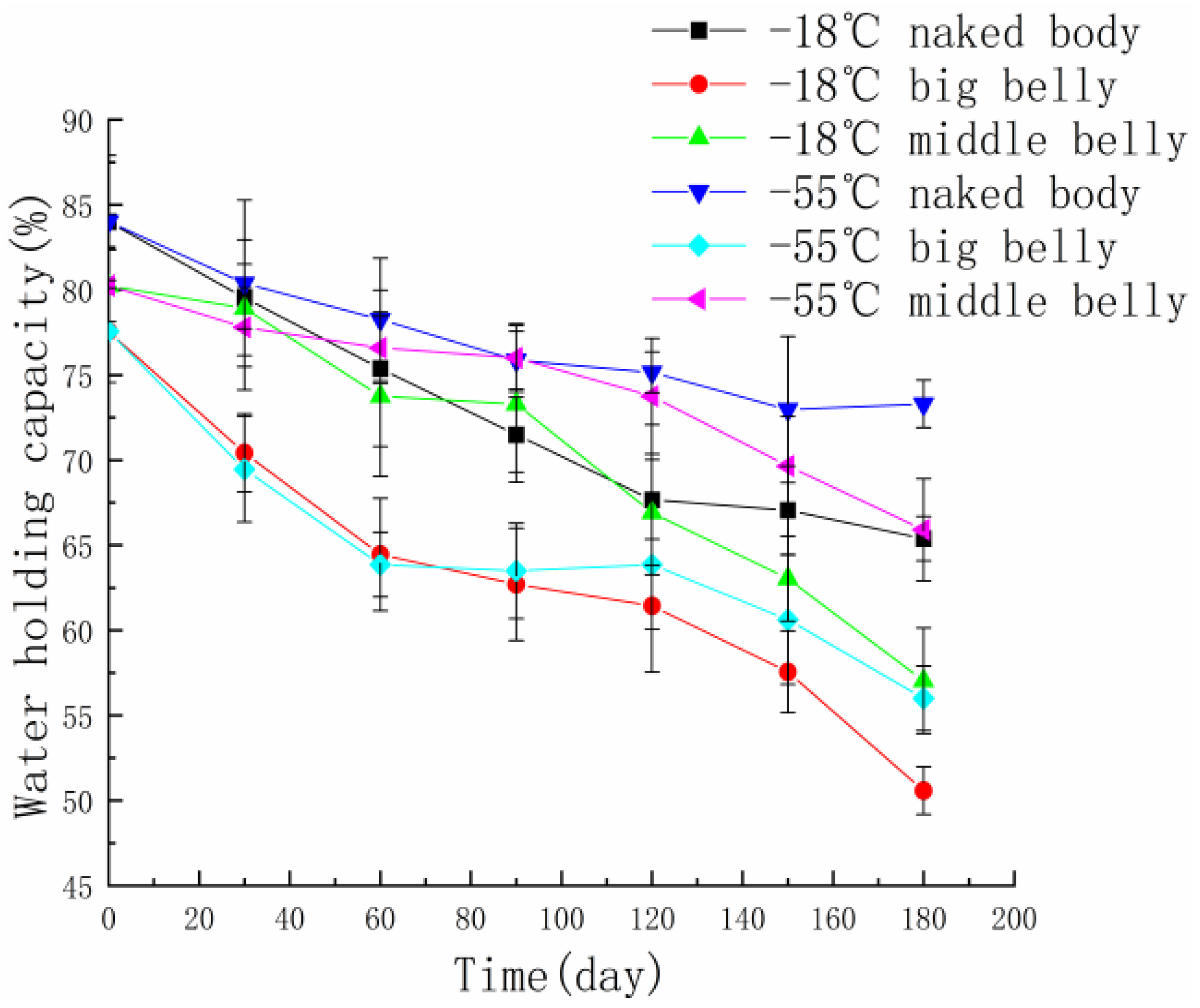
3.1. Changes of Muscle Water-Holding Capacity in Different Parts of Tuna
3.2. Changes in Muscle Color in Different Parts of Tuna
3.3. Changes in Muscle MDA in Different Parts of Tuna
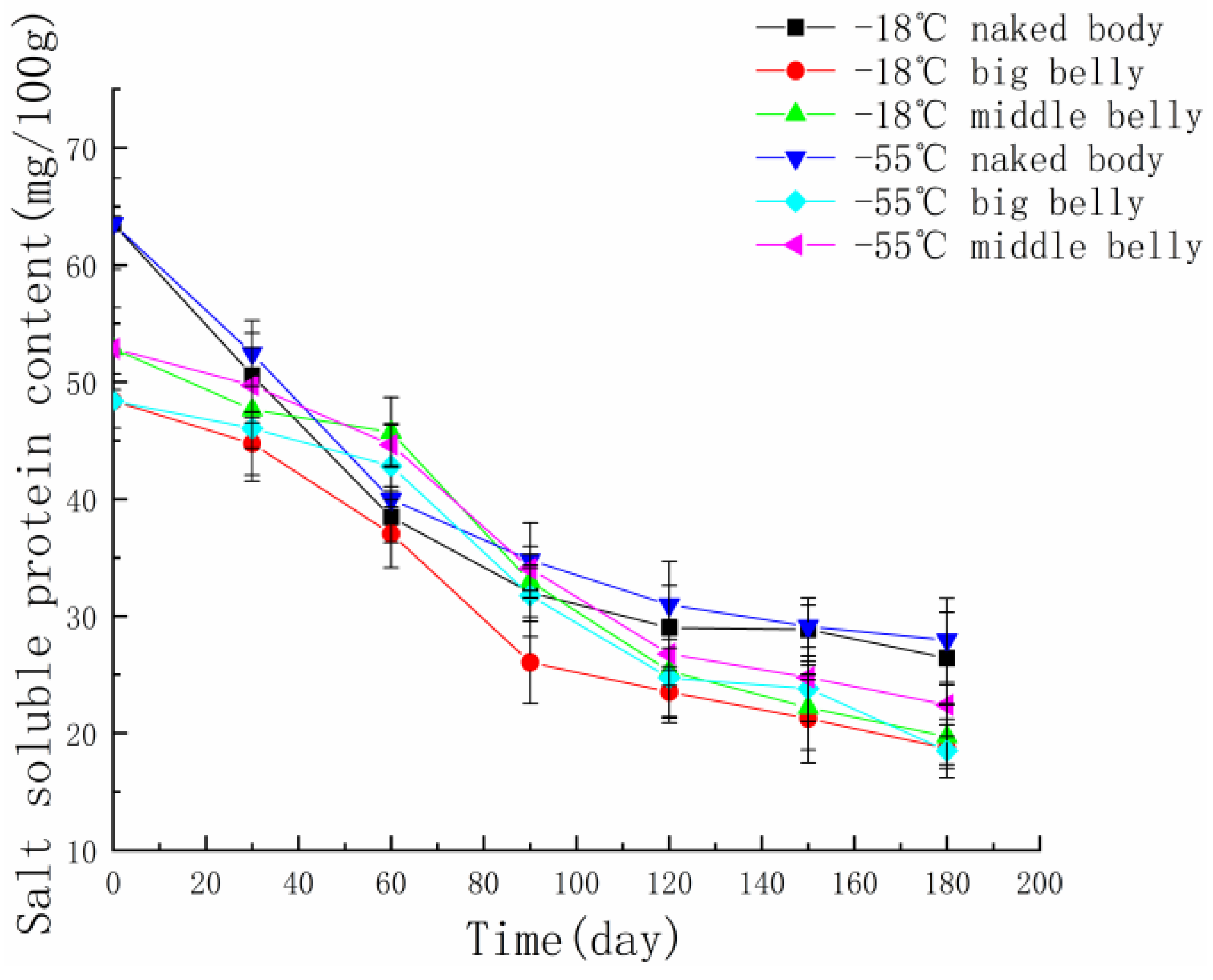
3.4. Changes in Salt-Soluble Protein Content in Different Parts of Tuna Muscle
3.5. FAA Content in Different Parts of Tuna Muscle
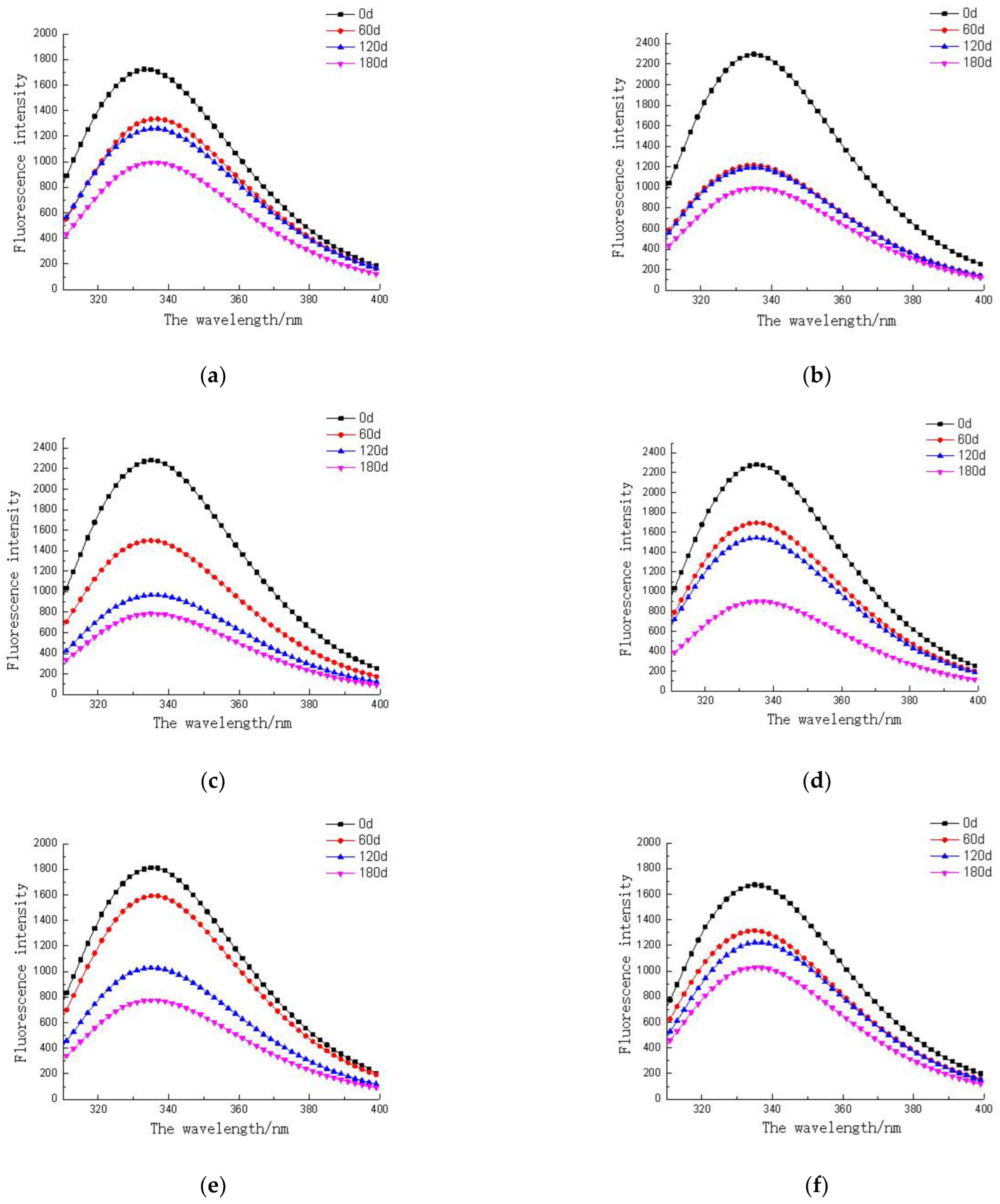
3.6. Endogenous Fluorescent Proteins in Different Parts of Tuna Muscle
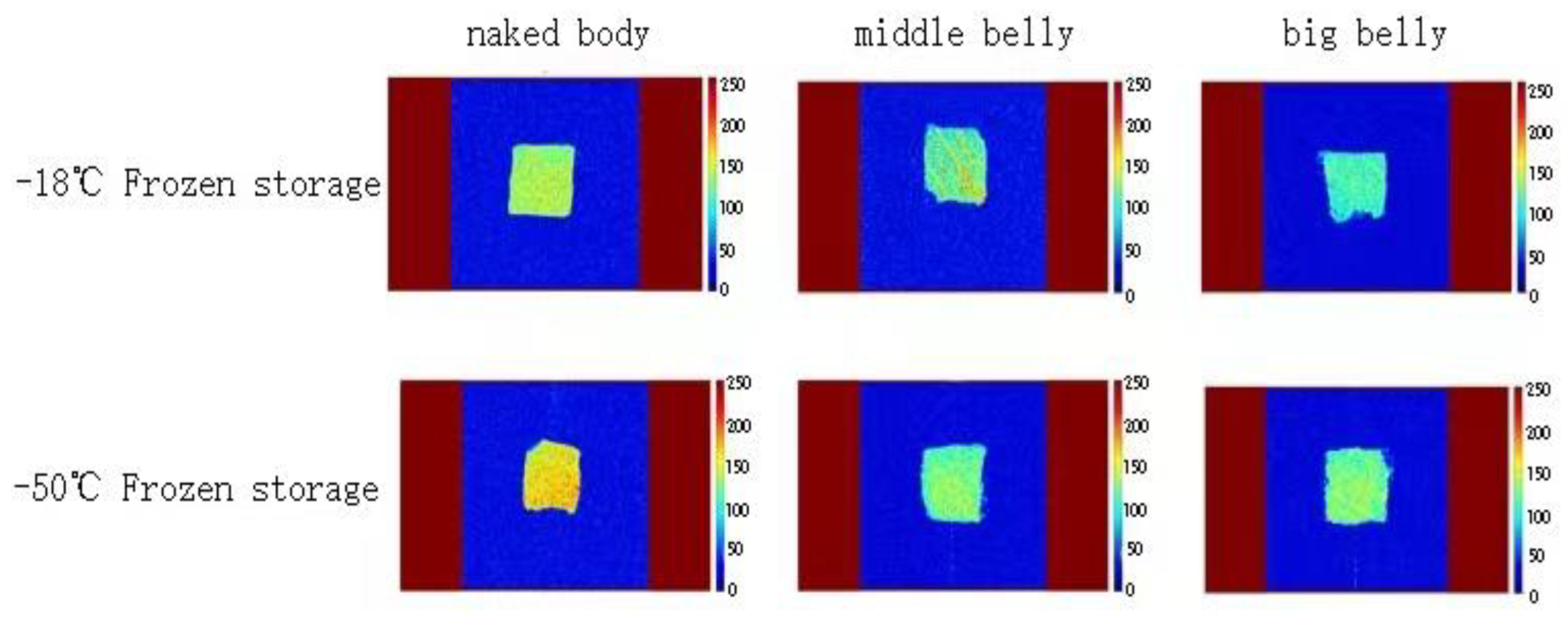
3.7. Water Distribution and Migration in Different Parts of Tuna Muscle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.Q.; Liu, H.Y.; Li, X.X.; Wei, Y.J.; Li, J.R. Effect of ultrasonic-assisted immersion freezing and quick-freezing on quality of sea bass during frozen storage. LWT 2022, 154, 112737. [Google Scholar] [CrossRef]
- Hafsa, M.; Muhammed, P.S.; Zynudheen, A.; Mudassir, A.; Sathish, K. Effect of beetroot peel dip treatment on the quality preservation of Deccan mahseer (Tor khudree) steaks during frozen storage (−18 °C). LWT 2021, 151, 112222. [Google Scholar]
- Astráin-Redín, L.; Abad, J.; Rieder, A.; Kirkhus, B.; Raso, J.; Cebrián, G.; Álvarez, I. Direct contact ultrasound assisted freezing of chicken breast samples. Ultrason. Sonochem. 2021, 70, 105319. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Han, M.L.; Tan, G.Z.; Zhu, W.H.; Li, X.P.; Li, J.R. Changes in quality characteristics of southern bluefin tuna (Thunnus maccoyii) during refrigerated storage and their correlation with color stability. LWT 2022, 154, 112715. [Google Scholar] [CrossRef]
- Lin, Y.-C.J.; Theodore, C.B. Embedding food in place and rural development: Insights from the Bluefin Tuna Cultural Festival in Donggang, Taiwan. J. Rural Stud. 2020, 79, 373–381. [Google Scholar] [CrossRef]
- Naho, N.; Ritsuko, W.; Hideto, F.; Ryusuke, T.; Shinji, K.; Emiko, O. Effect of long-term storage, ultra-low temperature, and freshness on the quality characteristics of frozen tuna meat. LWT 2022, 112, 270–280. [Google Scholar]
- Vivien, T.; Ina-Karina, K.; Madeleine, P.; Carsten, K. Effects of duration and temperature of frozen storage on the quality and food safety characteristics of pork after thawing and after storage under modified atmosphere. Meat Sci. 2021, 174, 108419. [Google Scholar]
- Jiang, Q.Q.; Jia, R.; Naho, N.; Hu, Y.Q.; Kazufumi, O.; Emiko, O. Changes in protein properties and tissue histology of tuna meat as affected by salting and subsequent freezing. J. Rural Stud. 2019, 271, 550–560. [Google Scholar] [CrossRef]
- Fatih, P.; Ozlem, S.; Can, A.; Mustafa, S. Some trace elements in front and rear dorsal ordinary muscles of wild and farmed bluefin tuna (Thunnus thynnus L. 1758) in the Turkish part of the eastern Mediterranean Sea. Food Chem. Toxicol. 2011, 49, 1006–1010. [Google Scholar]
- Zhu, M.M.; Zhang, J.; Jiao, L.X.; Ma, C.M.; Kang, Z.L.; Ma, H.J. Effects of freezing methods and frozen storage on physicochemical, oxidative properties and protein denaturation of porcine longissimus dorsi. LWT 2022, 153, 112529. [Google Scholar] [CrossRef]
- Leng, D.M.; Zhang, H.N.; Tian, C.Q.; Xu, H.B.; Li, P.R. The effect of magnetic field on the quality of channel catfish under two different freezing temperatures. Int. J. Refrig. 2022, 140, 49–56. [Google Scholar] [CrossRef]
- Qian, S.Y.; Hu, F.F.; Waris, M.; Li, X.; Zhang, C.H.; Christophe, B. The rise of thawing drip: Freezing rate effects on ice crystallization and myowater dynamics changes. Food Chem. 2022, 373, 131461. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jing, D.T.; Yu, D.W.; Xia, W.S.; Jiang, Q.X.; Xu, Y.S.; Yu, P.P. Differential roles of ice crystal, endogenous proteolytic activities and oxidation in softening of obscure pufferfish (Takifugu obscurus) fillets during frozen storage. Food Chem. 2019, 278, 452–459. [Google Scholar] [CrossRef]
- Li, X.Y.; Liu, H.X.; Yang, X.S.; Chi, H.; Huang, H.L.; Li, L.Z.; Rao, Y.L. Effects of Temperature on the Quality of Antarctic Krill (Euphausia superba) during Frozen Storage. Mod. Food Sci. Technol. 2014, 30, 191–195. [Google Scholar]
- Li, W.N.; Han, J.Z. Study on quality changes of goat meat under cold storage conditions with NMR. Sci. Technol. Food Ind. 2010, 31, 125–127. [Google Scholar]
- Ida, G.A.; Ulf, E.; Emil, V. Water properties and salt uptake in Atlantic salmon fillets as affected by ante-mortem stress, rigor mortis, and brine salting: A low-field 1H NMR and 1H/23Na MRI study. Food Chem. 2010, 120, 482–489. [Google Scholar]
- Bao, J.Q.; Xu, R.; Lu, H. Study on the T.T.T. Curve of the Back Muscle of Frozen Yellow fin Tuna. In Proceedings of the 2007 Academic Annual Meeting of Shanghai Refrigeration Society, Shanghai, China, 10–13 October 2007; pp. 125–127. [Google Scholar]
- Harnkarnsujarit, N.; Charoenrein, S. Influence of collapsed structure on stability of β-carotene in freeze-dried mangoes. Food Res. Int. 2011, 44, 3188–3194. [Google Scholar] [CrossRef]
- Jiang, H.Z.; Zhao, F.; Ma, Y.J.; Li, G.D.; Liu, M.; Zhou, D.Q. Effects of Different Freezing Temperatures on Physicochemical Properties and Freshness of Spanish Mackerel. Sci. Technol. Food Ind. 2007, 39, 258–262. [Google Scholar]
- Surendranath, P.S.; Poulson, J. Myoglobin chemistry and meat color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar]
- Cameron, F.; Sun, Q.; Richard, M.; Surendranath, P.S. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar]
- Yang, Q.; Xie, J. Effect of ultra high pressure on preservation of frozen hairtail. Food Ferment. Ind. 2015, 41, 200–206. [Google Scholar]
- Xu, K.H.; Liao, M.T.; Lin, S.S.; Zhao, Q.L.; Liu, S.C.; Feng, J.L.; Dai, Z.Y. Oxidation kinetics of lipid and myoglobin in bluefin tuna. J. Chin. Inst. Food Sci. Technol. 2015, 15, 64–71. [Google Scholar]
- Yang, J.S.; Lin, L.; Xia, S.Y.; Xie, C. Study on the mechanism of ultra-low temperature storage on the texture and biochemical characteristics of tuna meat. Oceanol. Limnol. Sin. 2015, 46, 828–832. [Google Scholar]
- Zhou, A.M.; Zeng, Q.X.; Liu, X.; Sun, Y.M. Physicochemical changes of frozen surimi protein in frozen storage and its influencing factors. Food Sci. 2003, 24, 153–157. [Google Scholar]
- Yang, J.S. Study on Cold Storage Technology of Tuna Muscle. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2012. [Google Scholar]
- Lv, X.J.; Liang, L.L.; Huang, H.J.; Qin, Y.; Ning, Z.X. Effect of free amino acid content on food flavor characteristics. Food Sci. 1996, 17, 10–12. [Google Scholar]
- Jin, Y. Study on the Flavor of Crab Meat. Master’s Thesis, Zhejiang Gongshang University, Hangzhou, China, 2011. [Google Scholar]
- Chen, J.L.; Shi, W.Z.; Chen, S.S.; Chen, S.H. Effect of different storage temperature on freshness and taste of grass carp meat. Sci. Technol. Food Ind. 2013, 38, 329–333. [Google Scholar]
- Chang, H.X.; Shi, Y.; Wang, H.; Huang, X.Q.; Li, R.P.; Bao, Z.Y.; Tu, Z.C. Effect of Ultrasonic Treatment on Physico-chemical Properties of Myofifi brillar Protein from Grass Carp. Food Sci. 2015, 36, 56–60. [Google Scholar]
- Lei, Y.T.; Shi, J.; Gui, P.; Feng, L.G.; Yu, X.P.; Luo, Y.K. Effects of glazing with tea polyphenols on the quality characteristics for Pacific white shrimp (Litopenaeus vannamei) during frozen storage. J. China Agric. Univ. 2018, 23, 92–99. [Google Scholar]
- Zhao, H.Q.; Wu, J.X.; Zhang, W.Y.; Lan, W.Q.; Liu, S.C.; Sun, X.H.; Xie, J. Effects of ultra-high pressure treatment on the quality and histological structure of frozen perch slices. Chin. J. High Press. Phys. 2017, 31, 494–504. [Google Scholar]
- Kelly, L.P.; Katja, R.; Henrik, J.A.; David, L.H. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar]
- Harnkarnsujarit, N.; Kawai, K.; Suzuki, T. Effects of Freezing Temperature and Water Activity on Microstructure, Color, and Protein Conformation of Freeze-Dried Bluefin Tuna (Thunnus orientalis). Food Bioprocess Technol. 2015, 8, 619–625. [Google Scholar] [CrossRef]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of melanosis and microbial growth in Pacific white shrimp (Litopenaeus vannamei) using high CO2 modified atmosphere packaging. Food Chem. 2020, 312, 126114. [Google Scholar] [CrossRef]
- Yang, H.X.; Liu, D.S.; Li, J.K.; He, Y.; Zhou, P. Effects of changes in protein and tissue structure of herring meat on fish quality under low temperature storage. Food Ferment. Ind. 2016, 42, 208–213. [Google Scholar]
- Miklos, R.; Mora-Gallego, H.; Larsen, F.H.; Serra, X.; Cheong, L.-Z.; Xu, X.; Arnau, J.; Lametsch, R. Influence of lipid type on water and fat mobility in fermented sausages studied by low-field NMR. Meat Sci. 2014, 96, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.T. Study on the Color Change of Bluefin Tuna Meat under Frozen Storage. Master’s Thesis, Zhejiang Gongshang University, Hangzhou, China, 2013. [Google Scholar]



| Type | −18 °C Naked Body | −18 °C Middle Belly | −18 °C Big Belly | −55 °C Naked Body | −55 °C Middle Belly | −55 °C Big Belly |
|---|---|---|---|---|---|---|
| Aspartic acid | 8.45278 | 28.5659 | 15.1902 | 7.82438 | 11.8069 | 9.36199 |
| threonine | 26.9676 | 62.6204 | 42.3936 | 26.1867 | 28.8607 | 24.9122 |
| serine | 6.08948 | 28.302 | 9.33835 | 6.92564 | 10.4408 | 7.08034 |
| glutamate | 29.402 | 77.3983 | 56.1695 | 27.7802 | 42.5536 | 44.1216 |
| glycine | 43.6959 | 98.0136 | 61.2816 | 39.8779 | 61.3309 | 36.0496 |
| alanine | 143.353 | 264.492 | 108.758 | 135.117 | 109.865 | 73.713 |
| valine | 36.3519 | 69.6088 | 47.6301 | 35.8909 | 32.9729 | 27.891 |
| methionine | 9.01322 | 38.6347 | 29.3612 | 10.7306 | 9.09695 | 16.0305 |
| isoleucine | 20.7363 | 36.0292 | 22.5213 | 21.1904 | 15.4014 | 13.831 |
| leucine | 35.9597 | 61.8645 | 39.3984 | 35.2674 | 27.7481 | 24.0869 |
| Tyrosine | 24.8361 | 54.2872 | 32.2359 | 23.0676 | 28.4776 | 21.9596 |
| Phenylalanine | 16.8455 | 45.6567 | 20.1593 | 14.4584 | 19.2527 | 10.7646 |
| Lysine | 264.279 | 1310.84 | 141.166 | 242.389 | 523.28 | 98.1079 |
| histidine | 8339.41 | 8802.48 | 9119.27 | 7127.83 | 9926.31 | 8101.62 |
| Arginine | 53.4648 | 36.7473 | 32.9904 | 44.2048 | 60.5516 | 20.2973 |
| total | 9058.857 | 11,015.54 | 9777.864 | 7798.741 | 10,907.85 | 8529.828 |
| Frozen Storage Mode | Total Peak Area (Water Content) | Bound Water (T21, %) | Immobilized Water (T22, %) | Free Water (T23, %) |
|---|---|---|---|---|
| −18 °C big belly | 7455.455 | 0.041843 | 0.941708 | 0.016449 |
| −18 °C middle belly | 8228.852 | 0.035899 | 0.941465 | 0.022635 |
| −18 °C naked body | 9767.239 | 0.032556 | 0.91532 | 0.052174 |
| −55 °C big belly | 7559.581 | 0.044403 | 0.919212 | 0.036385 |
| −55 °C middle belly | 8683.41 | 0.035597 | 0.890377 | 0.074526 |
| −55 °C naked body | 10,452.81 | 0.035959 | 0.957319 | 0.006723 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, H.; Xie, J.; Yu, W.; Sun, Y. Effects of Frozen Storage Temperature on Water-Holding Capacity and Physicochemical Properties of Muscles in Different Parts of Bluefin Tuna. Foods 2022, 11, 2315. https://doi.org/10.3390/foods11152315
Wang J, Zhang H, Xie J, Yu W, Sun Y. Effects of Frozen Storage Temperature on Water-Holding Capacity and Physicochemical Properties of Muscles in Different Parts of Bluefin Tuna. Foods. 2022; 11(15):2315. https://doi.org/10.3390/foods11152315
Chicago/Turabian StyleWang, Jinfeng, Hanwen Zhang, Jing Xie, Wenhui Yu, and Yuyao Sun. 2022. "Effects of Frozen Storage Temperature on Water-Holding Capacity and Physicochemical Properties of Muscles in Different Parts of Bluefin Tuna" Foods 11, no. 15: 2315. https://doi.org/10.3390/foods11152315
APA StyleWang, J., Zhang, H., Xie, J., Yu, W., & Sun, Y. (2022). Effects of Frozen Storage Temperature on Water-Holding Capacity and Physicochemical Properties of Muscles in Different Parts of Bluefin Tuna. Foods, 11(15), 2315. https://doi.org/10.3390/foods11152315







