Dimethyl Dicarbonate as a Food Additive Effectively Inhibits Geotrichum citri-aurantii of Citrus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathogen
2.2. Food Additive and Solution Preparation
2.3. Fruit
2.4. Effect of DMDC on Fungal Growth In Vitro
2.5. Effect on the Spore Germination of G. citri-aurantii
2.6. Effect of DMDC on Cell Membrane Permeability
2.7. Effect of DMDC on Cell Membrane Integrity
2.8. Detection of Intracellular ROS Levels and MDA Content
2.9. Scanning Electron Microscopy (SEM)
2.10. Transmission Electron Microscopy (TEM)
2.11. In Vivo Experiment
2.12. Determination of Enzyme Activity in Citrus
2.13. Statistical Analysis
3. Results
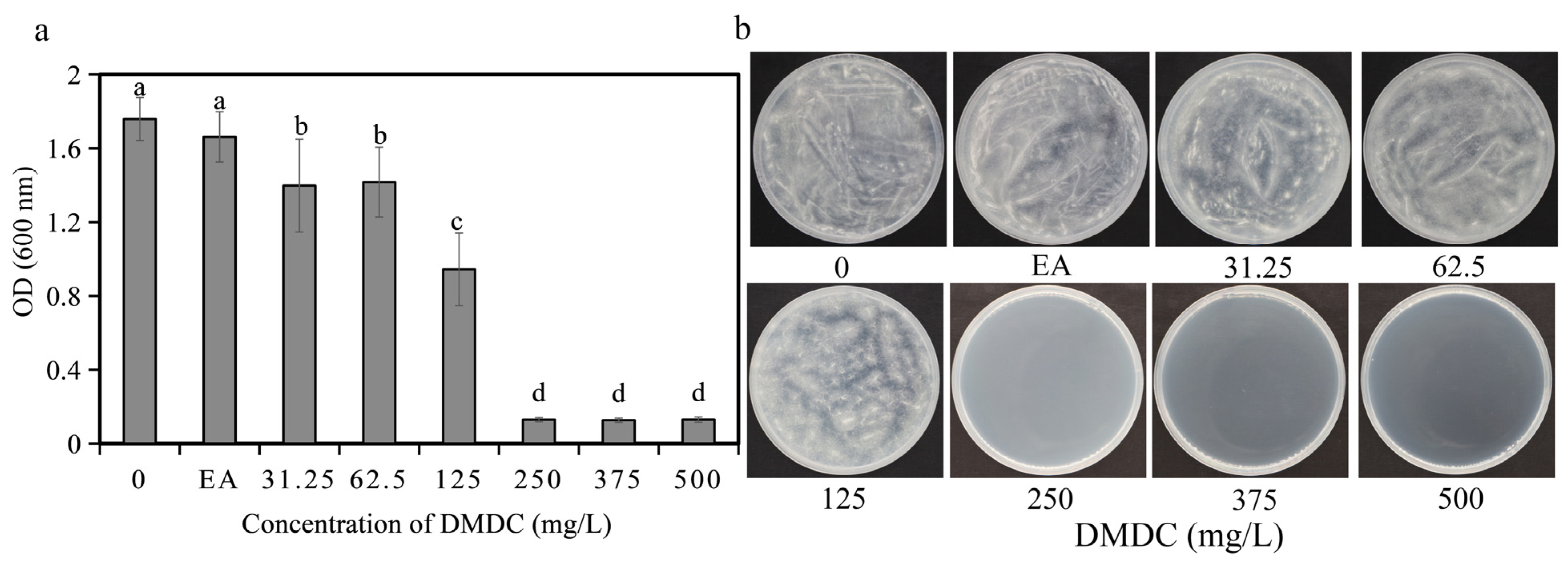
3.1. DMDC Inhibited G. citri-aurantii Mycelial Growth In Vitro
3.2. Effect of DMDC on Spore Germination
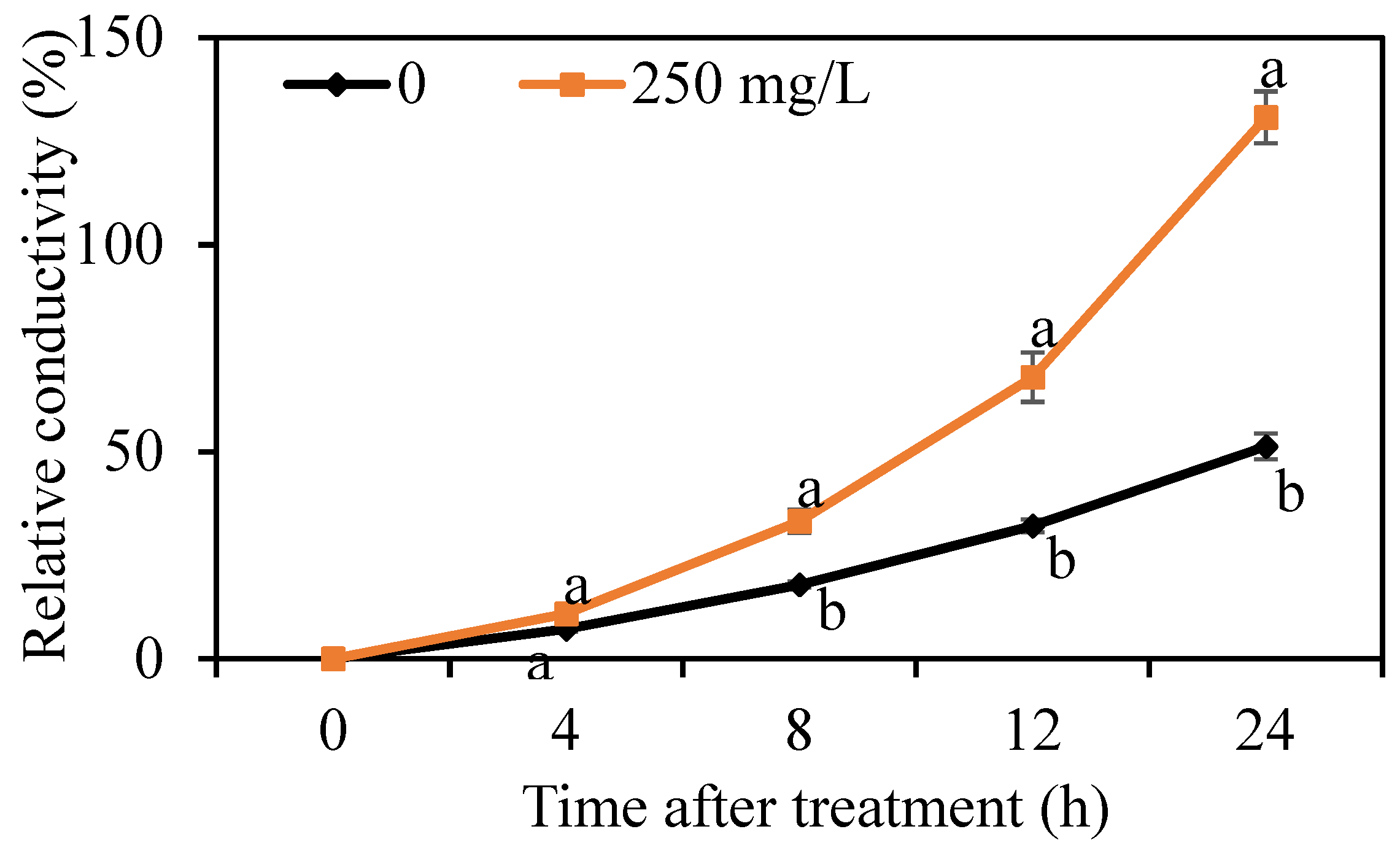
3.3. Effect of DMDC on the Cell Membrane Permeability of G. citri-aurantii
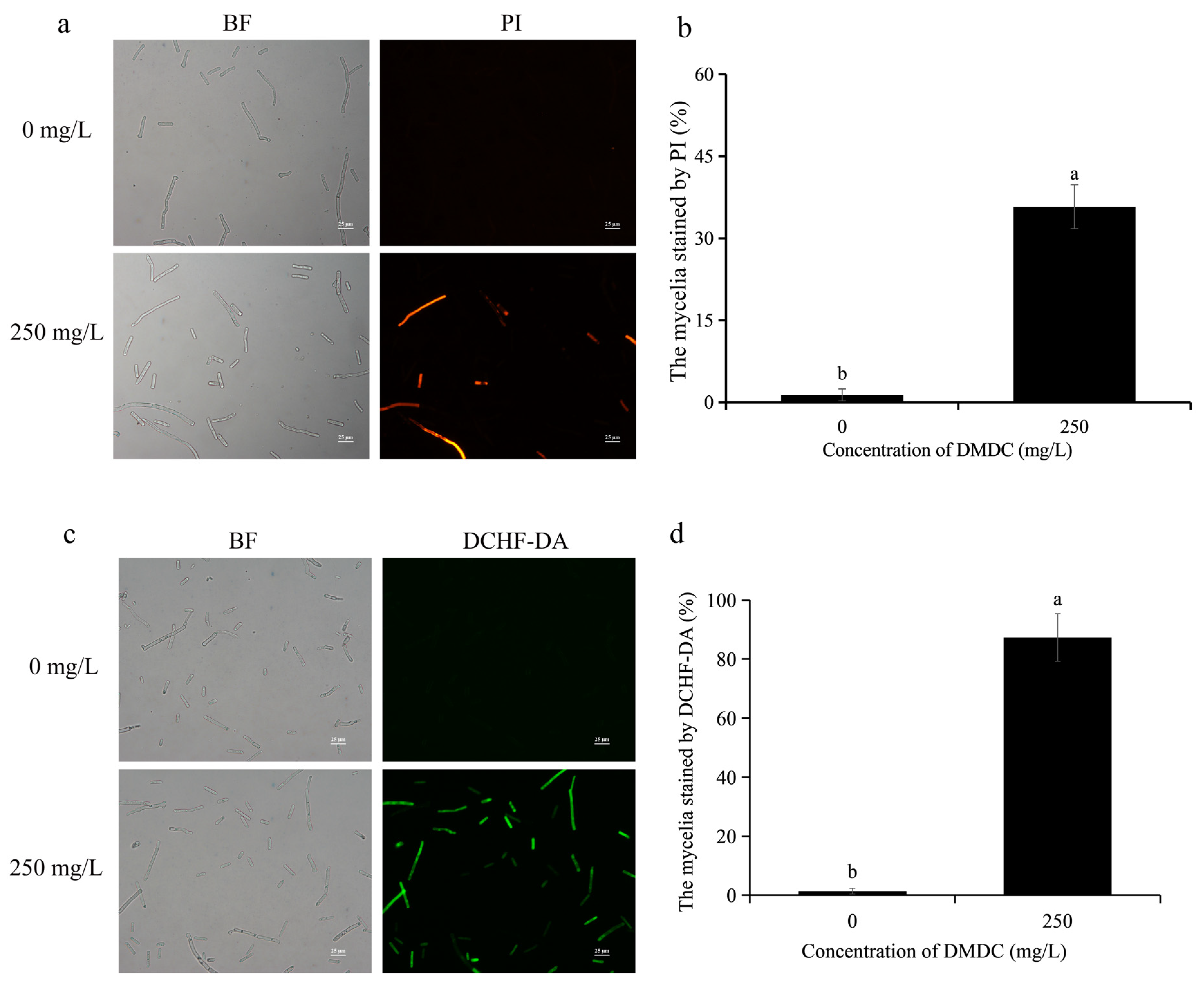
3.4. Effects of DMDC on the Cell Membrane Integrity of G. citri-aurantii
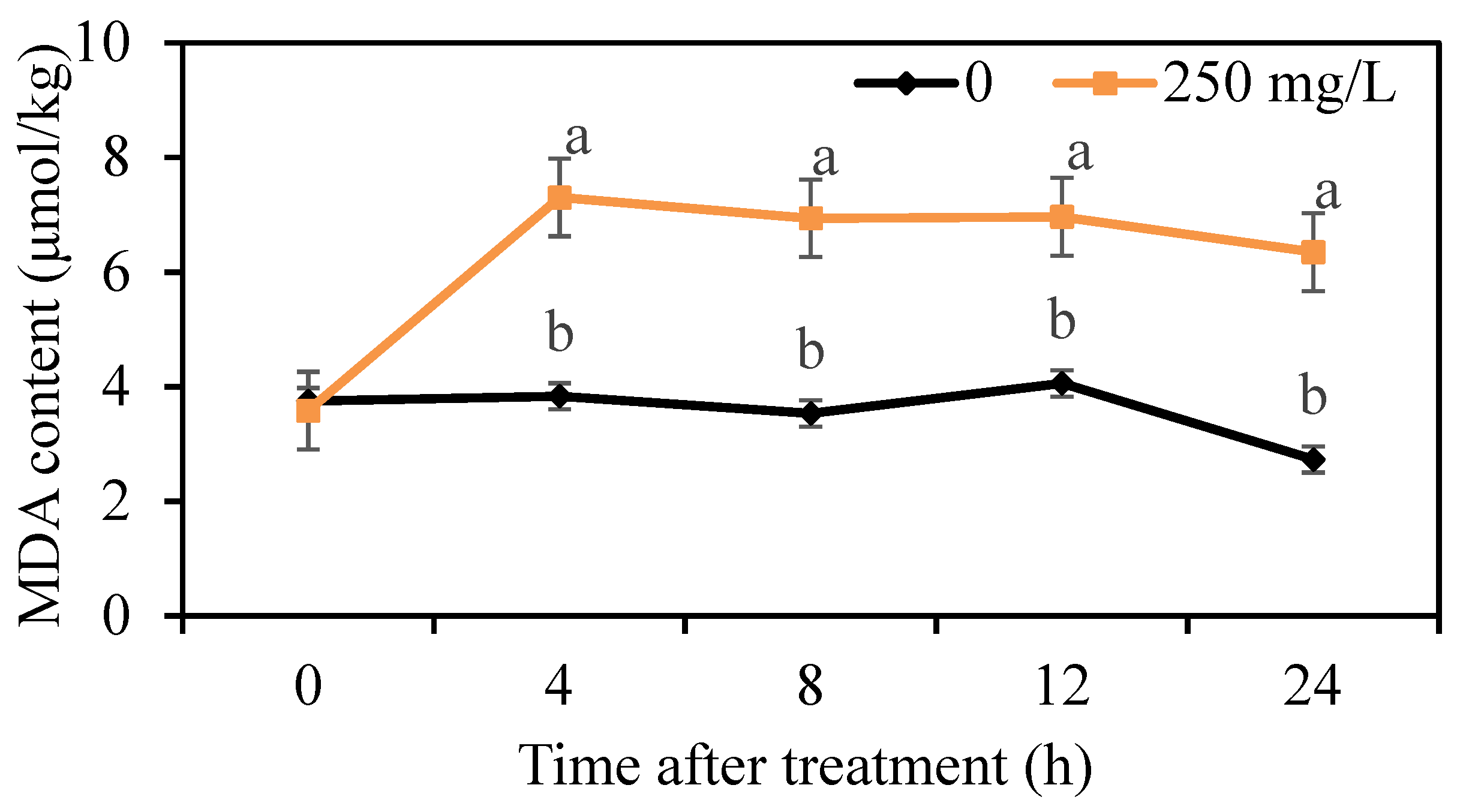
3.5. Effects of DMDC on Intracellular ROS Levels and MDA Content from G. citri-aurantii
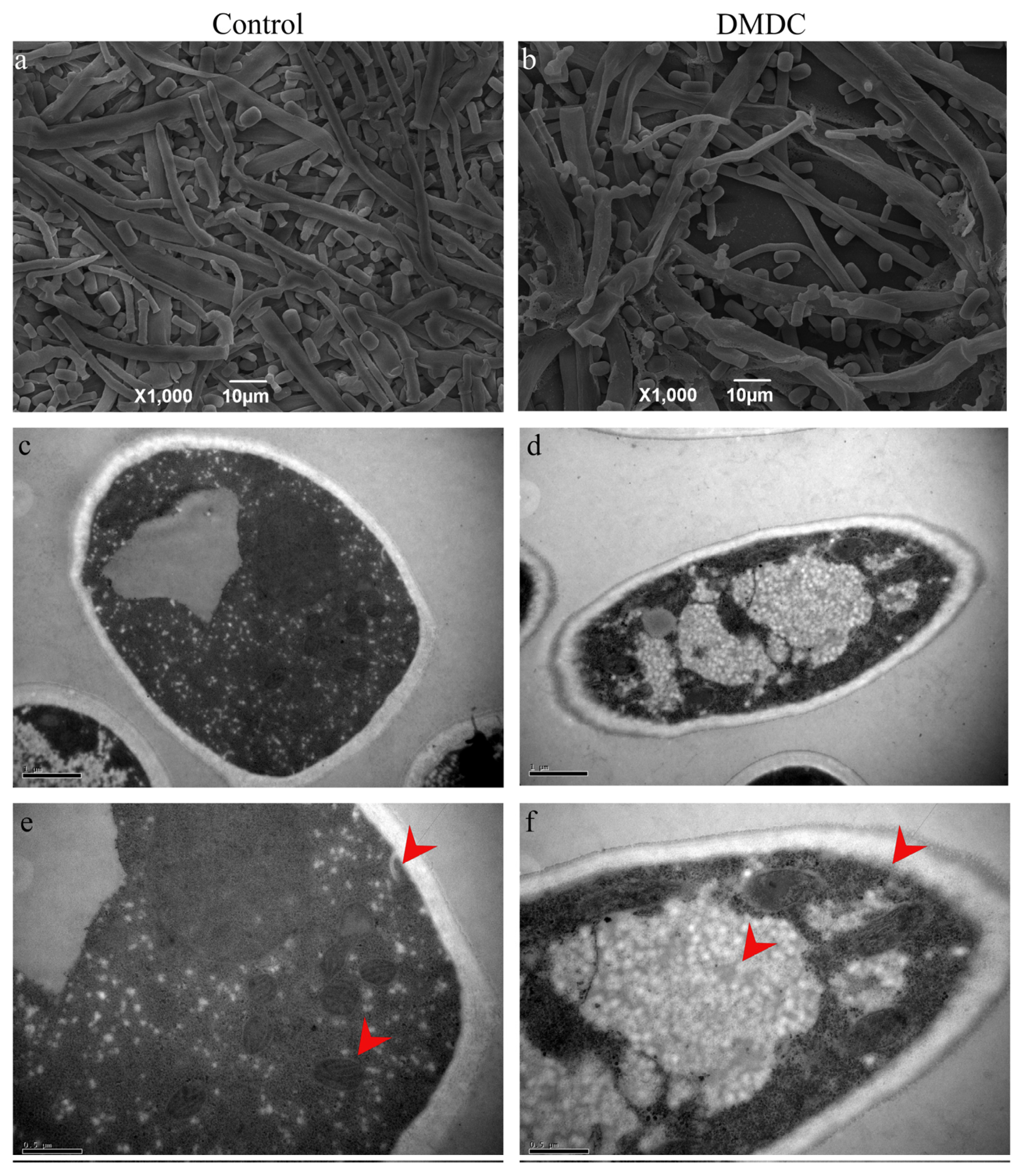
3.6. Effect of DMDC on Mycelium Morphology
3.7. Effect of DMDC on the Cell Ultrastructure
3.8. In Vivo Experiments
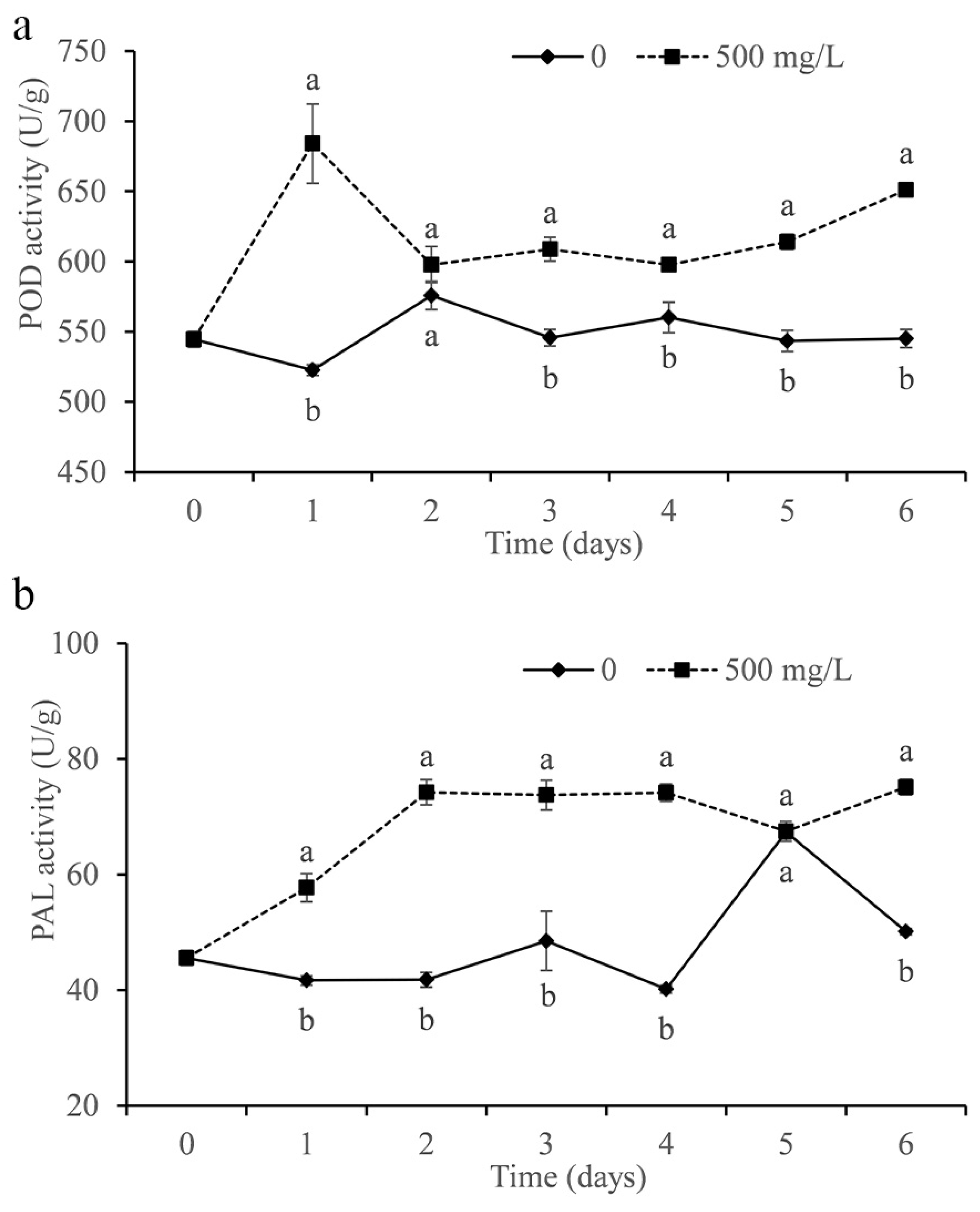
3.9. Determination of Citrus Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Y.; Chen, C.; Wan, C. Inhibition of Key Citrus Postharvest Fungal Strains by Plant Extracts In Vitro and In Vivo: A Review. Plants 2019, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Blay, V.; Taberner, V.; Perez-Gago, M.B.; Palou, L. Control of major citrus postharvest diseases by sulfur-containing food additives. Int. J. Food Microbiol. 2020, 330, 108713. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S.; Veldman, W.; Du Plooy, W. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind. Crops Prod. 2014, 61, 151–159. [Google Scholar] [CrossRef]
- Wang, W.; Liu, S.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of Citrus Post-harvest Green Molds, Blue Molds, and Sour Rot by the Cecropin A-Melittin Hybrid Peptide BP21. Front. Microbiol. 2018, 9, 2455. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Xu, Z.; Deng, R.; Huang, L.; Zheng, R.; Kong, Q. Peppermint Essential Oil Suppresses Geotrichum citri-aurantii Growth by Destructing the Cell Structure, Internal Homeostasis, and Cell Cycle. J. Agric. Food Chem. 2021, 69, 7786–7797. [Google Scholar] [CrossRef]
- Schirra, M.; D′Aquino, S.; Cabras, P.; Angioni, A. Control of postharvest diseases of fruit by heat and fungicides: Efficacy, residue levels, and residue persistence. A review. J. Agric. Food Chem. 2011, 59, 8531–8542. [Google Scholar] [CrossRef]
- Yin, C.; Liu, H.; Shan, Y.; Gupta, V.K.; Jiang, Y.; Zhang, W.; Tan, H.; Gong, L. Cytosporone B as a Biological Preservative: Purification, Fungicidal Activity and Mechanism of Action against Geotrichum citri-aurantii. Biomolecules 2019, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Yang, Y.; Zhu, X.; Yuan, P.; Gong, B.; Ding, S.; Shan, Y. Inhibitory mechanisms of cinnamic acid on the growth of Geotrichum citri-aurantii. Food Control 2022, 131, 108459. [Google Scholar] [CrossRef]
- Soto-Munoz, L.; Martinez-Blay, V.; Perez-Gago, M.B.; Fernandez-Catalan, A.; Argente-Sanchis, M.; Palou, L. Starch-based antifungal edible coatings to control sour rot caused by Geotrichum citri-aurantii and maintain postharvest quality of ‘Fino’ lemon. J. Sci. Food Agric. 2021, 102, 794–800. [Google Scholar] [CrossRef]
- Wang, W.; Deng, L.; Yao, S.; Zeng, K. Control of green and blue mold and sour rot in citrus fruits by the cationic antimicrobial peptide PAF56. Postharvest Biol. Technol. 2018, 136, 132–138. [Google Scholar] [CrossRef]
- Feng, L.; Wu, F.; Li, J.; Jiang, Y.; Duan, X. Antifungal activities of polyhexamethylene biguanide and polyhexamethylene guanide against the citrus sour rot pathogen Geotrichum citri-aurantii in vitro and in vivo. Postharvest Biol. Technol. 2011, 61, 160–164. [Google Scholar] [CrossRef]
- Wang, S.; Ruan, C.; Yi, L.; Deng, L.; Yao, S.; Zeng, K. Biocontrol ability and action mechanism of Metschnikowia citriensis against Geotrichum citri-aurantii causing sour rot of postharvest citrus fruit. Food Microbiol. 2020, 87, 103375. [Google Scholar] [CrossRef]
- Duan, X.; OuYang, Q.; Jing, G.; Tao, N. Effect of sodium dehydroacetate on the development of sour rot on Satsuma mandarin. Food Control 2016, 65, 8–13. [Google Scholar] [CrossRef]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of some Moroccan plants against Geotrichum candidum, the causal agent of postharvest citrus sour rot. Crop Prot. 2012, 35, 41–46. [Google Scholar] [CrossRef]
- Costa, A.; Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. Evaluation of the inhibitory effect of dimethyl dicarbonate (DMDC) against wine microorganisms. Food Microbiol. 2008, 25, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xiao, G.; Xu, Y.; Wu, J.; Wen, J. Effects of dimethyl dicarbonate (DMDC) on the fermentation of litchi juice by Lactobacillus casei as an alternative of heat treatment. J. Food Sci. 2014, 79, M947–M954. [Google Scholar] [CrossRef]
- Cheng, R.M.; Churey, J.J.; Worobo, R.W. Inactivation of Salmonella enterica and spoilage microorganisms in orange juice treated with dimethyl dicarbonate (DMDC). Int. J. Food Microbiol. 2018, 285, 152–157. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Xu, Y.; Wu, J.; Xiao, G. Effect of treatment with dimethyl dicarbonate on microorganisms and quality of Chinese cabbage. Postharvest Biol. Technol. 2013, 76, 139–144. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; Wang, Z.; Tian, Z.; Yang, F.; Lu, X.; Long, C.A. Genome sequencing and transcriptome analysis of Geotrichum citri-aurantii on citrus reveal the potential pathogenic- and guazatine-resistance related genes. Genomics 2020, 112, 4063–4071. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of sour rot in citrus fruit by three insect antimicrobial peptides. Postharvest Biol. Technol. 2019, 149, 200–208. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, K.; Fu, Y.; Ma, H.; Zhu, F. Toxic action and baseline sensitivity of boscalid against Penicillium digitatum. Crop Prot. 2020, 137, 105272. [Google Scholar] [CrossRef]
- Yang, Y.; OuYang, Q.; Li, L.; Shao, X.; Che, J.; Tao, N. Inhibitory effects of glutaraldehyde on Geotrichum citri-aurantii and its possible mechanism. J. Appl. Microbiol. 2019, 127, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, Y.; Yang, M. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014, 14, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, W.; Liu, S.; Ruan, C.; Yi, L.; Deng, L.; Yao, S.; Zeng, K. Effects of the peptide H-OOWW-NH2 and its derived lipopeptide C12-OOWW-NH2 on controlling of citrus postharvest green mold. Postharvest Biol. Technol. 2019, 158, 110979. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, C.; Chen, K.; Liu, P.; Fan, Q.; Zhao, J.; Long, C.A. Biocontrol and the mechanisms of Bacillus sp. w176 against postharvest green mold in citrus. Postharvest Biol. Technol. 2020, 159, 111022. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Dawood, D.H.; Safwat, N.A. Antifungal action and induction of resistance by beta-aminobutyric acid against Penicillium digitatum to control green mold in orange fruit. Pestic. Biochem. Physiol. 2021, 171, 104721. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Ji, S.; Chen, T.; Tian, S.; Qin, G. Enhancement of biocontrol efficacy of Cryptococcus laurentii by cinnamic acid against Penicillium italicum in citrus fruit. Postharvest Biol. Technol. 2019, 149, 42–49. [Google Scholar] [CrossRef]
- OuYang, Q.; Okwong, R.O.; Chen, Y.; Tao, N. Synergistic activity of cinnamaldehyde and citronellal against green mold in citrus fruit. Postharvest Biol. Technol. 2020, 162, 111095. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S. Effect of natamycin on Botrytis cinerea and Penicillium expansum—Postharvest pathogens of grape berries and jujube fruit. Postharvest Biol. Technol. 2019, 151, 134–141. [Google Scholar] [CrossRef]
- Cui, X.; Ma, D.; Liu, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. Magnolol inhibits gray mold on postharvest fruit by inducing autophagic activity of Botrytis cinerea. Postharvest Biol. Technol. 2021, 180, 111596. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Li, P.; Yang, Q.; Chen, K.; Zhao, L.; Apaliya, M.T.; Gu, X.; Zhang, H. Mechanisms of glycine betaine enhancing oxidative stress tolerance and biocontrol efficacy of Pichia caribbica against blue mold on apples. Biol. Control 2017, 108, 55–63. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, G.; Li, B.; Tian, S. Effect of Cinnamic Acid for Controlling Gray Mold on Table Grape and Its Possible Mechanisms of Action. Curr. Microbiol. 2015, 71, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Prisacaru, A.E. Effect of antioxidants on polyunsaturated fatty acids–review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montesinos-Herrero, C.; Moscoso-Ramírez, P.A.; Palou, L. Evaluation of sodium benzoate and other food additives for the control of citrus postharvest green and blue molds. Postharvest Biol. Technol. 2016, 115, 72–80. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, X.; Jing, G.; OuYang, Q.; Tao, N. Cinnamaldehyde inhibits the mycelial growth of Geotrichum citri-aurantii and induces defense responses against sour rot in citrus fruit. Postharvest Biol. Technol. 2017, 129, 23–28. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, K.; Ming, J. Control of blue and green mold decay of citrus fruit by Pichia membranefaciens and induction of defense responses. Sci. Hortic. 2012, 135, 120–127. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; Czymmek, K.J.; Li, H.; Shaw, J.B.; Buchko, G.W.; Shah, D.M. Antifungal symbiotic peptide NCR044 exhibits unique structure and multifaceted mechanisms of action that confer plant protection. Proc. Natl. Acad. Sci. USA 2020, 117, 16043–16054. [Google Scholar] [CrossRef]



| The Physiological Indicators | Treatments | Days after Storage | |||||
|---|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | 120 | ||
| Weight loss rate (%) | Control | 0.79 ± 0.07 Ae | 1.63 ± 0.14 Ad | 2.90 ± 0.23 Ac | 3.14 ± 0.26 Ac | 3.88 ± 0.34 Ab | 4.46 ± 0.38 Aa |
| DMDC | 0.83 ± 0.09 Ae | 1.69 ± 0.15 Ad | 2.98 ± 0.25 Ac | 3.26 ± 0.28 Ac | 4.05 ± 0.36 Ab | 4.70 ± 0.43 Aa | |
| Coloration index | Control | 11.47 ± 1.08 Aab | 12.08 ± 1.63 Aa | 11.42 ± 0.92 Aab | 11.14 ± 1.02 Ab | 11.21 ± 1.13 Aab | 11.02 ± 1.12 Ab |
| DMDC | 11.80 ± 1.05 Abc | 12.77 ± 1.23 Aa | 12.16 ± 0.90 Aab | 11.87 ± 1.18 Aab | 10.85 ± 0.86 Ac | 11.03 ± 1.40 Abc | |
| Total soluble solids (%) | Control | 10.34 ± 1.23 Aa | 11.42 ± 0.56 Aa | 11.60 ± 0.76 Aa | 10.88 ± 0.91 Aa | 11.26 ± 0.80 Aa | 10.86 ± 0.66 Aa |
| DMDC | 10.88 ± 0.72 Aa | 11.72 ± 0.63 Aa | 11.60 ± 0.64 Aa | 11.56 ± 0.61 Aa | 11.68 ± 1.55 Aa | 10.82 ± 0.32 Aa | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, D.; Wang, Y.; Yang, F.; Zhao, J.; Du, Y.; Tian, Z.; Long, C. Dimethyl Dicarbonate as a Food Additive Effectively Inhibits Geotrichum citri-aurantii of Citrus. Foods 2022, 11, 2328. https://doi.org/10.3390/foods11152328
Liu S, Zhang D, Wang Y, Yang F, Zhao J, Du Y, Tian Z, Long C. Dimethyl Dicarbonate as a Food Additive Effectively Inhibits Geotrichum citri-aurantii of Citrus. Foods. 2022; 11(15):2328. https://doi.org/10.3390/foods11152328
Chicago/Turabian StyleLiu, Shuqi, Deyao Zhang, Yuqing Wang, Fan Yang, Juan Zhao, Yujie Du, Zhonghuan Tian, and Chaoan Long. 2022. "Dimethyl Dicarbonate as a Food Additive Effectively Inhibits Geotrichum citri-aurantii of Citrus" Foods 11, no. 15: 2328. https://doi.org/10.3390/foods11152328
APA StyleLiu, S., Zhang, D., Wang, Y., Yang, F., Zhao, J., Du, Y., Tian, Z., & Long, C. (2022). Dimethyl Dicarbonate as a Food Additive Effectively Inhibits Geotrichum citri-aurantii of Citrus. Foods, 11(15), 2328. https://doi.org/10.3390/foods11152328





