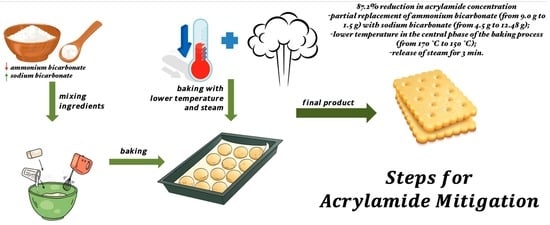
Mitigation of Acrylamide Content in Biscuits through Combined Physical and Chemical Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biscuit Preparation
2.2. Factorial Design of Experiment (FDOE)
2.3. Chemicals
2.4. Biscuit Size
2.5. Color Determination
2.6. Water Activity (aw)
2.7. Extraction Protocol for Acrylamide
2.8. LC-ESI-MS/MS Optimized Analytical Conditions
2.9. Evaluation of Linearity, Limit of Detection and Lower Limit of Quantification (Detectability), Intra-Day and Inter-Day Repeatability (Precision), and Recovery Test (Trueness)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimization of the Reference Industrial Biscuit (RIB)
3.2. Steam Release and Temperature Program Assessment
3.3. Validation of the MS/MS Parameters
3.4. Comparison between the Acrylamide Concentrations of the Eight FDOE Samples and the Reference Biscuits (RIB)
3.5. All Parameters Measured on the Eight FDOE Samples
3.6. Study of the Correlations among Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lingnert, H.; Grivas, S.; Jägerstad, M.; Skog, K.; Törnqvist, M.; Åman, P. Acrylamide in food: Mechanisms of formation and influencing factors during heating of foods. Scand. J. Nutr. 2002, 46, 159–172. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in human diet, its metabolism, toxicity, inactivation and the associated European Union legal regulations in food industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA J. 2012, 10, 2938. [Google Scholar] [CrossRef]
- Skog, K.I.; Murkovic, M.; Mottram, D.; van Boekel, T.; Zondervan, C.; van Klaveren, J.D. Heat-Generated Food Toxicants, Identification, Characterisation and Risk Minimisation; Project no. 506820 HEATOX; National Veterinary Institute: Oslo, Norway, 2007; Available online: https://edepot.wur.nl/4663 (accessed on 7 July 2022).
- Liu, Y.; Wang, P.; Chen, F.; Yuan, Y.; Zhu, Y.; Yan, H.; Hu, X. Role of plant polyphenols in acrylamide formation and elimination. Food Chem. 2015, 186, 46–53. [Google Scholar] [CrossRef] [PubMed]
- De Paola, E.L.; Montevecchi, G.; Masino, F.; Garbini, D.; Barbanera, M.; Antonelli, A. Determination of acrylamide in dried fruits and edible seeds using QuEChERS extraction and LC separation with MS detection. Food Chem. 2017, 217, 191–195. [Google Scholar] [CrossRef]
- Rannou, C.; Laroque, D.; Renault, E.; Prost, C.; Sérot, T. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res. Int. 2016, 90, 154–176. [Google Scholar] [CrossRef]
- Mesias, M.; Delgado-Andrade, C.; Morales, F.J. An updated view of acrylamide in cereal products. Curr. Res. Food Sci. 2022, 46, 100847. [Google Scholar] [CrossRef]
- Sarion, C.; Codina, G.G.; Dabija, A. Acrylamide in bakery products: A review on health risks, legal regulations and strategies to reduce its formation. Int. J. Environ. Res. Public Health 2021, 18, 4332. [Google Scholar] [CrossRef]
- Pal Murugan, M.; Agathian, G.; Semwal, A.D.; Sharma, G.K. A review on acrylamide mitigation strategies in various processed foods. Int. J. Adv. Res. 2016, 4, 1025–1040. [Google Scholar] [CrossRef] [Green Version]
- FoodDrinkEurope. Acrylamide Toolbox 2019. Available online: https://www.fooddrinkeurope.eu/wp-content/uploads/2021/05/FoodDrinkEurope_Acrylamide_Toolbox_2019.pdf (accessed on 7 July 2022).
- Anese, M.; Quarta, B.; Frias, J. Modelling the effect of asparaginase in reducing acrylamide formation in biscuits. Food Chem. 2011, 126, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Anese, M.; Quarta, B.; Peloux, L.; Calligaris, S. Effect of formulation on the capacity of l-asparaginase to minimize acrylamide formation in short dough biscuits. Food Res. Int. 2011, 44, 2837–2842. [Google Scholar] [CrossRef]
- Žilić, S.; Aktağ, I.G.; Dodig, D.; Filipović, M.; Gökmen, V. Acrylamide formation in biscuits made of different wholegrain flours depending on their free asparagine content and baking conditions. Food Res. Int. 2020, 132, 109109. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Amrein, T.M.; Graf, S.; Szalay, R.; Escher, F.; Amadò, R. Reducing the acrylamide content of a semi-finished biscuit on industrial scale. LWT-Food Sci. Technol. 2006, 39, 724–728. [Google Scholar] [CrossRef]
- Van Der Fels-Klerx, H.J.; Capuano, E.; Nguyen, H.T.; Ata ç Mogol, B.; Kocadağlı, T.; Göncüoğlu Taş, N.; Hamzalıoğlu, A.; Van Boekel, M.A.J.S.; Gökmen, V. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: NaCl and temperature–time profile effects and kinetics. Food Res. Int. 2014, 57, 210–217. [Google Scholar] [CrossRef]
- Claus, A.; Mongili, M.; Weisz, G.; Schieber, A.; Carle, R. Impact of formulation and technological factors on the acrylamide content of wheat bread and bread rolls. J. Cereal Sci. 2008, 47, 546–554. [Google Scholar] [CrossRef]
- Aarabi, F.; Seyedain Ardebili, M. The effect of sugar type and baking condition on formation of acrylamide in industrial rotary moulded biscuit. J. Food Meas. Char. 2020, 14, 2230–2239. [Google Scholar] [CrossRef]
- Galani, J.H.Y.; Patel, N.J.; Jayant, G.; Talati, J.G. Acrylamide-forming potential of cereals, legumes and roots and tubers analyzed by UPLC-UV. Food Chem. Toxicol. 2017, 108, 244–248. [Google Scholar] [CrossRef]
- Schouten, M.A.; Fryganas, C.; Tappi, S.; Romani, S.; Fogliano, V. The use of kidney bean flour with intact cell walls reduces the formation of acrylamide in biscuits. Food Control. 2022, 140, 109054. [Google Scholar] [CrossRef]
- Sung, W.C.; Chen, C.Y. Influence of cookies formulation on the formation of acrylamide. J. Food Sci. Nutr. Res. 2017, 5, 370–378. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Glicerina, V.; Rocculi, P.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Formation of acrylamide in biscuits during baking under different heat transfer conditions. LWT-Food Sci Technol. 2022, 153, 112541. [Google Scholar] [CrossRef]
- Dong, L.; Qiu, C.; Wei, F.; Yu, Z.; Zhang, Y.; Wang, S. The effect of microwave baking conditions on the quality of biscuits and the control of thermal processing hazards in the Maillard Reaction. Front. Nutr. 2022, 9, 825365. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, H.G.; Palazoğlu, T.K.; Miran, W.; Kocadağlı, T.; Gökmen, V. Evolution of surface temperature and its relationship with acrylamide formation during conventional and vacuum-combined baking of cookies. J. Food Eng. 2017, 197, 17–23. [Google Scholar] [CrossRef]
- McLaren, K. XIII—The development of the CIE 1976 (L* a* b*) uniform colour space and colour-difference formula. J. Soc. Dyers Colour. 1976, 92, 338–341. [Google Scholar] [CrossRef]
- Hunt, R.W.G.; Pointer, M.R. Measuring Colour, 4th ed.; Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Miller, J.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Ltd.: Harlow, UK, 2010. [Google Scholar]
- Wenzl, T.; Karasek, L.; Rosen, J.; Hellenaes, K.; Crews, C.; Castle, L.; Anklam, E. Collaborative trial validation study of two methods, one based on high performance liquid chromatography–tandem mass spectrometry and on gas chromatography–mass spectrometry for the determination of acrylamide in bakery and potato products. J. Chromatogr. A 2006, 1132, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bortolomeazzi, R.; Munari, M.; Anese, M.; Verardo, G. Rapid mixed mode solid phase extraction method for the determination of acrylamide in roasted coffee by HPLC–MS/MS. Food Chem. 2012, 135, 2687–2693. [Google Scholar] [CrossRef]
- Isleroglu, H.; Kemerli, T.; Sakin-Yilmazer, M.; Guven, G.; Ozdestan, O.; Uren, A.; Kaymak-Ertekin, F. Effect of steam baking on acrylamide formation and browning kinetics of cookies. J. Food Sci. 2012, 77, E257–E263. [Google Scholar] [CrossRef]
- Keramat, J.; LeBail, A.; Prost, C.; Jafari, M. Acrylamide in baking products: A review article. Food Bioproc. Tech. 2011, 4, 530–543. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Datta, A.K. Biscuit baking: A review. LWT-Food Sci. Technol. 2020, 131, 109726. [Google Scholar] [CrossRef]
- Fernandes, C.L.; Carvalho, D.O.; Guido, L.F. Determination of acrylamide in biscuits by high-resolution Orbitrap Mass Spectometry: A novel application. Foods 2019, 8, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arribas-Lorenzo, G.; Fogliano, V.; Morales, F.J. Acrylamide formation in a cookie system as influenced by the oil phenol profile and degree of oxidation. Eur. Food Res. Technol. 2009, 229, 63–72. [Google Scholar] [CrossRef]
- Simsek, S.; Martinez, M.O. Quality of dough and bread prepared with sea salt or sodium chloride. J. Food Process. Eng. 2016, 39, 44–52. [Google Scholar] [CrossRef]
- Sinesio, F.; Raffo, A.; Peparaio, M.; Moneta, E.; Civitelli, E.S.; Narducci, V.; Turfani, V.; Ferrari Nicoli, S.; Carcea, M. Impact of sodium reduction strategies on volatile compounds, sensory properties and consumer perception in commercial wheat bread. Food Chem. 2019, 301, 125252. [Google Scholar] [CrossRef]
- Riva, M. Approfondimenti: Il Colore degli Alimenti e la sua Misurazione 2003. Dipartimento di Scienze e Tecnologie Alimentari e Microbiologiche, University of Milan, Milan. Available online: https://documen.site/download/il-colore-degli-alimenti_pdf (accessed on 7 July 2022).
- Anese, M.; Valoppi, F.; Calligaris, S.; Lagazio, C.; Suman, M.; Manzocco, L.; Nicoli, M.C. Omega-3 enriched biscuits with low levels of heat-Induced toxicants: Effect of formulation and baking conditions. Food Bioproc. Tech. 2016, 9, 232–242. [Google Scholar] [CrossRef]


| Batches from FDOE | Rep. 1 | Rep. 2 | Rep. 3 |
|---|---|---|---|
| 1 | 1 | 2 | 8 |
| 2 | 2 | 3 | 1 |
| 3 | 3 | 4 | 2 |
| 4 | 4 | 5 | 3 |
| 5 | 5 | 6 | 4 |
| 6 | 6 | 7 | 5 |
| 7 | 7 | 8 | 6 |
| 8 | 8 | 1 | 7 |
| Analyte | ESI | MW (g/mol) | Parent Ions (m/z) | Product Ions (m/z) | Dwell Times (msec) | Fragmentor Voltage (V) | Collision Energy (V) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Acrylamide | + | 71.08 | 72.1 (M + H)+ | 55 | 80 | 70 | 8 | |||
| Acrylamide d3 | + | 74.10 | 75.1 (M + H)+ | 58 | 80 | 70 | 8 | |||
| Linearity | Detectability | Trueness | Precision | |||||||
| Range of concentration (mg/L) | R2 (aqueous solutions of acrylamide of known concentration) | R2 (analyte-free biscuit matrix with addition of known acrylamide concentrations) | LOD (mg/L) | LLOQ (mg/L) | % recovery | Intra-day on a biscuit sample (n = 5) (RDS %) | Intra-day using a standard solution of acrylamide (n = 5) (RDS %) | Inter-day on a biscuit sample (n = 5) (RDS %) | ||
| Acrylamide | 0.02 ÷ 1.00 | 0.995 | 0.998 | 0.03 | 0.10 | 98.31 | 5.57 | 2.61 | 6.16 | |
| Samples | Thickness (mm) | Length (mm) | Width (mm) | L* | a* | b* | aw | Acrylamide (mg/kgFM) | Percentage Reduction Compared with RIB (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.7 ± 0.5 | 80.7 ± 1.4 | 27.6 ± 2.2 | 75.41 ± 0.91 | 6.36 ± 0.62 | 30.53 ± 0.68 | 0.325 ± 0.018 | 0.229 ± 0.010 | 87.2 |
| 2 | 5.6 ± 0.5 | 80.4 ± 1.1 | 26.5 ± 1.6 | 75.81 ± 0.89 | 6.25 ± 0.47 | 30.67 ± 0.60 | 0.271 ± 0.034 | 0.363 ± 0.005 | 79.7 |
| 3 | 5.7 ± 0.6 | 80.4 ± 1.7 | 26.6 ± 1.6 | 74.39 ± 1.17 | 7.27 ± 0.63 | 31.35 ± 0.62 | 0.298 ± 0.026 | 0.401 ± 0.007 | 77.6 |
| 4 | 5.6 ± 0.7 | 80.0 ± 1.2 | 27.4 ± 1.6 | 74.61 ± 1.07 | 7.00 ± 0.69 | 31.21 ± 0.74 | 0.244 ± 0.035 | 0.455 ± 0.003 | 74.6 |
| 5 | 6.0 ± 0.8 | 80.6 ± 1.4 | 27.6 ± 2.0 | 76.94 ± 1.03 | 5.76 ± 0.53 | 27.69 ± 0.85 | 0.384 ± 0.039 | 0.372 ± 0.004 | 79.2 |
| 6 | 5.3 ± 0.4 | 79.8 ± 1.7 | 27.2 ± 1.8 | 77.25 ± 1.00 | 5.91 ± 0.73 | 27.91 ± 1.03 | 0.257 ± 0.036 | 0.470 ± 0.030 | 73.7 |
| 7 | 6.1 ± 0.4 | 79.0 ± 1.3 | 26.8± 1.4 | 76.62 ± 0.97 | 6.36 ± 0.64 | 28.60 ± 0.80 | 0.337 ± 0.029 | 0.478 ± 0.010 | 73.3 |
| 8 | 5.8 ± 0.6 | 80.0 ± 1.8 | 26.7 ± 1.6 | 76.60 ± 0.94 | 6.65 ± 0.74 | 29.04 ± 1.09 | 0.245 ± 0.053 | 0.658 ± 0.092 | 63.2 |
| RIB | 5.9 ± 0.6 | 79.1 ± 1.3 | 27.9 ± 1.6 | 71.71 ± 2.26 | 9.64 ± 1.09 | 33.10 ± 1.11 | 0.109 ± 0.031 | 1.788 ± 0.111 |
| Thickness (mm) | Length (mm) | Width (mm) | L* | a* | b* | aw | Acrylamide (mg/kgFM) | |
|---|---|---|---|---|---|---|---|---|
| TP | n.s. | n.s. | n.s. | n.s. | 0.0238 | n.s. | n.s. | 0.0356 |
| SRT | 0.0574 | n.s. | n.s. | n.s. | n.s. | n.s. | 0.0022 | n.s. |
| AB | n.s. | n.s. | n.s. | 0.0016 | 0.0659 | 0.0008 | n.s. | 0.0312 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | RIB | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | |||||||||
| 2 | 0.43 | ||||||||
| 3 | 1.87 | 1.59 | |||||||
| 4 | 1.51 | 1.23 | 0.37 | ||||||
| 5 | 3.23 | 3.29 | 4.41 | 4.71 | |||||
| 6 | 3.13 | 3.23 | 4.36 | 4.67 | 0.42 | ||||
| 7 | 2.23 | 2.28 | 3.36 | 3.66 | 1.03 | 1.14 | |||
| 8 | 1.86 | 1.93 | 2.97 | 3.25 | 1.50 | 1.66 | 0.53 | ||
| RIB | 3.82 | 3.99 | 3.60 | 3.62 | 6.70 | 6.89 | 5.46 | 5.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Faro, E.; Salerno, T.; Montevecchi, G.; Fava, P. Mitigation of Acrylamide Content in Biscuits through Combined Physical and Chemical Strategies. Foods 2022, 11, 2343. https://doi.org/10.3390/foods11152343
Lo Faro E, Salerno T, Montevecchi G, Fava P. Mitigation of Acrylamide Content in Biscuits through Combined Physical and Chemical Strategies. Foods. 2022; 11(15):2343. https://doi.org/10.3390/foods11152343
Chicago/Turabian StyleLo Faro, Emanuela, Tommaso Salerno, Giuseppe Montevecchi, and Patrizia Fava. 2022. "Mitigation of Acrylamide Content in Biscuits through Combined Physical and Chemical Strategies" Foods 11, no. 15: 2343. https://doi.org/10.3390/foods11152343