Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus B8 Isolated from Sichuan Pickle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Propagation
2.2. Crude EPS Extraction and Purification
2.3. Chemical Composition Characterization Assays
2.4. Determination of MW
2.5. Monosaccharide Composition
2.6. Linkage Analysis by Methylation
2.7. FTIR and NMR Spectroscopy Analysis
2.8. Thermal Stability Evaluation
2.9. Examination of X-ray Diffractometry, Particle Size, and Zeta Potential
2.10. Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM)
2.11. Emulsifying Activities of the EPSs
2.12. Analysis of the Antioxidant Activities of LPB8-0 and LPB8-1 In Vitro
2.12.1. DPPH Scavenging Activity
2.12.2. ABTS Radical Scavenging Activity
2.12.3. Hydroxyl Free Radical Scavenging Activity
2.13. Statistical Analysis
3. Results and Discussion
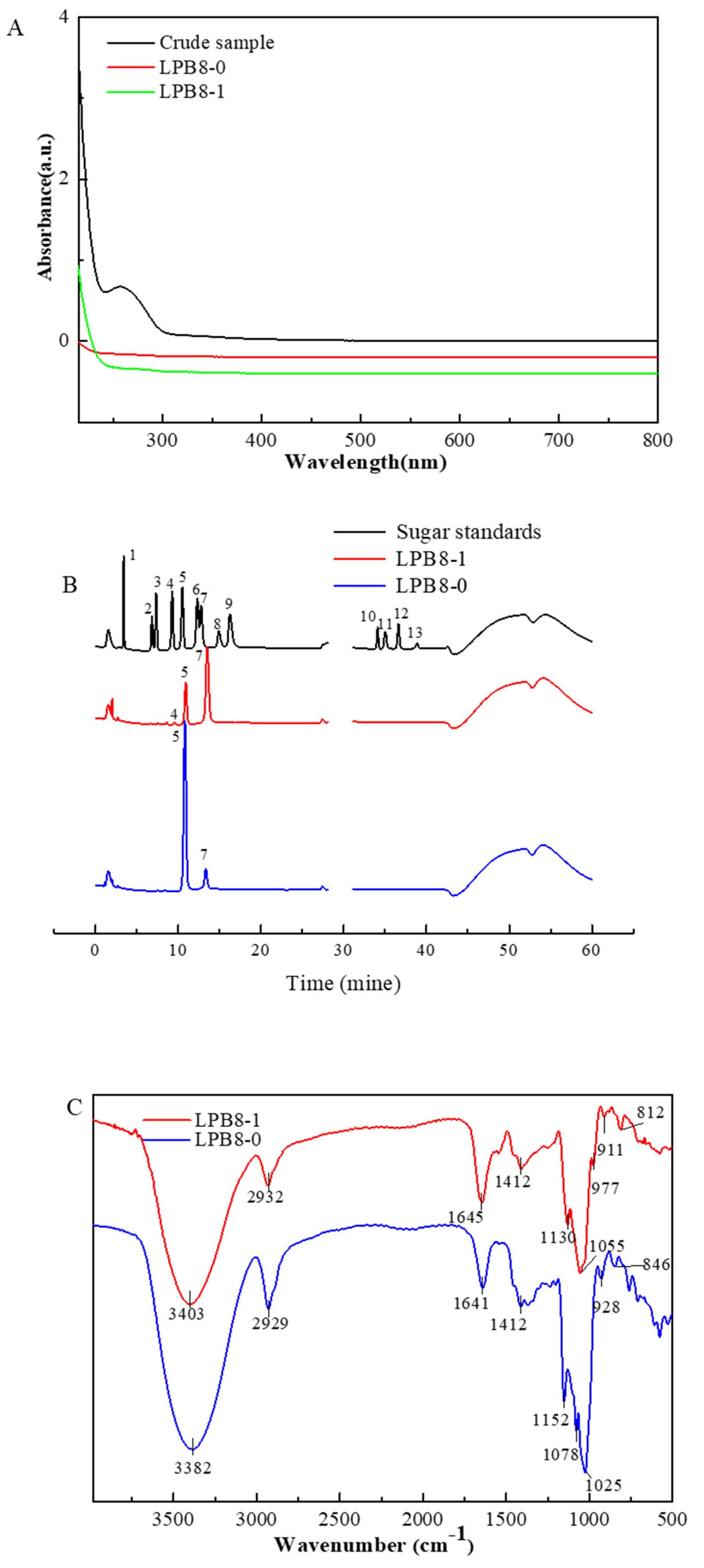
3.1. Extraction, Purification, and Chemical Composition of the EPSs
3.2. MW Distribution and Monosaccharide Composition Analysis of the EPSs
3.3. Functional Groups and Glycosidic Linkages
3.4. NMR Analysis
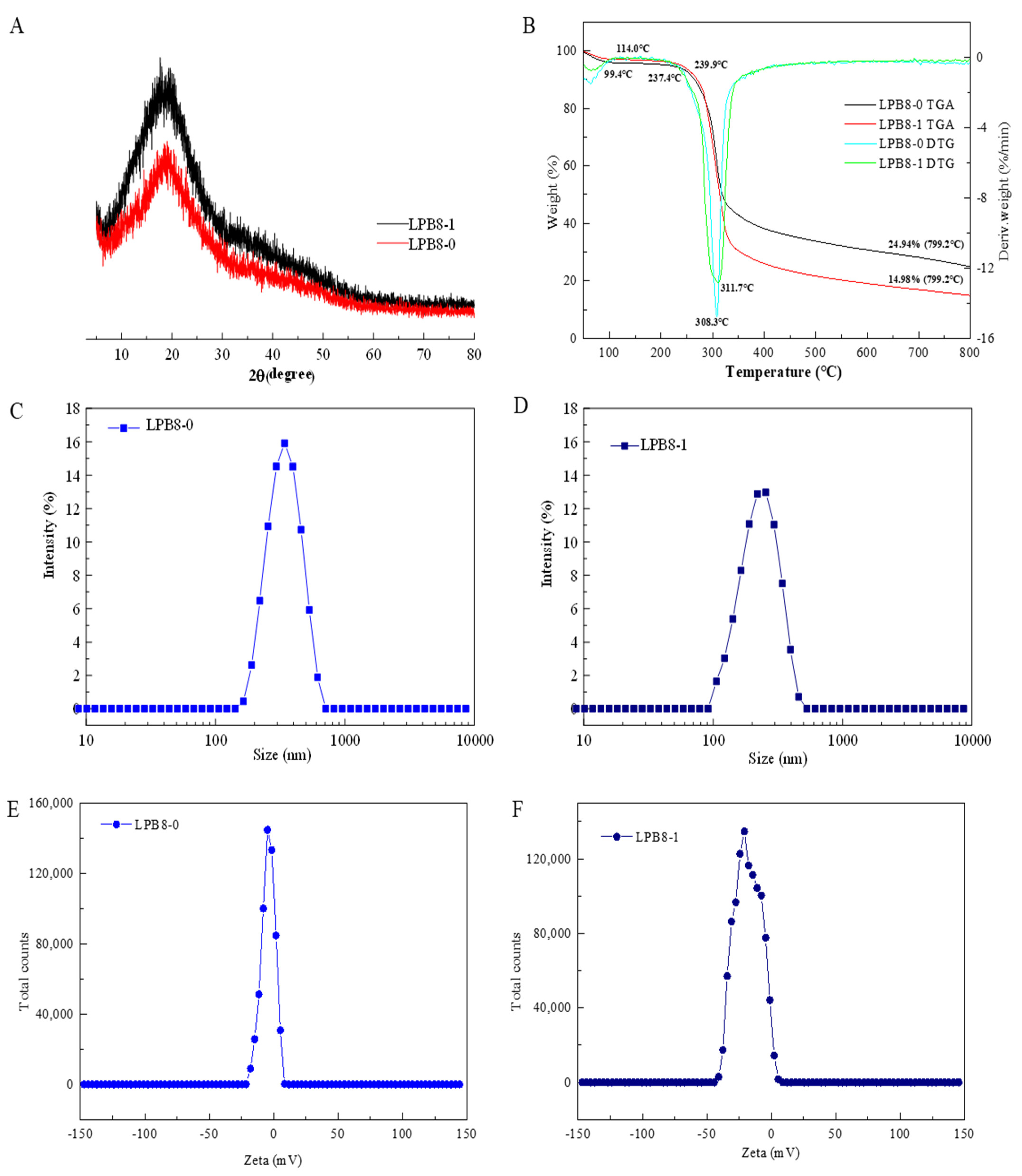
3.5. Crystalline Features and Thermal Stability
3.6. Particle Size and Zeta Potential Examination
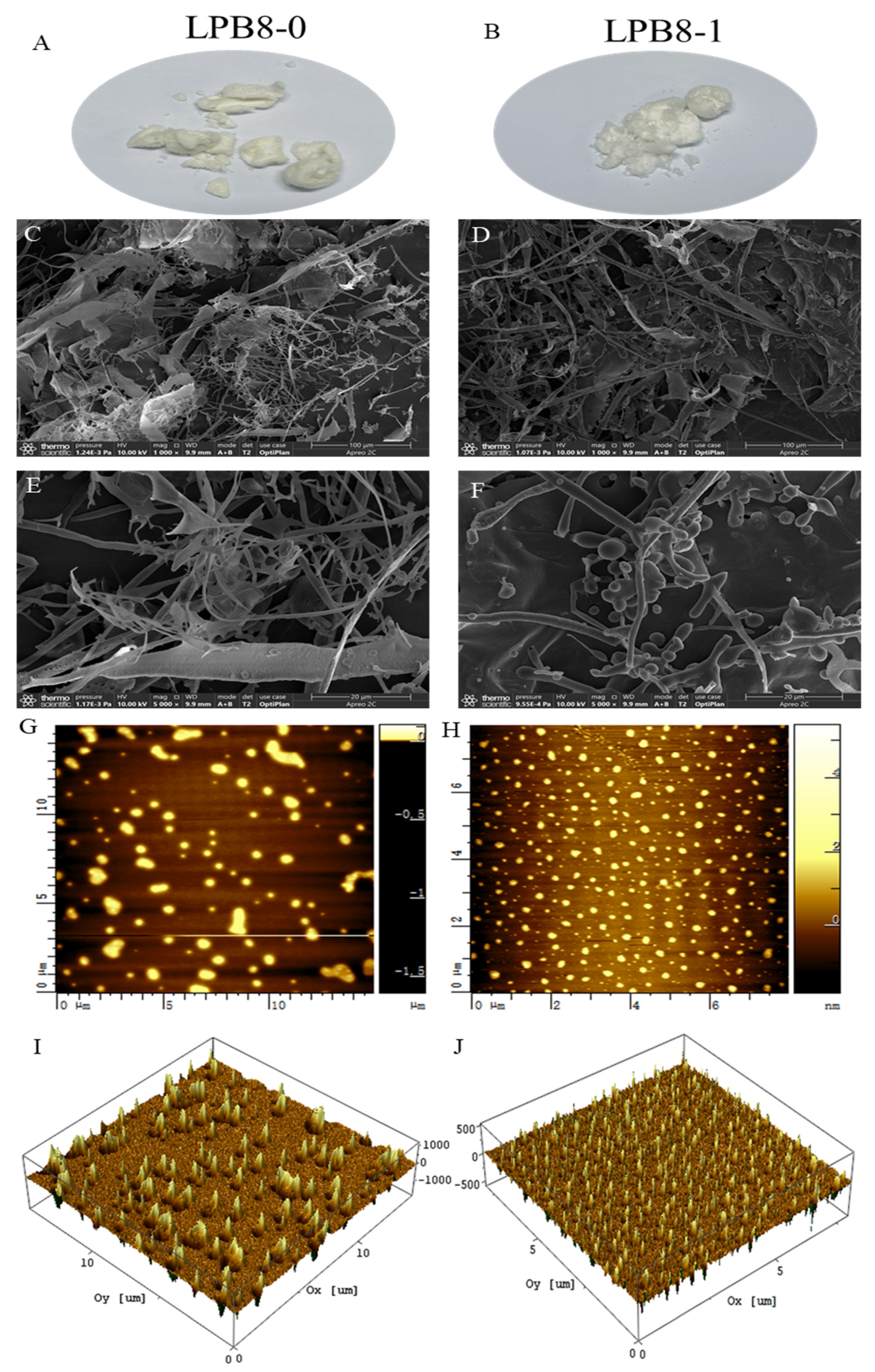
3.7. Morphology Characteristics
3.8. Emulsifying Activity and Emulsion Stability of the EPSs
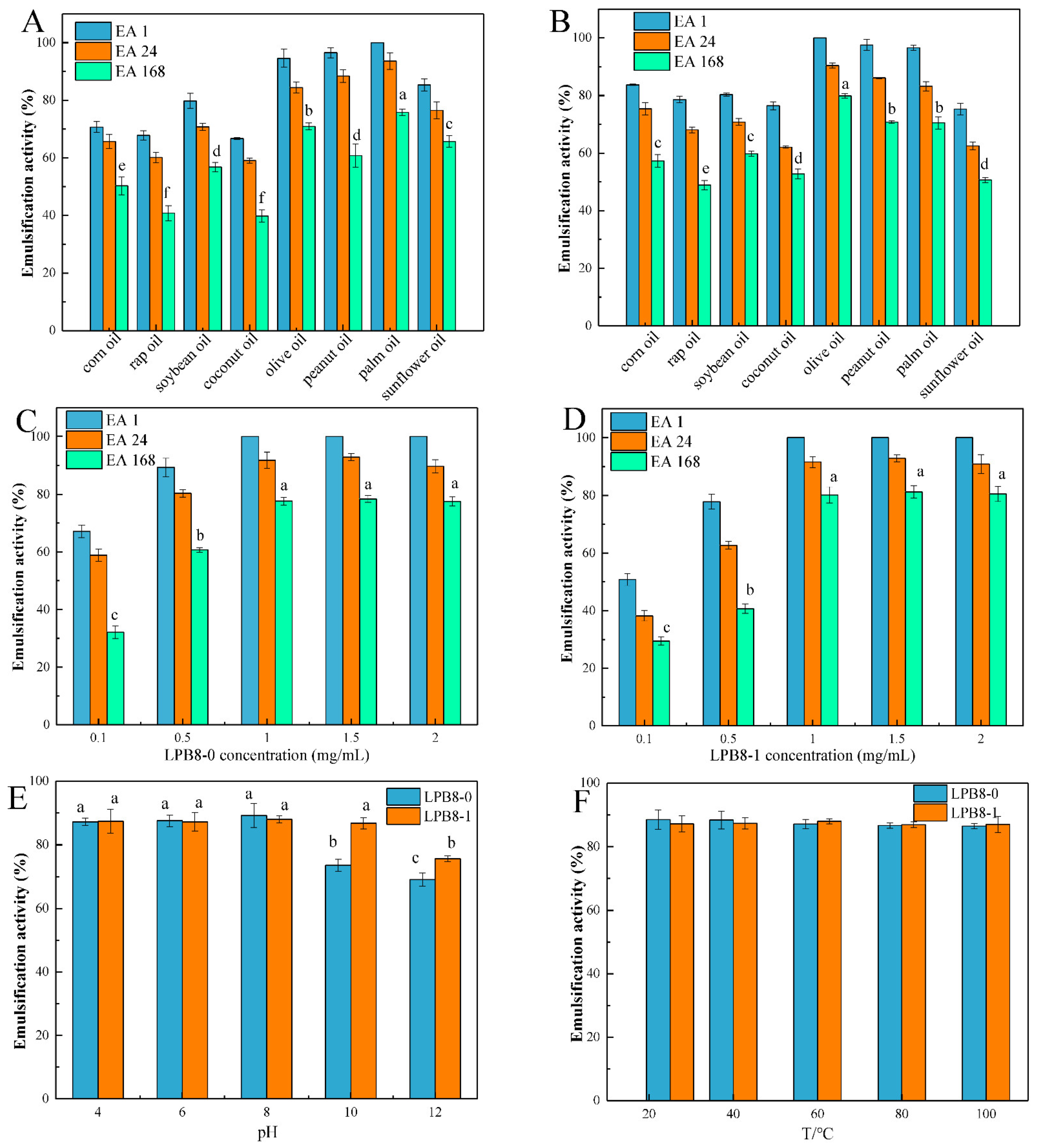
3.8.1. Emulsifying Activities with Several Edible Oils
3.8.2. Effects of EPS Concentration, pH, and Temperature on Emulsifying Activity
3.9. Antioxidant Activities of LPB8-0 and LPB8-1
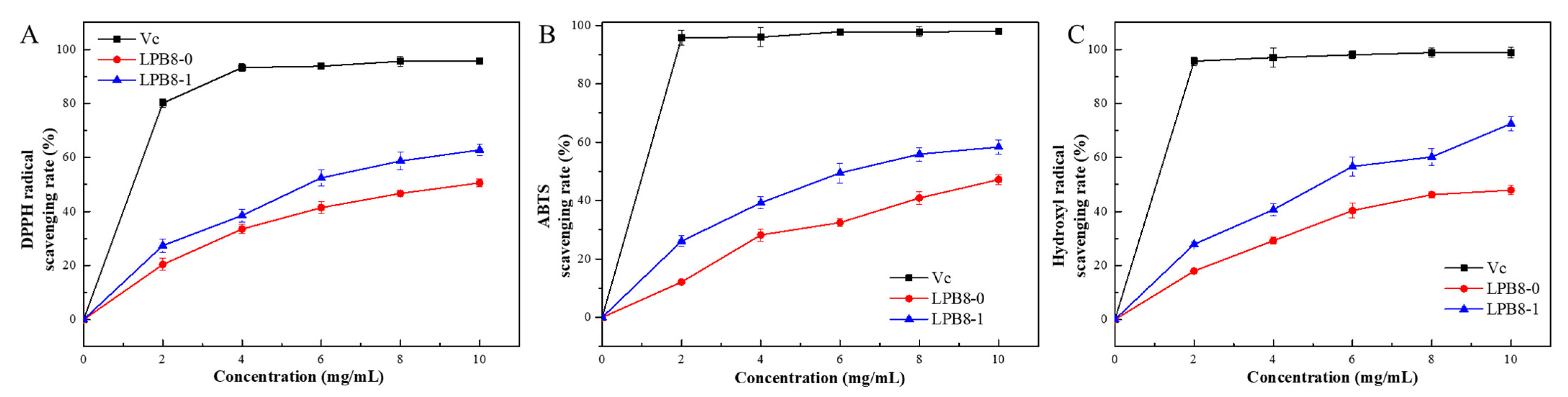
3.9.1. DPPH Radical Scavenging Activities of LPB8-0 and LPB8-1
3.9.2. ABTS Radical Scavenging Activities of LPB8-0 and LPB8-1
3.9.3. Hydroxyl Free Radical Scavenging Abilities of LPB8-0 and LPB8-1
3.9.4. Correlation between Structure and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riaz Rajoka, M.S.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus exopolysaccharides: New perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Rahbar Saadat, Y.; Yari Khosroushahi, A.; Pourghassem Gargari, B. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohyd. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Rajoka, M.S.R.; Mehwish, H.M.; Kitazawa, H.; Barba, F.J.; Berthelot, L.; Umair, M.; Zhao, L. Techno-functional properties and immunomodulatory potential of exopolysaccharide from Lactiplantibacillus plantarum MM89 isolated from human breast milk. Food Chem. 2022, 377, 131954. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Siddiq, M.; Haobin, Z.; Zhu, J.; Yan, L.; Shi, J. Identification, characterization, and probiotic potential of Lactobacillus rhamnosus isolated from human milk. LWT Food Sci. Technol. 2017, 84, 271–280. [Google Scholar] [CrossRef]
- Yang, X.; Ren, Y.; Zhang, L.; Wang, Z.; Li, L. Structural characteristics and antioxidant properties of exopolysaccharides isolated from soybean protein gel induced by lactic acid bacteria. LWT Food Sci. Technol. 2021, 150, 111811. [Google Scholar] [CrossRef]
- Hu, S.M.; Zhou, J.M.; Zhou, Q.Q.; Li, P.; Xie, Y.Y.; Zhou, T.; Gu, Q. Purification, characterization and biological activities of exopolysaccharides from Lactobacillus rhamnosus ZFM231 isolated from milk. LWT Food Sci. Technol. 2021, 147, 111561. [Google Scholar] [CrossRef]
- Bomfim, V.B.; Pereira Lopes Neto, J.H.; Leite, K.S.; de Andrade Vieira, É.; Iacomini, M.; Silva, C.M.; Cardarelli, H.R. Partial characterization and antioxidant activity of exopolysaccharides produced by Lactobacillus plantarum CNPC003. LWT Food Sci. Technol. 2020, 127, 109349. [Google Scholar] [CrossRef]
- Cao, F.; Liang, M.; Liu, J.; Liu, Y.; Renye, J.A., Jr.; Qi, P.X.; Ren, D. Characterization of an exopolysaccharide (EPS-3A) produced by Streptococcus thermophilus ZJUIDS-2-01 isolated from traditional yak yogurt. Int. J. Biol. Macromol. 2021, 192, 1331–1343. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, G.; Li, X.; Zhang, S.; Tian, Y.; Peng, B. Structural elucidation and physicochemical characteristics of a novel high-molecular-weight fructan from halotolerant Bacillus sp. SCU-E108. Food Chem. 2021, 365, 130496. [Google Scholar] [CrossRef]
- Yan, J.K.; Li, L.; Wang, Z.M.; Leung, P.H.; Wang, W.Q.; Wu, J.Y. Acidic degradation and enhanced antioxidant activities of exopolysaccharides from Cordyceps sinensis mycelial culture. Food Chem. 2009, 117, 641–646. [Google Scholar] [CrossRef]
- Nasir, A.; Sattar, F.; Ashfaq, I.; Lindemann, S.R.; Chen, M.H.; Van den Ende, W.; Anwar, M.A. Production and characterization of a high molecular weight levan and fructooligosaccharides from a rhizospheric isolate of Bacillus aryabhattai. LWT Food Sci. Technol. 2020, 123, 109093. [Google Scholar] [CrossRef]
- Jiang, G.; Gan, L.; Li, X.; He, J.; Zhang, S.; Chen, J.; Tian, Y. Characterization of Structural and Physicochemical Properties of an Exopolysaccharide Produced by Enterococcus sp. F2 From Fermented Soya Beans. Front. Microbiol. 2021, 12, 744007. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.N.; Wang, Y.J.; Li, R.L.; Li, S.Q.; Zhang, M.; He, C.W.; Chen, H.X. The structural characteristic of acidic-hydrolyzed corn silk polysaccharides and its protection on the H2O2-injured intestinal epithelial cells. Food Chem. 2021, 356, 129691. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. T 2018, 9, 155–176. [Google Scholar] [CrossRef]
- Park, S.; Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Hu, X.; Wang, M.H. Cellular antioxidant properties of nontoxic exopolysaccharide extracted from Lactobacillales (Weissella cibaria) isolated from Korean kimchi. LWT Food Sci. Technol. 2022, 154, 112727. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, K.; Yin, S.; Liu, S.; Zhu, Y.; Yang, Y.; Wang, C. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int. J. Biol. Macromol. 2019, 134, 516–526. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 67, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, M.; Zhang, J.; Yue, Y.; Wu, N.; Geng, L.; Wang, J. Structural characteristics and immune-enhancing activity of an extracellular polysaccharide produced by marine Halomonas sp. 2E1. Int. J. Biol. Macromol. 2021, 183, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Galiwango, E.; Tamiello-Rosa, C.; Abdullah, H.; Hamed, F. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int. J. Biol. Macromol. 2020, 144, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Lei, M.; Zhou, Y.; Zhong, X.; Liu, Y.; Hou, J.; Wu, W. Structural characterization of two novel polysaccharides from Gastrodia elata and their effects on Akkermansia muciniphila. Int. J. Biol. Macromol. 2021, 186, 501–509. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Li, D.; Tian, J.; Xiao, L.; Kwok, L.Y.; Sun, Z. Structure characterization, antioxidant capacity, rheological characteristics and expression of biosynthetic genes of exopolysaccharides produced by Lactococcus lactis subsp. lactis IMAU11823. Food Chem. 2022, 384, 132566. [Google Scholar] [CrossRef]
- Shi, W.; Zhong, J.; Zhang, Q.; Yan, C. Structural characterization and antineuroinflammatory activity of a novel heteropolysaccharide obtained from the fruits of Alpinia oxyphylla. Carbohyd. Polym. 2020, 229, 115405. [Google Scholar] [CrossRef] [PubMed]
- Insulkar, P.; Kerkar, S.; Lele, S.S. Purification and structural-functional characterization of an exopolysaccharide from Bacillus licheniformis PASS26 with in-vitro antitumor and wound healing activities. Int. J. Biol. Macromol. 2018, 120, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, M.; Jayaraman Uthaya, C.; Thangavel, M.; Annadurai, V.; Rajendran, R.; Gurusamy, A. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohyd. Polym. 2020, 227, 115369. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohyd. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Zhao, F.; Zhang, L.; Han, Y.; Zhou, Z. Isolation and characterization of dextran produced by Lactobacillus sakei L3 from Hubei sausage. Carbohyd. Polym. 2019, 223, 115111. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Olaimat, A.; Esposito, G.; Itsaranuwat, P.; Osaili, T.; Liu, S.Q. Physicochemical, bioactive and rheological properties of an exopolysaccharide produced by a probiotic Pediococcus pentosaceus M41. Carbohyd. Polym. 2020, 229, 115462. [Google Scholar] [CrossRef]
- Ayyash, M.; Abu-Jdayil, B.; Itsaranuwat, P.; Almazrouei, N.; Galiwango, E.; Esposito, G.; Najjar, Z. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: Structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020, 333, 127418. [Google Scholar] [CrossRef]
- Wang, B.H.; Cao, J.J.; Zhang, B.; Chen, H.Q. Structural characterization, physicochemical properties and alpha-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohyd. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Sathiyanarayanan, G.; Balaji, P.; Mathivanan, K.; Pugazhendhi, A.; Ma, Y.; Thirumurugan, R. Structural characterization, functional and biological activities of an exopolysaccharide produced by probiotic Bacillus licheniformis AG-06 from Indian polyherbal fermented traditional medicine. Int. J. Biol. Macromol. 2021, 174, 144–152. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Yan, Y.; Shi, M.; Liu, Y. Comparison of structural characterization and antioxidant activity of polysaccharides from jujube (Ziziphus jujuba Mill.) fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohyd. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef]
- Pei, F.; Ma, Y.; Chen, X.; Liu, H. Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromol. 2020, 161, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Maalej, H.; Hmidet, N.; Boisset, C.; Bayma, E.; Heyraud, A.; Nasri, M. Rheological and emulsifying properties of a gel-like exopolysaccharide produced by Pseudomonas stutzeri AS22. Food Hydrocolloid. 2016, 52, 634–647. [Google Scholar] [CrossRef]
- Gomaa, M.; Yousef, N. Optimization of production and intrinsic viscosity of an exopolysaccharide from a high yielding Virgibacillus salarius BM02: Study of its potential antioxidant, emulsifying properties and application in the mixotrophic cultivation of Spirulina platensis. Int. J. Biol. Macromol. 2020, 149, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Kodali, V.P.; Das, S.; Sen, R. An exopolysaccharide from a probiotic: Biosynthesis dynamics, composition and emulsifying activity. Food Res. Int. 2009, 42, 695–699. [Google Scholar] [CrossRef]
- Han, Y.; Liu, E.; Liu, L.; Zhang, B.; Wang, Y.; Gui, M.; Li, P. Rheological, emulsifying and thermostability properties of two exopolysaccharides produced by Bacillus amyloliquefaciens LPL061. Carbohyd. Polym. 2015, 115, 230–237. [Google Scholar] [CrossRef]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Xing, H.; Du, R.; Zhao, F.; Han, Y.; Xiao, H.; Zhou, Z. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. Int. J. Biol Macromol. 2018, 112, 1208–1216. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, S.W.; Nie, S.P.; Chen, Y.; Wang, Y.X.; Xie, M.Y. Identification of pivotal components on the antioxidant activity of polysaccharide extract from Ganoderma atrum. Bioact. Carbohyd. Diet. Fibre. 2016, 7, 9–18. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, W.; Wen, P.; Shen, M.; Li, H.; Ren, Y.; Xie, J. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocolloid. 2020, 100, 105412. [Google Scholar] [CrossRef]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Xie, M.Y. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. 2016, 56, 60–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, H.; Wen, C.; Zhang, J.; He, Y.; Ma, H.; Duan, Y. Purification, characterization, antioxidant and immunological activity of polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020, 136, 109345. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Ye, X.; Han, Y.; Zhao, M.; Chen, S.; Liu, X.; Wang, W. Structure analysis of polysaccharides purified from Cyclocarya paliurus with DEAE-Cellulose and its antioxidant activity in RAW264.7 cells. Int. J. Biol. Macromol. 2020, 157, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhou, C.; Zeng, Y.; Zhang, H.; Hossen, M.A.; Dai, J.; Liu, Y. Structures, physicochemical and bioactive properties of polysaccharides extracted from Panax notoginseng using ultrasonic/microwave-assisted extraction. LWT Food Sci. Technol. 2022, 154, 112446. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Chen, Z.; Gao, X.; Yuan, G.; Pan, Y.; Chen, H. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, alpha-amylase and alpha-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res. Int. 2018, 103, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Cheng, H.; Xu, Z.; Yuan, M.; Huang, Y.; Liao, J.; Ding, C. Panax notoginseng polysaccharide increases stress resistance and extends lifespan in Caenorhabditis elegans. J. Funct. Foods. 2018, 45, 15–23. [Google Scholar] [CrossRef]


| Factions | Carbohydrate Content (%) | Protein Content (%) | Sulfates (%) | Mw (Da) |
|---|---|---|---|---|
| LPB8-0 | 96.2 ± 1.0% | Nd * | Nd * | 1.12 × 104 |
| LPB8-1 | 99.1 ± 0.5% | Nd * | Nd * | 1.78 × 105 |
| Time (min) | Methylated Sugars | Deduced Linkages | Molar Ratios |
|---|---|---|---|
| 8.9 | 2,3,4,6-Me4-Manp | T-Manp-(1→ | 36.00 |
| 12.3 | 2,4,6-Me3-Manp | →3)-Manp-(1→ | 11.94 |
| 12.4 | 3,4,6-Me3-Manp | →2)-Manp-(1→ | 17.31 |
| 13.6 | 2,3,4-Me3-Manp | →6)-Manp-(1→ | 2.58 |
| 13.7 | 2,3,4-Me3-Glcp | →6)-Glcp-(1→ | 2.93 |
| 14.1 | 2,3,6-Me3-Glcp | →4)-Glcp-(1→ | 11.11 |
| 17.9 | 2,4-Me2-Manp | →3,6)-Manp-(1→ | 0.97 |
| 18.2 | 3,4-Me2-Manp | →2,6)-Manp-(1→ | 10.10 |
| 18.2 | 3,4-Me2-Glcp | →2,6)-Glcp-(1→ | 6.74 |
| 21.4 | 4-Me-Manp | →2,3,6)-Manp-(1→ | 0.32 |
| Sugar Residue | Chemical Shifts δ (ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 | H-6′ | |
| A: →4)-α-D-Glcp-(1→ | 5.43/101.28 | 3.66/72.05 | 4.01/72.64 | 3.67/73.08 | 3.83/74.25 | 3.93/61.84 | 3.69 |
| B: →2)-α-D-Manp-(1→ | 5.33/102.29 | 4.16/79.80 | 3.96/71.68 | 3.79/68.01 | 3.92/74.49 | 3.80/62.38 | 3.66 |
| C: →3)-α-D-Manp-(1→ | 5.19/103.62 | 4.11/71.36 | 3.92/74.74 | 3.68/68.24 | 3.85/74.64 | 3.94/62.46 | 3.70 |
| D: α-D-Manp-(1→ | 5.18/103.54 | 4.10/71.29 | 3.91/72.07 | 3.67/68.24 | 3.82/74.72 | 3.93/62.23 | 3.69 |
| E: →2,6)-α-D-Manp-(1→ | 5.16/99.72 | 4.07/80.27 | 3.80/71.91 | 3.70/68.55 | 3.83/74.57 | 3.92/68.08 | 3.78 |
| F: →2,6)-α-D-Manp-(1→ | 5.13/99.64 | 4.06/80.19 | 3.80/71.76 | 3.71/68.63 | 3.82/74.62 | 3.93/68.32 | 3.78 |
| G: α-D-Manp-(1→ | 5.09/103.39 | 4.10/71.44 | 3.91/71.68 | 3.68/68.32 | 3.81/74.57 | 3.92/62.46 | 3.69 |
| H: →3,6)-α-D-Manp-(1→ | 5.08/103.62 | 4.28/71.05 | 3.89/75.05 | 3.78/67.85 | 3.95/74.72 | 3.84/68.01 | 3.79 |
| I: →6)-α-D-Manp-(1→ | 5.05/103.54 | 4.28/71.21 | 3.97/71.76 | 3.79/67.85 | 3.95/74.41 | 3.82/68.16 | 3.77 |
| J: →2,6)-β-D-Glcp-(1→ | 4.93/101.04 | 4.03/79.72 | 3.65/71.32 | 3.91/70.82 | 3.80/74.18 | 3.91/68.24 | 3.71 |
| K: →6)-β-D-Glcp-(1→ | 4.56/104.56 | 3.37/74.64 | 3.54/77.14 | 3.67/69.26 | 3.78/74.80 | 3.97/67.77 | 3.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.; Li, R.; He, J.; Yang, L.; Chen, J.; Xu, Z.; Zheng, B.; Yang, Y.; Xia, Z.; Tian, Y. Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus B8 Isolated from Sichuan Pickle. Foods 2022, 11, 2327. https://doi.org/10.3390/foods11152327
Jiang G, Li R, He J, Yang L, Chen J, Xu Z, Zheng B, Yang Y, Xia Z, Tian Y. Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus B8 Isolated from Sichuan Pickle. Foods. 2022; 11(15):2327. https://doi.org/10.3390/foods11152327
Chicago/Turabian StyleJiang, Guangyang, Ran Li, Juan He, Li Yang, Jia Chen, Zhe Xu, Bijun Zheng, Yichen Yang, Zhongmei Xia, and Yongqiang Tian. 2022. "Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus B8 Isolated from Sichuan Pickle" Foods 11, no. 15: 2327. https://doi.org/10.3390/foods11152327
APA StyleJiang, G., Li, R., He, J., Yang, L., Chen, J., Xu, Z., Zheng, B., Yang, Y., Xia, Z., & Tian, Y. (2022). Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus B8 Isolated from Sichuan Pickle. Foods, 11(15), 2327. https://doi.org/10.3390/foods11152327





