Optimization of Osmotic Dehydration of White Mushrooms by Response Surface Methodology for Shelf-Life Extension and Quality Improvement of Frozen End-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Osmotic Dehydration
2.3. Calculation for Mass Transport Parameters for Osmotic Dehydration
2.4. Physicochemical Measurements during Osmotic Treatment
2.5. Experimental Design
2.6. Frozen Storage—Determination of Color Change and Drip Loss
2.7. Statistical Analysis
3. Results and Discussion
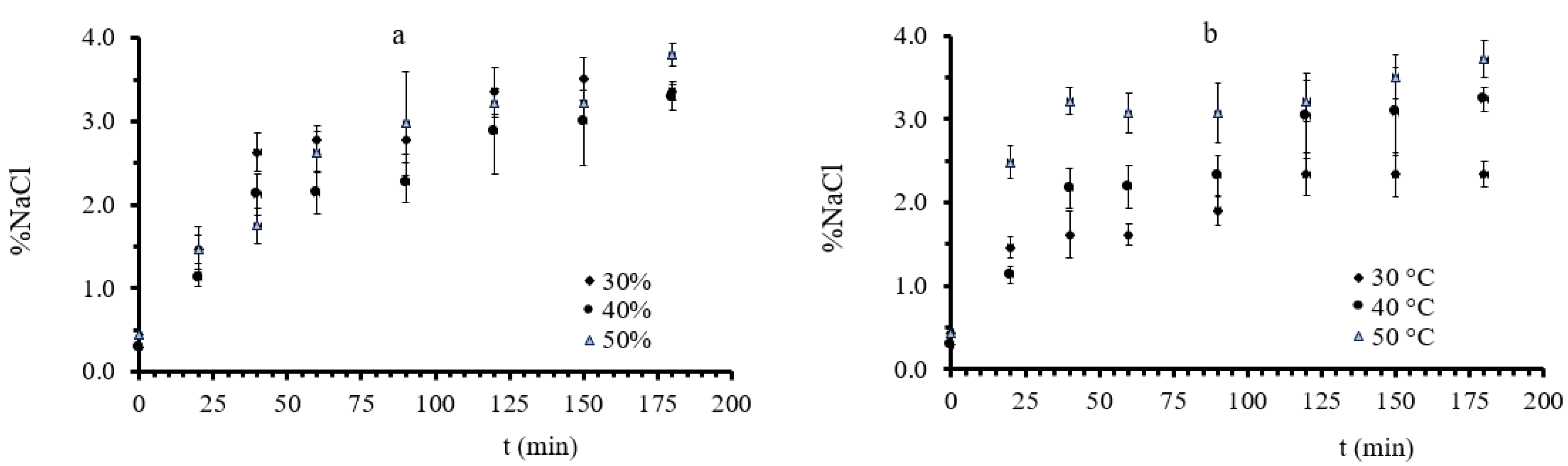
3.1. Mass Transfer during Osmotic Dehydration of Button Mushrooms
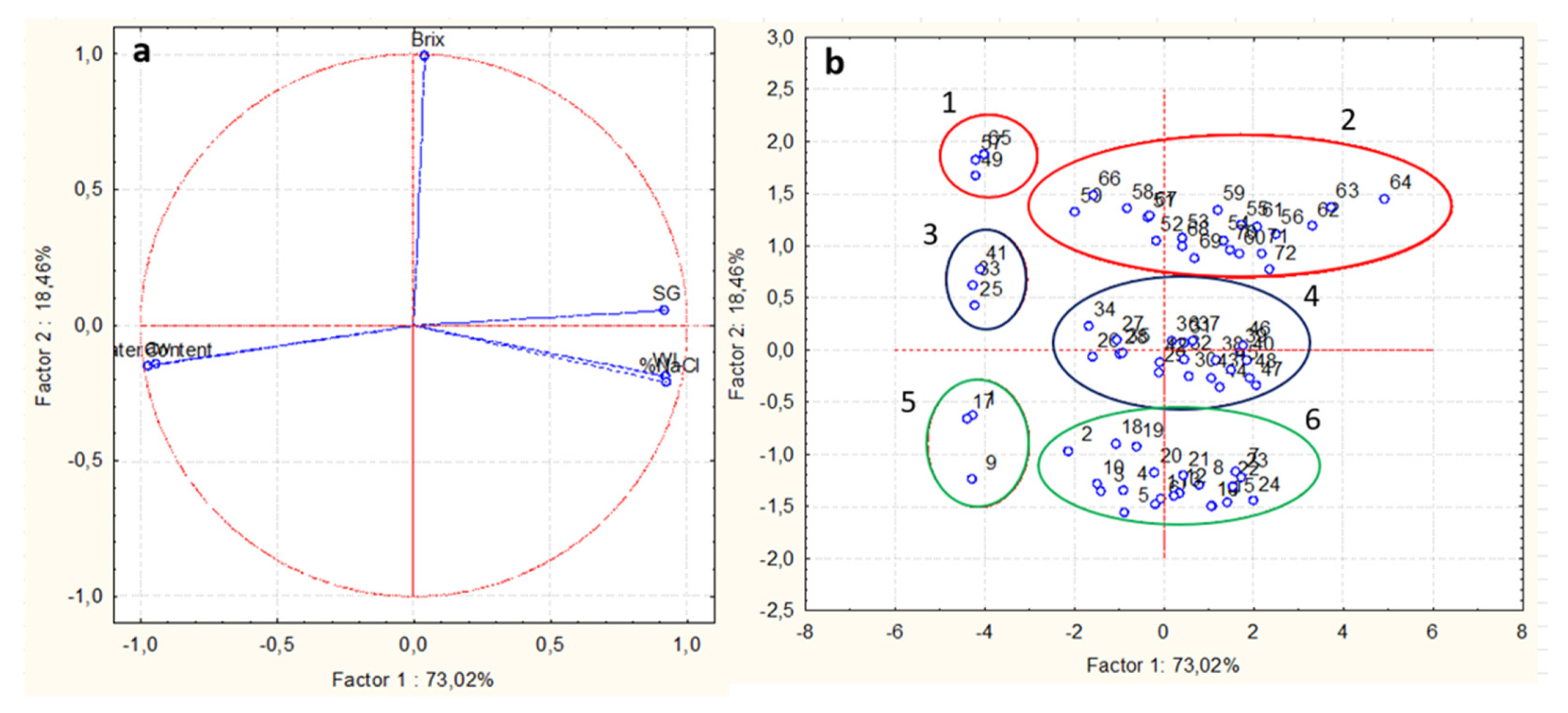
Principal Component Analysis (PCA)
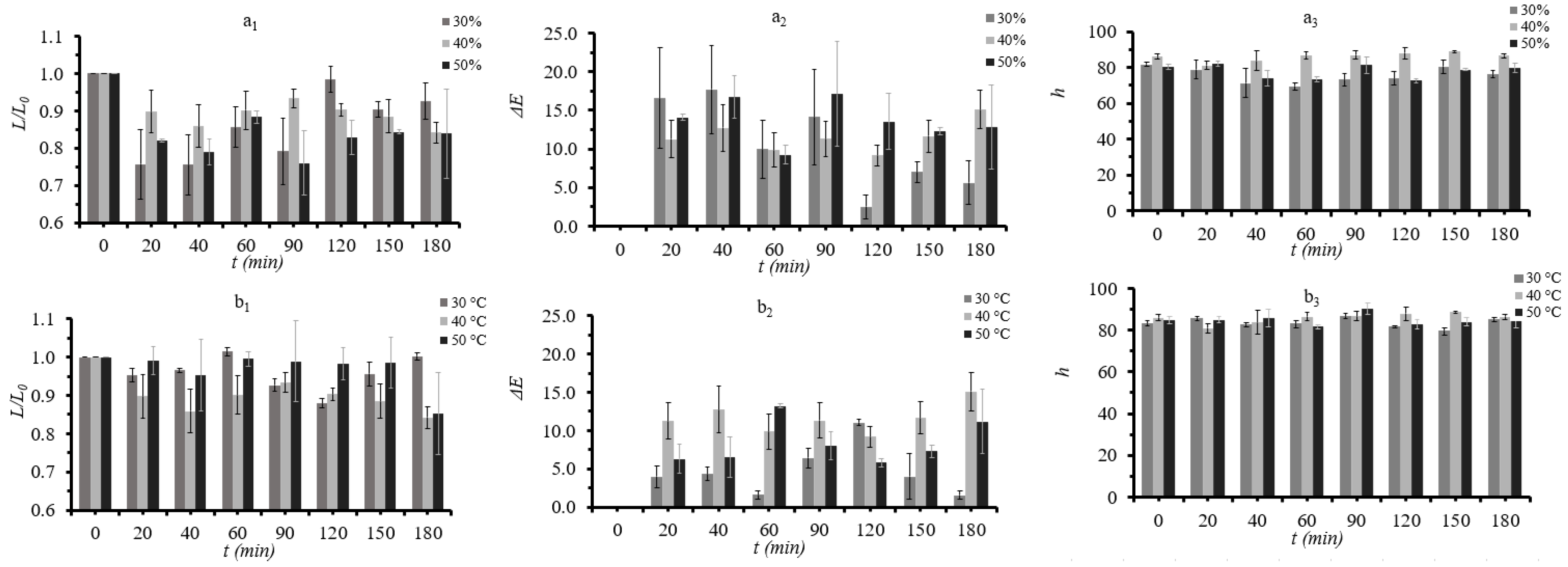
3.2. Quality Evaluation during Osmotic Dehydration of Button Mushrooms
3.3. Determination of Parameter Interactions during Osmotic Dehydration (OD)
3.4. Optimization and Validation of Process Conditions Based on Mass Transfer and Quality Requirements
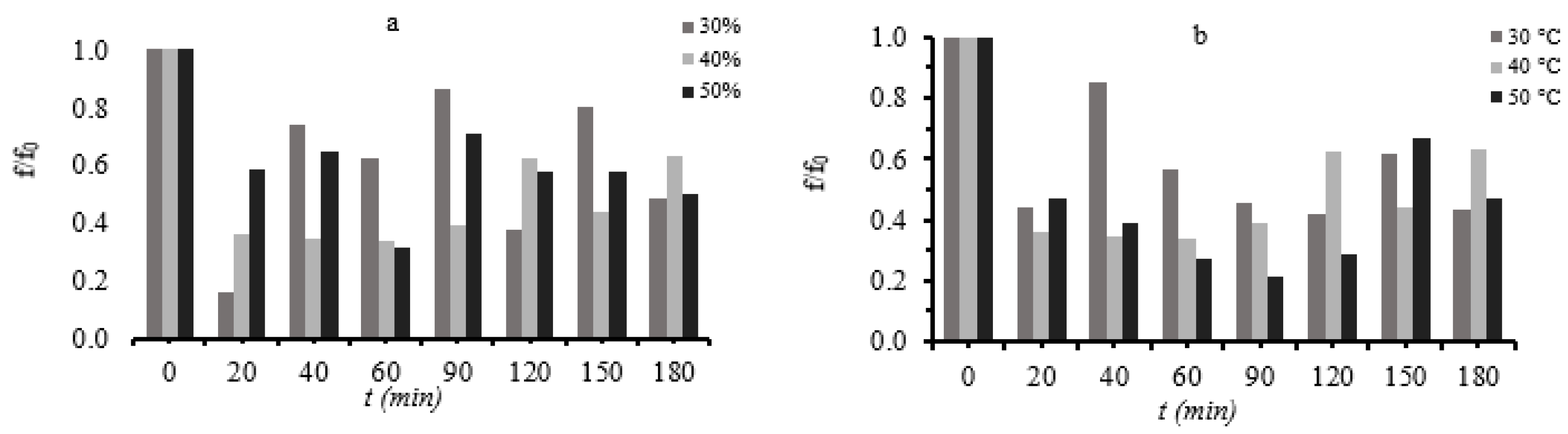
3.5. Assessment of Storage Stability under Frozen Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, M.P.; Nikhanj, P.; Sodhi, H.S. Physico-chemical and nutritional studies on vinegar dips for shelf life extension of Agaricus Bisporus. J. Food Process. Preserv. 2020, 44, e14706. [Google Scholar] [CrossRef]
- Sinha, S.K.; Upadhyay, T.K.; Sharma, S.K. Nutritional-Medicinal Profile and Quality Categorization of Fresh White Button Mushroom. Biointerface Res. Appl. Chem. 2021, 11, 8669–8685. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef] [PubMed]
- Montes, A.P.; Rangel-Vargas, E.; Lorenzo, J.M.; Romero, L.; Santos, E.M. Edible mushrooms as a novel trend in the development of healthier meat products. Curr. Opin. Food Sci. 2020, 37, 118–124. [Google Scholar] [CrossRef]
- Cha, M.H.; Heo, J.Y.; Lee, C.; Lo, Y.M.; Moon, B. Quality and sensory characterization of white jelly mushroom (Tremella fuciformis) as a meat substitute in pork patty formulation. J. Food Process. Preserv. 2014, 38, 2014–2019. [Google Scholar] [CrossRef]
- Van Ba, H.; Seo, H.W.; Cho, S.H.; Kim, Y.S.; Kim, J.H.; Ham, J.S.; Park, B.Y.; Pil Nam, S. Antioxidant and anti-foodborne bacteria activities of shiitake by-product extract in fermented sausages. Food Control 2016, 70, 201–209. [Google Scholar] [CrossRef]
- Summers, A.; Ezike, A.; Smith, P.; Frutchey, R.; Leslie, L.; Paredes, S.; Alvarado, C.; Karani, S.; Taylor, J.; Cheskin, L. Acceptance of a mushroom-soy-beef blended burger among school-aged children. Health Behav. Policy Rev. 2017, 4, 274–281. [Google Scholar] [CrossRef]
- Choe, J.; Lee, J.; Jo, K.; Jo, C.; Song, M.; Jung, S. Application of winter mushroom powder as an alternative to phosphates in emulsion-type sausages. Meat Sci. 2018, 143, 114–118. [Google Scholar] [CrossRef]
- Jo, K.; Lee, J.; Jung, S. Quality characteristics of low-salt chicken sausage supplemented with a winter mushroom powder. Korean J. Food Sci. Anim. Resour. 2018, 38, 768–779. [Google Scholar]
- Chun, S.; Chambers, E., IV; Chambers, D. Perception of pork patties with shiitake (Lentinus edode P.) mushroom powder and sodium tripolyphosphate as measured by Korean and United States consumers. J. Sens. Stud. 2005, 20, 156–166. [Google Scholar] [CrossRef]
- Devi, S.; Zhang, M.; Ju, R.; Bhandari, B. Water loss and partitioning of the oil fraction of mushroom chips using ultrasound-assisted vacuum frying. Food Biosci. 2020, 38, 100753. [Google Scholar] [CrossRef]
- Martine, B.; Gaëlle, L.; Ronan, G. Post-harvest treatment with citric acid or hydrogen peroxide to extend the shelf life of fresh sliced mushrooms. LWT 2000, 33, 285–289. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Hanula, M.; Wojtasik-Kalinowska, I.; Stelmasiak, A.; Zalewska, M.; Półtorak, A.; Wierzbicka, A. Packaging in a high O2 or air atmospheres and in microperforated films effects on quality of button mushrooms stored at room temperature. Agriculture 2020, 10, 479. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.-Y.; Sun, D.-W. Recent Advances in Quality Preservation of Postharvest Mushrooms (Agaricus bisporus): A Review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Bobo-García, G.; Arroqui, C.; Merino, G.; Vírseda, P. Antibrowning Compounds for Minimally Processed Potatoes: A Review. Food Rev. Int. 2019, 36, 529–546. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Pedreira, A.J.R.M.; Cristianini, L.B.; Guidi, M.F.; Capato, M.O.; Ávila, P.F.; Goldbeck, R.; Kamimura, E.S. Application of soluble fibres in the osmotic dehydration of pineapples and reuse of effluent in a beverage fermented by water kefir. LWT 2020, 132, 109819. [Google Scholar] [CrossRef]
- Gamboa-Santos, J.; Campañone, L.A. Application of osmotic dehydration and microwave drying to strawberries coated with edible films. Dry. Technol. 2019, 37, 1002–1012. [Google Scholar] [CrossRef]
- Janowicz, M.; Ciurzyńska, A.; Lenart, A. Effect of Osmotic Pretreatment Combined with Vacuum Impregnation or High Pressure on the Water Diffusion Coefficients of Convection Drying: Case Study on Apples. Foods 2021, 10, 2605. [Google Scholar] [CrossRef]
- Pacheco-Angulo, H.; Herman-Lara, E.; García-Alvarado, M.A.; Ruiz-López, I.I. Mass transfer modeling in osmotic dehydration: Equilibrium characteristics and process dynamics under variable solution concentration and convective boundary. Food Bioprod. Process. 2016, 97, 88–99. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Hoque, M.E.; Rahman, S.; Hasanuzzaman, M. Osmotic dehydration of pumpkin using response surface methodology—infuences of operating conditions on water loss and solute gain. J. Bioprocess Biotech. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sangeeta; Hathan, B.S. Studies on Mass Transfer and Diffusion Coefficients in Elephant Foot Yam (Amorphophallus spp.) during Osmotic Dehydration in Sodium Chloride Solution. J. Food Process. Preserv. 2016, 40, 521–530. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Giannakourou, M.C. Modelling dehydration of apricot in a non-conventional multi-component osmotic solution: Effect on mass transfer kinetics and quality characteristics. J. Food Sci. Technol. 2018, 55, 4079–4089. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.L.; Corrêa, J.L.G.; da Silva Araújo, C.; Vimercati, W.C. Effect of osmotic agent and vacuum application on mass exchange and qualitative parameters of osmotically dehydrated strawberries. J. Food Process. Preserv. 2022, 46, 16621. [Google Scholar] [CrossRef]
- Lopez, M.; Morais, R.; Morais, A. Flavonoid enrichment of fresh-cut apple through osmotic dehydration-assisted impregnation. Br. Food J. 2021, 123, 820–832. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.K.; Pantelaiaki, K.; Andreou, V.; Katsaros, G.J.; Taoukis, P.S. Osmotic pretreatment for the production of novel dehydrated tomatoes and cucumbers. J. Food Process. Preserv. 2019, 43, e13968. [Google Scholar] [CrossRef]
- Osae, R.; Essilfie, G.; Alolga, R.N.; Akaba, S.; Song, X.; Owusu-Ansah, P.; Zhou, C. Application of non-thermal pretreatment techniques on agricultural products prior to drying: A review. J. Sci. Food Agric. 2020, 100, 2585–2599. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Le Maguer, M. Osmotic dehydration of foods: Mass transfer and modeling aspects. Food Rev. Int. 2002, 18, 305–335. [Google Scholar] [CrossRef]
- Ramya, V.; Jain, N.K. A review on osmotic dehydration of fruits and vegetables: An integrated approach. J. Food Process Eng. 2016, 40, e12440. [Google Scholar] [CrossRef]
- Assis, F.R.; Morais, R.M.S.C.; Morais, A.M.M.B. Osmotic Dehydration combined with freeze drying of apple cubes and comparison with microwave drying and hot air drying. Adv. Food Sci. 2018, 2, 39–47. [Google Scholar] [CrossRef]
- Sulistyawati, T.; Verkerk, R.; Fogliano, V.; Dekker, M. Modelling the kinetics of osmotic dehydration of mango: Optimizing process conditions and pre-treatment for health aspects. J. Food Eng. 2020, 280, 109985. [Google Scholar] [CrossRef]
- Haque, R.; Hosain, M.; Kamal, M.; Islam, R.; Mondal, S.C. Optimization of osmotic dehydration parameters of banana under pulsed microwave conditions by response surface methodology. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1053–1059. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Staniszewska, I.; Zielinska, D.; Zhou, Y.-H.; Nowak, K.W.; Xiao, H.-W.; Pan, Z.; Zielinska, M. Combined Hot Air and Microwave-Vacuum Drying of Cranberries: Effects of Pretreatments and Pulsed Vacuum Osmotic Dehydration on Drying Kinetics and Physicochemical Properties. Food Bioprocess Tech. 2020, 13, 1848–1856. [Google Scholar] [CrossRef]
- Kroehnke, J.; Szadzińska, J.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Musielak, G.; Mierzwa, D. Osmotic dehydration and convective drying of kiwifruit (Actinidia deliciosa)—The influence of ultrasound on process kinetics and product quality. Ultrason. Sonochem. 2021, 71, 105377. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Hosain, M.; Rahman, M.A.; Kamal, K.; Mondal, S.C.; Islam, M. Modeling and optimization of the pulsed vacuum osmotic dehydration (pvod) process of carrots in a ternary solution by response surface methodology. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 424–430. [Google Scholar] [CrossRef]
- Dehghannya, J.; Bozorghi, S.; Heshmati, M.K. Low temperature hot air drying of potato cubes subjected to osmotic dehydration and intermittent microwave: Drying kinetics, energy consumption and product quality indexes. Heat Mass Transf. 2017, 54, 929–954. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Lazou, A.E.; Dermesonlouoglou, E.K. Optimization of Osmotic Dehydration of Tomatoes in Solutions of Non-Conventional Sweeteners by Response Surface Methodology and Desirability Approach. Foods 2020, 9, 1393. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Paraskevopoulou, E.; Andreou, V.; Taoukis, P. Osmotic Dehydration for the Production of Novel Pumpkin Cut Products of Enhanced Nutritional Value and Sustainability. Appl. Sci. 2020, 10, 6225. [Google Scholar] [CrossRef]
- Suput, D.Z.; Filipović, V.S.; Lončar, B.L.; Nićetin, M.R.; Knežević, V.M.; Lazarević, J.M.; Plavšić, D.V. Modeling of mushrooms (Agaricus bisporus) osmotic dehydration process in sugar beet molasses. Food Feed Res. 2020, 47, 175–187. [Google Scholar] [CrossRef]
- González-Pérez, J.E.; López-Méndez, E.M.; Luna-Guevara, J.J.; Ruiz-Espinosa, H.; Ochoa Velasco, C.E.; Ruiz-López, I.I. Analysis of mass transfer and morphometric characteristics of white mushroom (Agaricus bisporus) pilei during osmotic dehydration. J. Food Eng. 2019, 240, 120–132. [Google Scholar] [CrossRef]
- Pei, F.; Xiao, K.; Chen, L.; Yang, W.; Zhao, W.; Fang, Y.; Ma, N.; Mariga, A.M.; Hu, Q. Mass transfer characteristics during ultrasound-assisted osmotic dehydration of button mushroom (Agaricus bisporus). J. Food Sci. Technol. 2019, 56, 2213–2223. [Google Scholar] [CrossRef]
- Singhal, S.; Rasane, P.; Kaur, S.; Singh, J.; Gupta, N. Thermal degradation kinetics of bioactive compounds in button mushroom (Agaricus bisporus) during tray drying process. J. Food Process Eng. 2020, 43, e13555. [Google Scholar] [CrossRef]
- Kumar, K.; Barmanray, A.; Kumar, S. Shelf-Life Studies on Osmo-Air Dried White Button Mushroom (Agaricus Bisporus L.). Curr. Res. Nutr. Food Sci. 2017, 5, 144–153. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Q.; Wang, L.; Zhang, B.; Zhang, W.; Yang, W.; Hu, Q.; Pei, F. Prediction of soluble solid content of Agaricus bisporus during ultrasound-assisted osmotic dehydration based on hyperspectral imaging. LWT 2020, 122, 109030. [Google Scholar] [CrossRef]
- Anshu, S.; Anju, B. Effect of osmotic dehydration on quality of oyster mushrooms. Int. J. Chem. Stud. 2018, 6, 1601–1605. [Google Scholar]
- Tolera, K.D.; Abera, S. Nutritional quality of Oyster Mushroom (Pleurotus Ostreatus) as affected by osmotic pretreatments and drying methods. Food Sci. Nutr. 2017, 5, 989–996. [Google Scholar] [CrossRef]
- Ren, A.; Pan, S.; Li, W.; Chen, G.; Duan, X. Effect of Various Pretreatments on Quality Attributes of Vacuum-Fried Shiitake Mushroom Chips. J. Food Qual. 2018, 2018, 4510126. [Google Scholar] [CrossRef] [Green Version]
- González-Pérez, J.E.; López-Méndez, E.M.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Mass transfer and morphometric characteristics of fresh and osmodehydrated white mushroom pilei during convective drying. J. Food Eng. 2019, 262, 181–188. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, L.; Xu, Y.; Dong, J.; Zhao, Y.; Yuan, Y. Optimization of Processing Parameters for Lettuce Vacuum Osmotic Dehydration Using Response Surface Methodology. Pol. J. Food Nutr. Sci. 2018, 68, 15–23. [Google Scholar] [CrossRef]
- Mirzayi, B.; Heydari, A.; Jabbari, A. The effects of Sucrose/NaCl/Time interactions on the osmotic dehydration of banana slices. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Ramya, H.G.; Kumar, S. Evaluation of Mass Transfer Kinetics for Osmotic Dehydration of Oyster Mushrooms (Pleurotus sajorcaju) in Salt-sugar Solution. Proc. Natl. Acad. Sci. India Sect. B 2015, 85, 517–525. [Google Scholar] [CrossRef]
- Gupta, P.; Bhat, A.; Chauhan, H.; Ahmed, N.; Malik, A. Osmotic Dehydration of Button Mushroom. Int. J. Food Ferment. Technol. 2015, 5, 177–182. [Google Scholar] [CrossRef]
- Mehta, B.K.; Kumari, M.; Surabhi; Jain, S.K. Osmotic Dehydration as a Pre-treatment Before Hot Air Drying of Mushroom (Agaricus bisporus). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1341–1349. [Google Scholar]
- Samira, A.; Hadi, P.S. A study of drying kinetics of button mushroom pre-treated through osmotic dehydration. Iran. J. Biosyst. Eng. (Iran. J. Agric. Sci.) 2016, 47, 569–575. [Google Scholar]
- Katsoufi, S.; Lazou, A.E.; Giannakourou, M.C.; Krokida, M.K. Mass transfer kinetics and quality attributes of osmo-dehydrated candied pumpkins using nutritious sweeteners. J. Food Sci. Technol. 2017, 54, 3338–3348. [Google Scholar] [CrossRef]
- Pathare, P.; Opara, U.; Al-Said, F. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Nishinari, K.; Kohyama, K.; Kumagai, H.; Funami, T.; Bourne, M.C. Parameters of texture profile analysis. Food Sci. Technol. 2013, 19, 519–521. [Google Scholar] [CrossRef] [Green Version]
- Costa, N.; Lourenço, J. A comparative study of multiresponse optimization criteria working ability. Chemom. Intell. Lab. Syst. 2014, 138, 171–177. [Google Scholar] [CrossRef]
- Costa, N.R.; Lourenço, J.; Pereira, Z.L. Desirability function approach: A review and performance evaluation in adverse conditions. Chemom. Intell. Lab. Syst. 2011, 107, 234–244. [Google Scholar] [CrossRef]
- Islam, N.; Zhang, M.; Adhikari, B.; Cheng, X.; Xu, B.-G. The effect of ultrasound-assisted immersion freezing on selected physicochemical properties of mushrooms. Int. J. Refrig. 2014, 42, 121–133. [Google Scholar] [CrossRef]
- Tiroutchelvame, D.; Prakash Maran, J.; Pragalyaashree, M.M. Response surface analysis and optimization of osmotic dehydration of Musa acuminata slices. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1016–1020. [Google Scholar] [CrossRef] [Green Version]
- Lazou, A.; Dermesonlouoglou, E.K.; Giannakourou, M.C. Modeling and Evaluation of the Osmotic Pretreatment of Tomatoes (S. lycopersicum) with Alternative Sweeteners for the Production of Candied Products. Food Bioprocess Technol. 2020, 13, 948–961. [Google Scholar] [CrossRef]
- de Mello, R., Jr.; Corrêa, J.L.G.; Lopes, F.J.; de Souza, A.U.; da Silva, K.C.R. Kinetics of the pulsed vacuum osmotic dehydration of green fig (Ficus carica L.). Heat Mass Transf. 2019, 55, 1685–1691. [Google Scholar] [CrossRef]
- Checmarev, G.; Casales, M.R.; Yeannes, M.I. Analysis of applicability of Peleg model to the cooking-infusion of mackerel (Scomber japonicus) slices. Food Sci. Technol. 2013, 33, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Tortoe, C.; Orchard, J.; Beezer, A. Effect of agitation and antagonism between sucrose and sodium chloride on mass transfer during osmo-dehydration in plant materials. Int. Food Res. 2009, 16, 521–530. [Google Scholar]
- Kowalska, H.; Marzec, A.; Domian, E.; Masiarz, E.; Ciurzyńska, A.; Galus, S.; Małkiewicz, A.; Lenart, A.; Kowalska, J. Physical and Sensory Properties of Japanese Quince Chips Obtained by Osmotic Dehydration in Fruit Juice Concentrates and Hybrid Drying. Molecules 2020, 25, 5504. [Google Scholar] [CrossRef]
- Rai, R.; Rani, P.; Tripathy, P.P. Osmo-air drying of banana slices: Multivariate analysis, process optimization and product quality characterization. J. Food Sci. Technol. 2022, 59, 2430–2447. [Google Scholar] [CrossRef]
- Mune, M.A.M.; Minka, S.R. Production and characterization of cowpea protein hydrolysate with optimum nitrogen solubility by enzymatic hydrolysis using pepsin. J. Sci. Food Agric. 2017, 97, 2561–2568. [Google Scholar] [CrossRef]
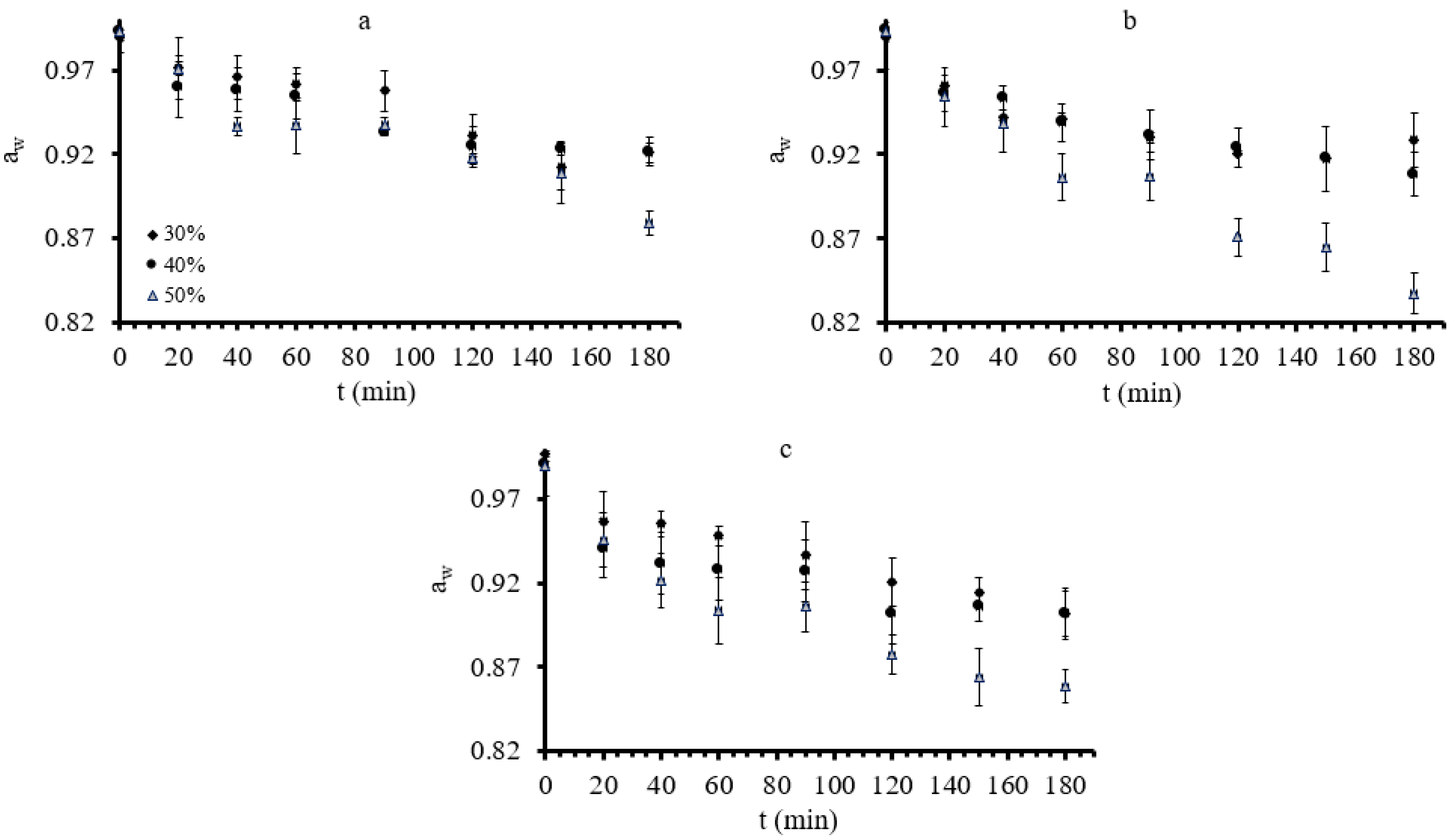
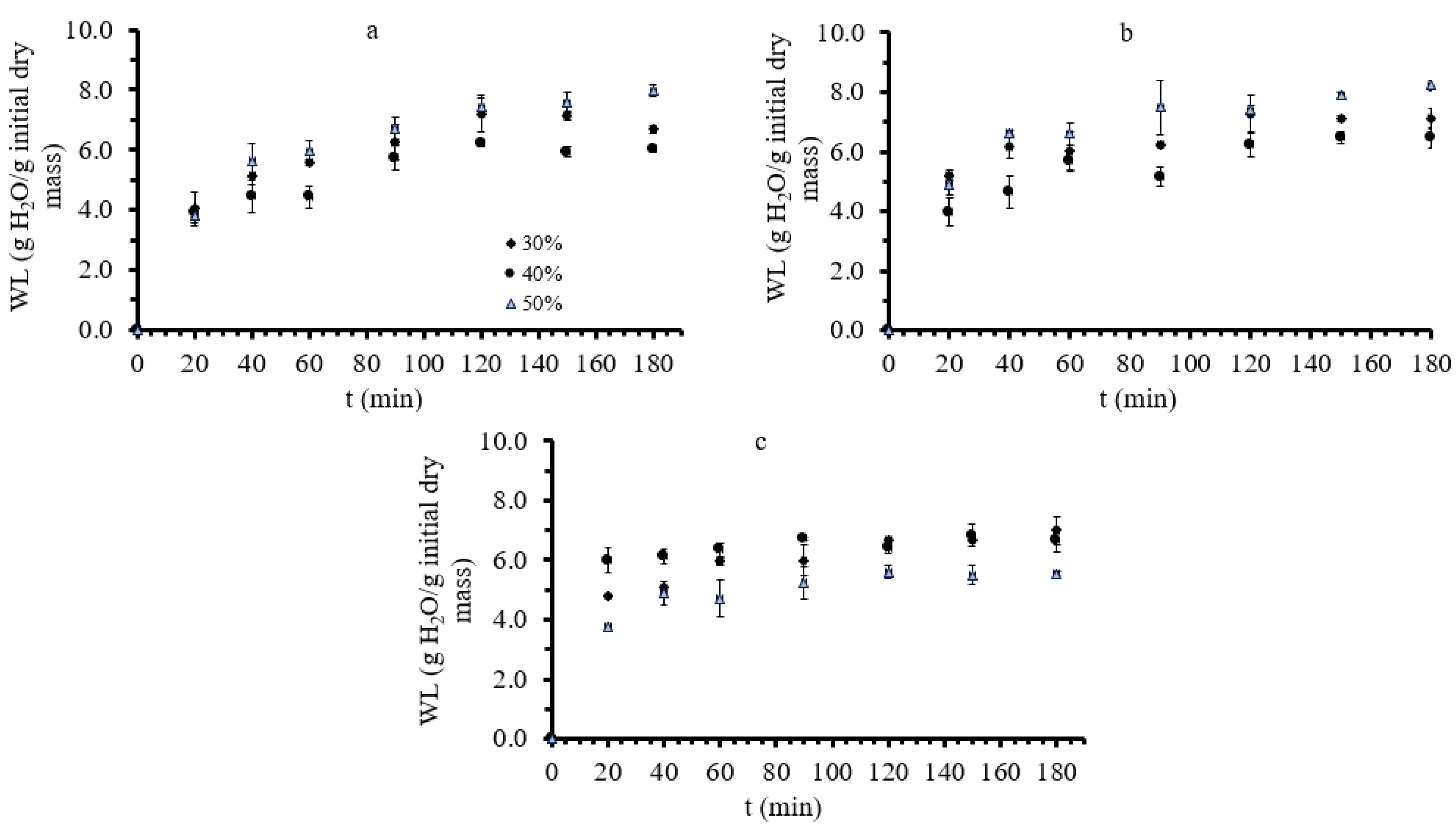
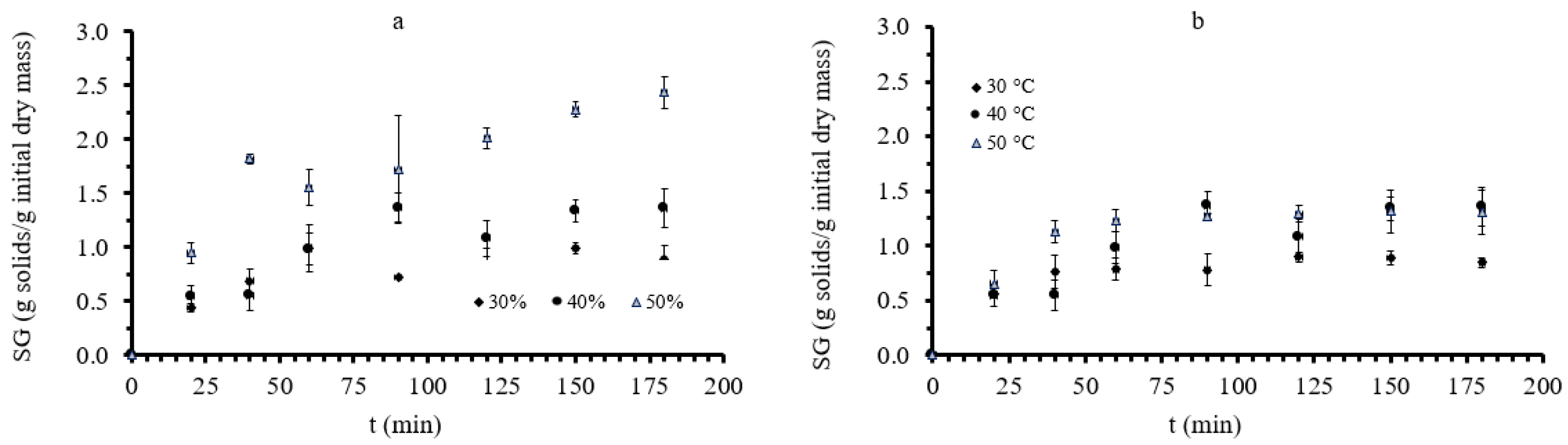
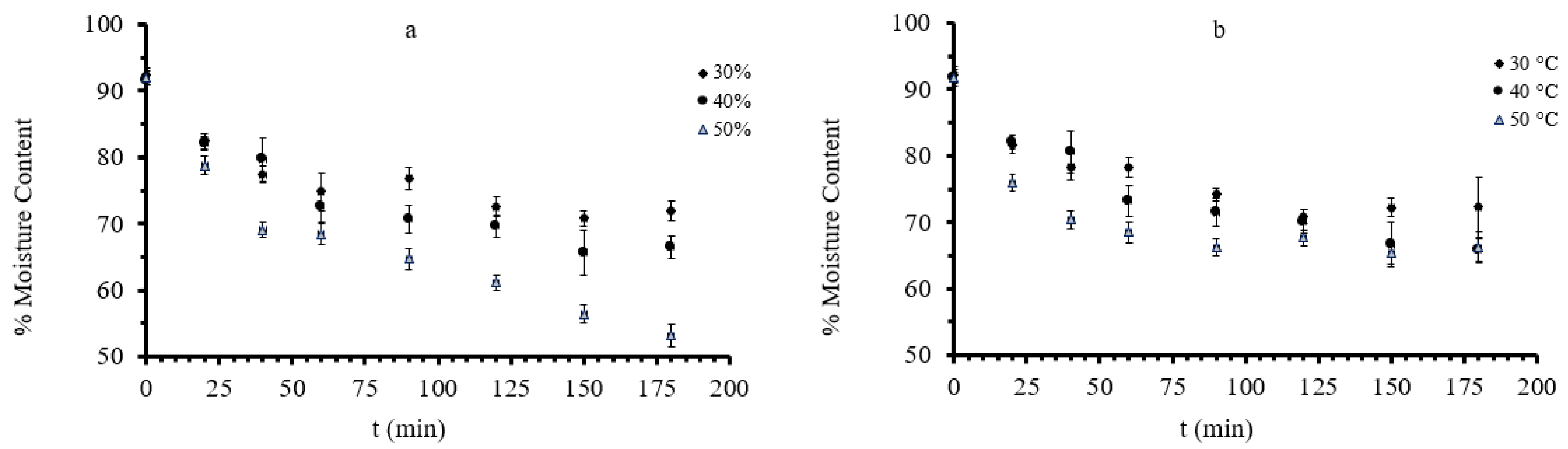
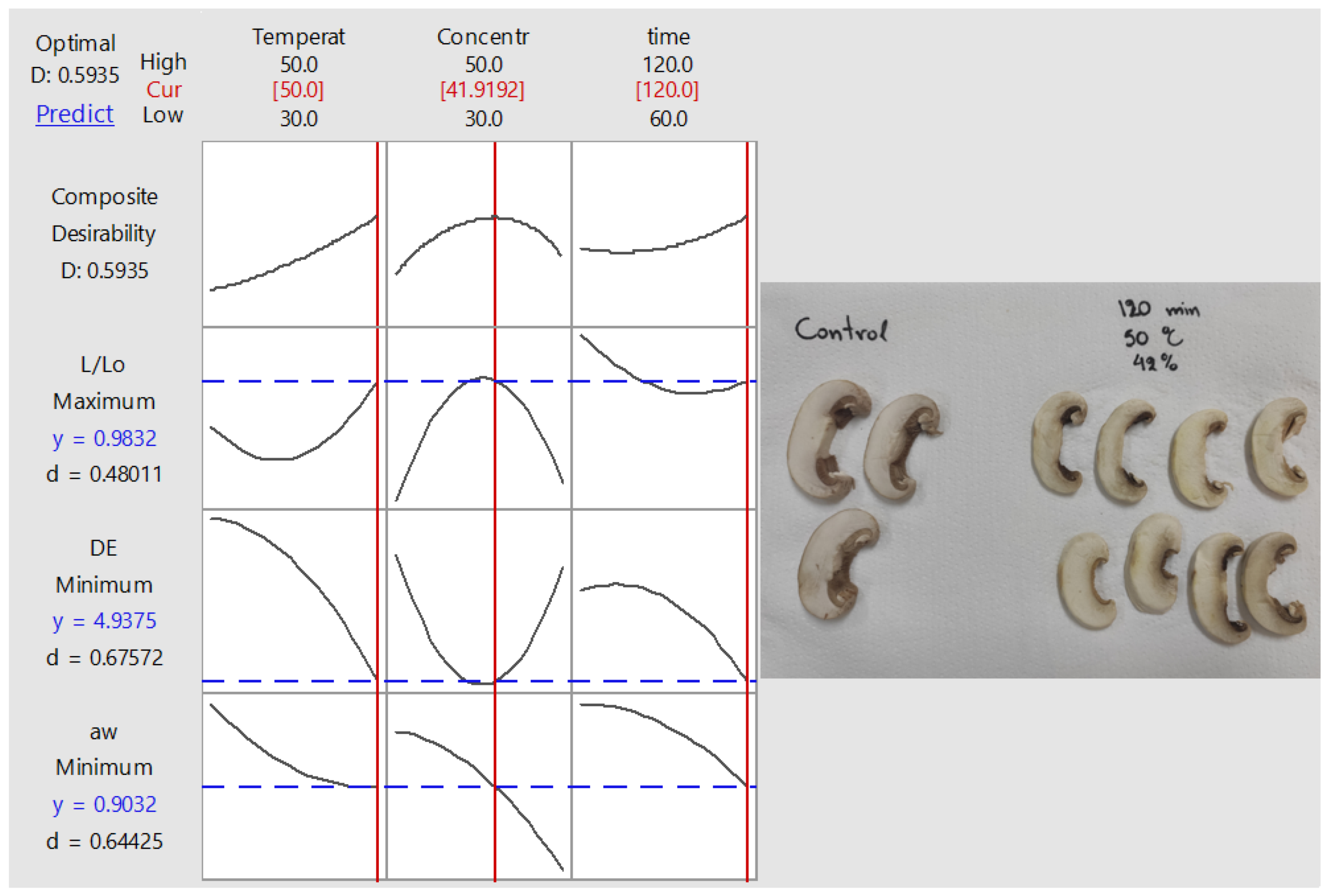
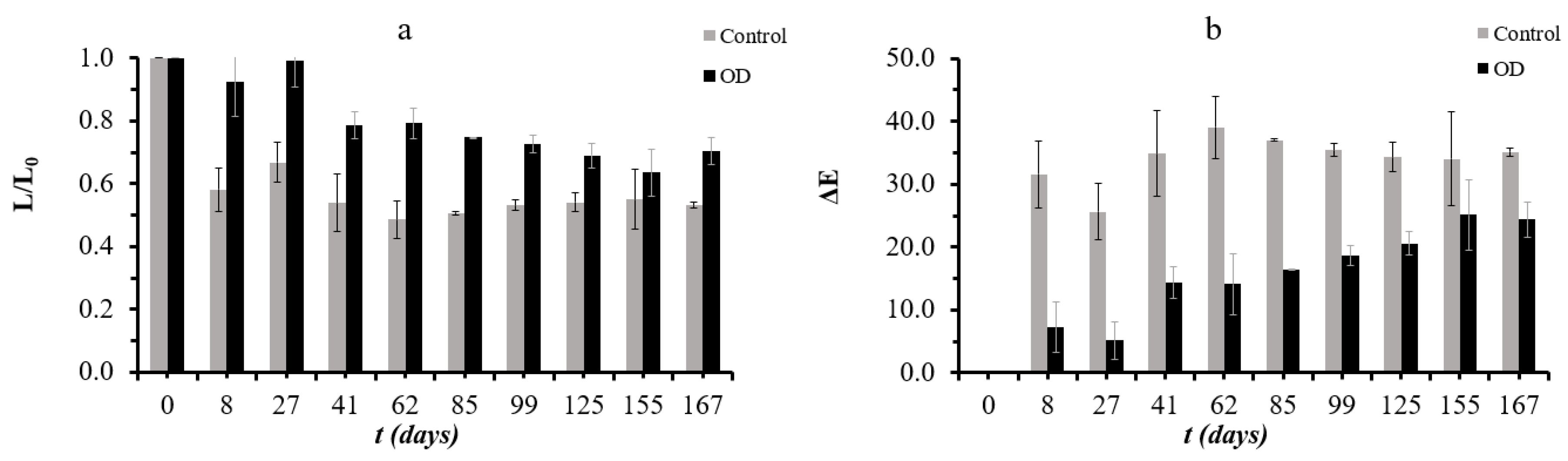
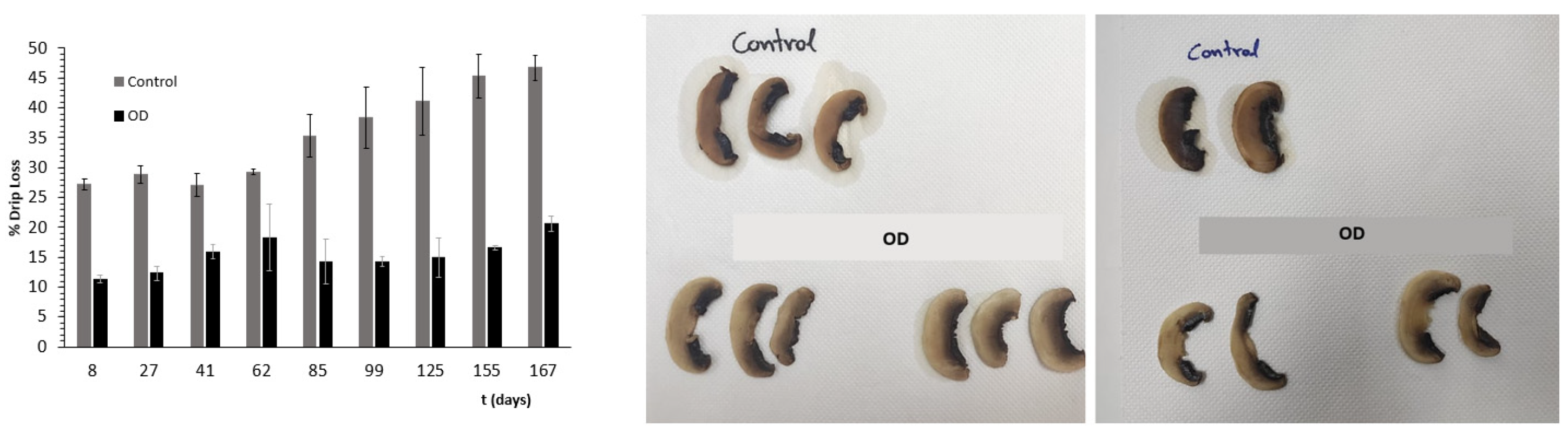
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | |
|---|---|---|---|---|---|---|
| %MC | −0.9734 | −0.1497 | 0.0700 | 0.0595 | −0.0792 | 0.1238 |
| aw | −0.9381 | −0.1480 | 0.2405 | 0.1547 | −0.0993 | −0.0804 |
| WL | 0.9211 | −0.1891 | 0.1162 | 0.2811 | 0.1500 | 0.0267 |
| SG | 0.9169 | 0.0533 | 0.3468 | −0.1753 | −0.0704 | 0.0214 |
| %NaCl | 0.9241 | −0.2127 | −0.1429 | 0.1086 | −0.2618 | 0.0005 |
| °Brix | 0.0377 | 0.9907 | 0.0193 | 0.1185 | −0.0506 | 0.0107 |
| Coefficient | % MC | aw | °Brix | WL | SG | L/Lo | ΔΕ | %NaCl |
|---|---|---|---|---|---|---|---|---|
| Constant a0 | 117.8113 * | 0.915714 * | −21.0144 * | 0.338394 * | −7.47012 * | 0.246284 * | 27.69284 * | −5.08150 |
| a1 | −1.4216 * | −0.007706 * | 0.9035 * | 0.474699 | 0.29925 * | 0.004986 * | 0.64629 | 0.35387 * |
| a2 | −0.1856 | 0.007221 * | 1.3535 * | −0.272870 * | 0.11342 | 0.039845 * | −2.69240 * | 0.02108 |
| a3 | 0.0578 | 0.000843 | −0.0591 | 0.023802 | −0.00463 | −0.005140 | 0.55270 | −0.02118 |
| a11 | 0.0148 | 0.000069 | −0.0075 | −0.003324 | −0.00257 * | −0.000127 | −0.00507 | −0.0038 * |
| a22 | 0.0029 | −0.000085 * | −0.0042 | 0.005273 | −0.00039 | −0.000441 * | 0.02897 | 0.00051 |
| a33 | −0.0004 | −0.000007 | −0.0001 | 0.000182 | −0.00003 | 0.000033 | −0.00182 | 0.00013 |
| a12 | −0.0047 | −0.000049 | −0.0100 * | −0.003453 | −0.00218 * | 0.000025 | 0.00736 | −0.00170 |
| a13 | 0.0019 | −0.00000 | 0.0011 | −0.000902 | 0.00007 | 0.000025 | −0.00620 | 0.00028 |
| a23 | −0.0033 | −0.000001 | 0.0006 | −0.000122 | 0.00023 | −0.000050 | 0.00052 | −0.00061 |
| R2 | 0.869 | 0.843 | 0.983 | 0.752 | 0.801 | 0.721 | 0.698 | 0.795 |
| Predicted Value | Experimental Value | % Error | |
|---|---|---|---|
| aw | 0.8981 | 0.9173 ± 0.01 | 2.09 |
| WL | 5.7817 | 5.6622 ± 0.15 | −2.11 |
| SG | 1.4393 | 1.2091 ± 0.19 | −19.04 |
| DE | 5.0417 | 6.0783 ± 2.42 | 16.94 |
| L/L0 | 0.9898 | 0.9202 ± 0.03 | −7.57 |
| NaCl | 3.1319 | 2.9220 ± 0.15 | −7.18 |
| °Brix | 35.4648 | 36.5 ± 0.87 | 2.84 |
| % MC | 66.7719 | 68.5142 ± 1.54 | 2.54 |
| f/f0 | 0.3792 | 0.4569 ± 0.15 | 17.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulou, N.A.; Pavlidis, V.-A.; Giannakourou, M.C. Optimization of Osmotic Dehydration of White Mushrooms by Response Surface Methodology for Shelf-Life Extension and Quality Improvement of Frozen End-Products. Foods 2022, 11, 2354. https://doi.org/10.3390/foods11152354
Stavropoulou NA, Pavlidis V-A, Giannakourou MC. Optimization of Osmotic Dehydration of White Mushrooms by Response Surface Methodology for Shelf-Life Extension and Quality Improvement of Frozen End-Products. Foods. 2022; 11(15):2354. https://doi.org/10.3390/foods11152354
Chicago/Turabian StyleStavropoulou, Natalia A., Vassilis-Aggelos Pavlidis, and Maria C. Giannakourou. 2022. "Optimization of Osmotic Dehydration of White Mushrooms by Response Surface Methodology for Shelf-Life Extension and Quality Improvement of Frozen End-Products" Foods 11, no. 15: 2354. https://doi.org/10.3390/foods11152354
APA StyleStavropoulou, N. A., Pavlidis, V.-A., & Giannakourou, M. C. (2022). Optimization of Osmotic Dehydration of White Mushrooms by Response Surface Methodology for Shelf-Life Extension and Quality Improvement of Frozen End-Products. Foods, 11(15), 2354. https://doi.org/10.3390/foods11152354






