Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Ultrasonic-Assisted Extraction (UAE)
2.4. Pressurized Liquid Extraction (PLE)
2.5. Extraction Yield, Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.6. In Vitro Assays
2.6.1. Anti-Cholinergic Activity
2.6.2. LOX Inhibitory Activity
2.6.3. ROS and RNS Scavenging Capacity
2.7. Blood–Brain Barrier (BBB)
2.8. HPLC-Q-TOF-MS/MS Analysis
3. Results and Discussion
3.1. In Vitro Neuroprotection of UAE Extracts
3.2. PLE Optimization
3.3. Extraction Yield, TPC and TFC Analyses of PLE Extracts
3.4. In Vitro Neuroprotection of PLE Extracts
3.4.1. Anti-Cholinergic Activity
3.4.2. Anti-Inflammatory Activity
3.4.3. Antioxidant Activity
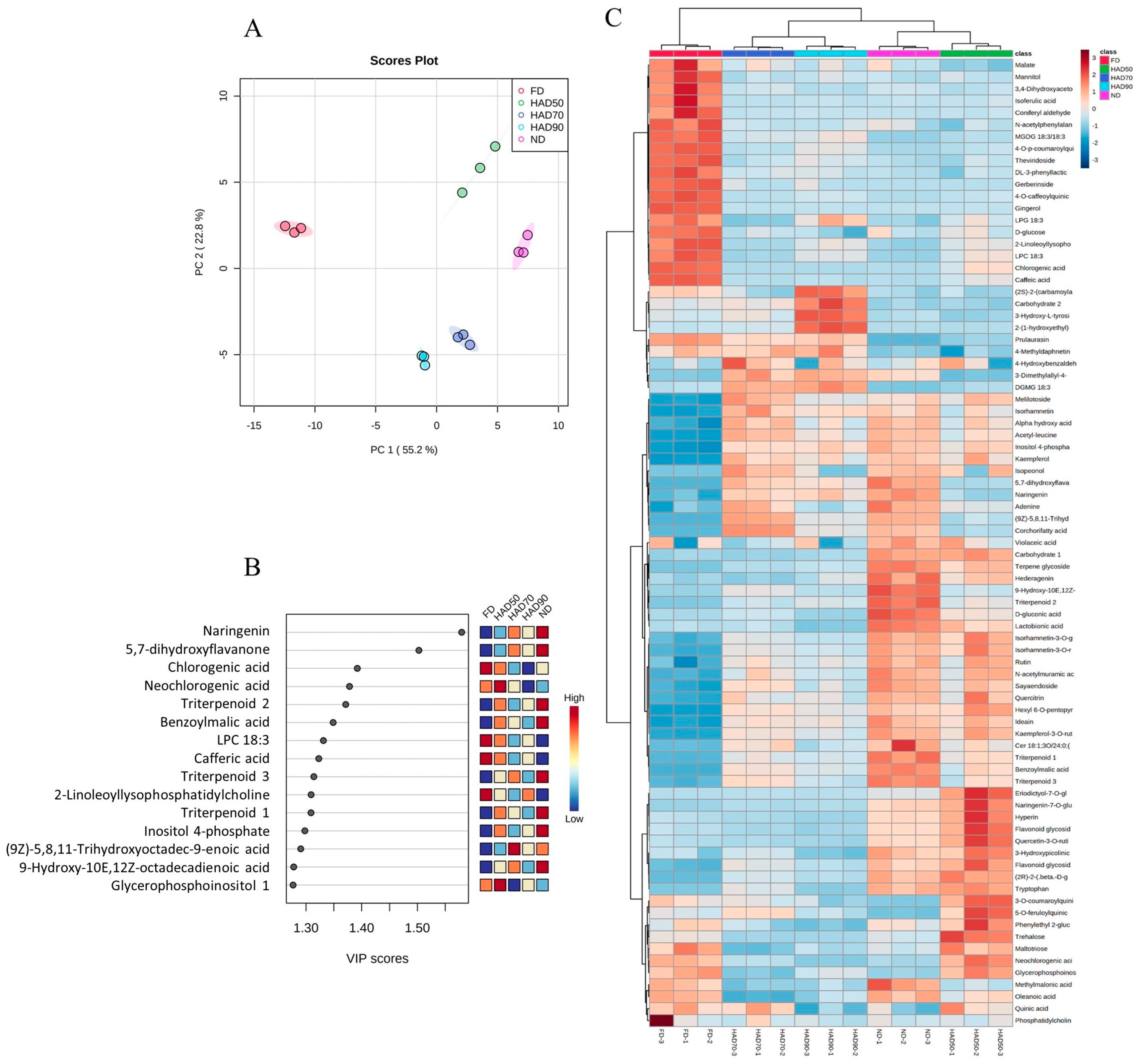
3.5. Characterization of Metabolites in Thinned Peach Extracts from PLE
3.6. Correlation between Metabolites and Neuroprotective Potential
3.7. BBB Permeability Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, S.; Rosa-Neto, P.; Morais, J.A.; Webster, C. World Alzheimer Report 2021: Journey through the Diagnosis of Dementia; Alzheimer’s Disease International: London, UK, 2021. [Google Scholar]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, J.B.; Strosznajder, R.P. The Lipoxygenases: Their Regulation and Implication in Alzheimer’s Disease. Neurochem. Res. 2015, 41, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Napolitano, M.; Tedesco, I.; Moccia, S.; Milito, A.; Luigi Russo, G. Neuroprotective Role of Natural Polyphenols. Curr. Top. Med. Chem. 2016, 16, 1943–1950. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Wu, X.; Xu, Y. Polyphenol metabolic diversity of Chinese peach and nectarine at thinned and ripe stages by UPLC-ESI-Q-TOF-MS combined with multivariate statistical analysis. J. Food Compos. Anal. 2020, 90, 103502. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Liu, X.; Wu, X.; Liu, J. Immunomodulation effects of polyphenols from thinned peach treated by different drying methods on RAW264.7 cells through the NF-κB and Nrf2 pathways. Food Chem. 2020, 340, 127931. [Google Scholar] [CrossRef]
- Guo, C.; Li, X.; Bi, J.; Lü, J.; Wu, X.; Lü, Y.; Xu, Y. Research achievements in bioactive components, functional properties and applications of thinned fruits. Food Sci. 2020, 41, 303–309. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Xu, Y.; Hu, J. Investigation on the phenolic composition, related oxidation and antioxidant activity of thinned peach dried by different methods. LWT-Food Sci. Technol. 2021, 147, 111573. [Google Scholar] [CrossRef]
- Tripodo, G.; Ibáñez, E.; Cifuentes, A.; Gilbert-López, B.; Fanali, C. Optimization of pressurized liquid extraction by response surface methodology of Goji berry (Lycium barbarum L.) phenolic bioactive compounds. Electrophoresis 2018, 39, 1673–1682. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Transport across the Blood-Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In vitro neuroprotective potential of terpenes from industrial orange juice by-products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef]
- Whent, M.; Ping, T.; Kenworthy, W.; Yu, L. High-Throughput Assay for Detection of Soybean Lipoxygenase-1. J. Agric. Food Chem. 2010, 58, 12602–12607. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-C.; Tang, Y.-L.; Lin, S.-M.; Liew, Y.-F. Evaluation of peroxynitrite-scavenging capacities of several commonly used fresh spices. Food Chem. 2010, 119, 1102–1107. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Kidoń, M.; Grabowska, J. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT-Food Sci. Technol. 2021, 136, 110302. [Google Scholar] [CrossRef]
- Suárez-Montenegro, Z.J.; Ballesteros-Vivas, D.; Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. Neuroprotective Potential of Tamarillo (Cyphomandra betacea) Epicarp Extracts Obtained by Sustainable Extraction Process. Front. Nutr. 2021, 8, 769617. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Alvarez-Rivera, G.; Gallego, R.; Bittencourt Fagundes, M.; Valdés, A.; Mendiola, J.A.; Ibánez, E.; Cifuentes, A. Neuroprotective potential of terpenoid-rich extracts from orange juice by-products obtained by pressurized liquid extraction. Food Chem. X 2021, 13, 100242. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic Compounds Prevent Alzheimer’s Pathology through Different Effects on the Amyloid-β Aggregation Pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef]
- Kamran, M.; Kousar, R.; Ullah, S.; Khan, S.; Umer, M.F.; Rashid, H.U.; Khan, Z.; Khattak, M.I.K.; Rehman, M.U. Taxonomic Distribution of Medicinal Plants for Alzheimer’s Disease: A Cue to Novel Drugs. Int. J. Alzheimer’s Dis. 2020, 2020, 7603015. [Google Scholar] [CrossRef]
- Fernandes, F.; Barroso, M.F.; De Simone, A.; Emriková, E.; Dias-Teixeira, M.; Pereira, J.P.; Chlebek, J.; Fernandes, V.C.; Rodrigues, F.; Andrisano, V.; et al. Multi-target neuroprotective effects of herbal medicines for Alzheimer’s disease. J. Ethnopharmacol. 2022, 290, 115107. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of Hot-Air and Freeze-Drying on the Quality Attributes of Dried Pomegranate (Punica granatum L.) Arils during Long-Term Cold Storage of Whole Fruit. Agriculture 2020, 10, 493. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Dek, M.S.; Hairuddin, M.R. Effect of Freeze-Drying on the Antioxidant Compounds and Antioxidant Activity of Selected Tropical Fruits. Int. J. Mol. Sci. 2011, 12, 4678. [Google Scholar] [CrossRef]
- Rózek, A.; García-Pérez, J.V.; López, F.; Güell, C.; Ferrando, M. Infusion of grape phenolics into fruits and vegetables by osmotic treatment: Phenolic stability during air drying. J. Food Eng. 2010, 99, 142–150. [Google Scholar] [CrossRef]
- Madrau, M.A.; Piscopo, A.; Sanguinetti, A.M.; Del Caro, A.; Poiana, M.; Romeo, F.V.; Piga, A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur. Food Res. Technol. 2008, 228, 441–448. [Google Scholar] [CrossRef]
- Katalinić, M.; Rusak, G.; Domaćinović Barović, J.; Sinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef]
- Błaszczak, W.; Latocha, P.; Jeż, M.; Wiczkowski, W. The impact of high-pressure processing on the polyphenol profile and anti-glycaemic, anti-hypertensive and anti-cholinergic activities of extracts obtained from kiwiberry (Actinidia arguta) fruits. Food Chem. 2021, 343, 128421. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase inhibition by plant extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.; Porto, G.; Cabrita, E.J.; Marques, M.M.; Fernandes, E. Inhibition of LOX by flavonoids: A structure-activity relationship study. Eur. J. Med. Chem. 2014, 72, 137–145. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Basilio Heredia, J. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of Convective and Vacuum–Microwave Drying on the Bioactive Compounds, Color, and Antioxidant Capacity of Sour Cherries. Food Bioprocess Technol. 2013, 7, 829–841. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. Formation of ester and amine derivatives of 5-O-caffeoylquinic acid in the process of its simulated extraction. J. Agric. Food Chem. 2012, 60, 12289–12295. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite Profiling of Hydroxycinnamate Derivatives in Plasma and Urine after the Ingestion of Coffee by Humans: Identification of Biomarkers of Coffee Consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Mandrioli, R.; Angeloni, C.; Freschi, M.; Malaguti, M.; Hrelia, S.; Lugli, S.; Gennari, F.; Muzzi, E.; et al. Fruit Quality Characterization of New Sweet Cherry Cultivars as a Good Source of Bioactive Phenolic Compounds with Antioxidant and Neuroprotective Potential. Antioxidants 2020, 9, 677. [Google Scholar] [CrossRef]
- Lee, K.-W.; Im, J.-Y.; Woo, J.-M.; Grosso, H.; Kim, Y.-S.; Cristovao, A.C.; Sonsalla, P.K.; Schuster, D.S.; Jalbut, M.M.; Fernandez, J.R.; et al. Neuroprotective and Anti-inflammatory Properties of a Coffee Component in the MPTP Model of Parkinson’s Disease. Neurotherapeutics 2013, 10, 143–153. [Google Scholar] [CrossRef]
- Wu, Y.; Shamoto-Nagai, M.; Maruyama, W.; Osawa, T.; Naoi, M. Phytochemicals prevent mitochondrial membrane permeabilization and protect SH-SY5Y cells against apoptosis induced by PK11195, a ligand for outer membrane translocator protein. J. Neural Transm. 2016, 124, 89–98. [Google Scholar] [CrossRef]
- Jiang, H.-W.; Lin, J.; Wang, G.-M.; Zhang, J.-J.; Gu, S.-S.; Cao, L.; Chen, Y.; Wang, L.; Jiao, H.; Zhu, W.-L.; et al. Acetophenone derivatives from the root bark of Cynanchum wilfordii as potential neuroprotective agents. Phytochem. Lett. 2018, 24, 179–183. [Google Scholar] [CrossRef]
- Tan, H.P.; Wong, D.Z.H.; Ling, S.K.; Chuah, C.H.; Kadir, H.A. Neuroprotective activity of galloylated cyanogenic glucosides and hydrolysable tannins isolated from leaves of Phyllagathis rotundifolia. Fitoterapia 2012, 83, 223–229. [Google Scholar] [CrossRef]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. NeuroReport 2001, 12, 3871–3875. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some Pro-oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Li, W.; Meng, G.; Wang, P.; Liao, W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 2016, 4, 219–229. [Google Scholar] [CrossRef]
- Zhu, J.; Yi, X.; Zhang, J.; Chen, S.; Wu, Y. Rapid screening of brain-penetrable antioxidants from natural products by blood-brain barrier specific permeability assay combined with DPPH recognition. J. Pharm. Biomed. Anal. 2018, 151, 42–48. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef]


| Samples | AChE | BChE | LOX |
|---|---|---|---|
| FD | 547.3 ± 40 a | 894.1 ± 43 a | 151.9 ± 19 a |
| ND | 391.1 ± 36 bc | 942.5 ± 99 a | 131.2 ± 15 ab |
| HAD50 | 425.6 ± 43 b | 844.6 ± 55 a | 104.2 ± 6 bc |
| HAD70 | 359.5 ± 19 c | 567.3 ± 7 b | 163.1 ± 12 a |
| HAD90 | 231.1 ± 20 d | 282.6 ± 20 c | 93.6 ± 10 c |
| No. (Unit) | Temp. (°C) | Solvent Composition | Yield (%) | TPC (mg GAE/g) | AChE (IC50 µg/mL) | LOX (IC50 µg/mL) | ROS (IC50 µg/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 115 | Water | 38.5 | 22.9 ± 2 | 1634.8 ± 120 | 237.4 ± 15 | 12.4 ± 0.9 |
| 2 | 50 | Water | 31.4 | 13.8 ± 0.6 | 1868.7 ± 129 | 288.3 ± 33 | 16.7 ± 0.1 |
| 3 | 115 | 50% ethanol | 46.9 | 37.4 ± 3 | 1255.4 ± 75 | 385.3 ± 29 | 9.2 ± 0.4 |
| 4 | 115 | Ethanol | 48.5 | 37.6 ± 2 | 1259.1 ± 93 | 546.5 ± 49 | 9.2 ± 0.5 |
| 5 | 115 | 50% ethanol | 43.7 | 36.1 ± 1 | 1174.0 ± 114 | 393.0 ± 31 | 9.6 ± 0.9 |
| 6 | 50 | Ethanol | 34.2 | 29.1 ± 0.5 | 1377.9 ± 130 | 717.3 ± 68 | 12.1 ± 0.8 |
| 7 | 50 | 50% ethanol | 38.4 | 26.0 ± 2 | 1340.0 ± 58 | 476.5 ± 34 | 11.7 ± 1 |
| 8 * | 180 | 50% ethanol | 79.3 | 100.1 ± 5 | 229.5 ± 22 | 65.4 ± 5 | 3.9 ± 0.2 |
| 9 | 115 | 50% ethanol | 42.0 | 38.3 ± 0.9 | 1205.5 ± 120 | 377.4 ± 41 | 9.3 ± 0.9 |
| 10 | 180 | Ethanol | 60.1 | 81.4 ± 3 | 347.3 ± 35 | 83.4 ± 6 | 5.7 ± 0.7 |
| 11 | 180 | Water | 77.0 | 82.3 ± 3 | 384.8 ± 32 | 50.0 ± 5 | 3.6 ± 0.4 |
| 12 | 115 | 50% ethanol | 41.2 | 40.7 ± 0.5 | 1196.5 ± 113 | 325.1 ± 16 | 9.5 ± 0.4 |
| 13 # | 200 | 50% ethanol | 80.0 | 91.3 ± 2 | 285.5 ± 12 | 72.9 ± 6 | 4.2 ± 0.3 |
| Samples | Yield (%) | TPC (mg GAE/g) | TFC (mg QE/g) | AChE (IC50 µg/mL) | BChE (IC50 µg/mL) | LOX (IC50 µg/mL) | ROS (IC50 µg/mL) | RNS (%) |
|---|---|---|---|---|---|---|---|---|
| FD | 77.6 ± 0.3 b | 100.7 ± 2 a | 15.1 ± 0.5 a | 238.4 ± 8 e | 273.6 ± 4 e | 63.0 ± 3 e | 3.9 ± 0.2 e | 42.3 ± 1 b |
| ND | 80.3 ± 2 ab | 58.5 ± 1 d | 5.2 ± 0.1 d | 447.3 ± 5 a | 553.3 ± 7 a | 96.0 ± 4 c | 7.5 ± 0.3 a | 15.2 ± 0.2 e |
| HAD50 | 78.8 ± 2 ab | 63.2 ± 2 cd | 5.6 ± 0.2 cd | 426.9 ± 4 b | 482.6 ± 2 b | 221.4 ± 4 a | 6.2 ± 0.1 b | 21.5 ± 0.9 d |
| HAD70 | 80.5 ± 1 ab | 65.1 ± 1 c | 6.4 ± 0.3 c | 377.4 ± 7 c | 424.5 ± 5 c | 186.8 ± 2 b | 5.4 ± 0.2 c | 26.6 ± 2 c |
| HAD90 | 82.4 ± 0.2 a | 91.3 ± 3 b | 11.2 ± 0.4 b | 260.3 ± 6 d | 318.9 ± 5 d | 81.3 ± 7 d | 4.8 ± 0.3 d | 38.9 ± 3 b |
| Galantamine | 1.2 ± 0.1 f | 16.1 ± 1 f | ||||||
| Quercetin | 17.2 ± 2 f | |||||||
| Ascorbic acid | 3.3 ± 0.6 e | 83.8 ± 4 a |
| TPC | TFC | ACHE | BCHE | LOX | ROS | RNS | |
|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.99 ** | −0.98 ** | −0.95 ** | −0.67 ** | −0.89 ** | 0.95 ** |
| TFC | 0.99 ** | 1 | −0.96 ** | −0.93 ** | −0.70 ** | −0.88 ** | 0.93 ** |
| Retention Time (min) | Metabolite Name | Adduct Type or Fragment | m/z | Formula | Chemical Subclass |
|---|---|---|---|---|---|
| 0.811 | 3-O-coumaroylquinic acid | [M-H]− | 337.0791 | C16H18O8 | Alcohols and polyols |
| 1.780 | 4-O-caffeoylquinic acid | [M-H-H2O]−/ [M-H-C7H10O5]− | 335.0780/ 161.0244 | C16H18O9 | Alcohols and polyols |
| 2.258 | 4-O-p-coumaroylquinic acid | [M-H-H2O]− | 319.0828 | C16H18O7 | Alcohols and polyols |
| 1.072 | 5-O-feruloylquinic acid | [M-H]− | 367.1035 | C17H20O9 | Alcohols and polyols |
| 1.018 | Chlorogenic acid | [M-H]−/ [M-H-C9H6O3]−/ [2M-H]− | 353.0888/ 191.0564/ 707.1846 | C16H18O9 | Alcohols and polyols |
| 0.238 | Inositol 4-phosphate | [M-H]− | 259.0246 | C6H13O9P | Alcohols and polyols |
| 0.572 | Neochlorogenic acid | [2M-H]−/ [M-H-C7H10O5]−/ [M-H-C8H10O7]−/ [M-H]− | 707.1853/ 179.0353/ 135.0446/ 353.0890 | C16H18O9 | Alcohols and polyols |
| 0.259 | Quinic acid | [M-H]−/ [2M-H]− | 191.0590/ 383.1260 | C7H12O6 | Alcohols and polyols |
| 1.214 | Alpha hydroxy acid 1 ((4E)-8-hydroxy-4-(1-hydroxypropan-2-ylidene)-10-oxatricyclo[7.2.1.0]dodecane-8-carboxylic acid) | [M-H]− | 281.1395 | C15H22O5 | Alpha hydroxy acids and derivatives |
| 0.487 | (2S)-2-(carbamoylamino)-4-(methylsulfanyl)butanoic acid | [2M-H]− | 383.1107 | C6H12N2O3S | Amino acids, peptides, and analogues |
| 0.415 | 3-Hydroxy-L-tyrosine | [M-H]− | 196.0623 | C9H11NO4 | Amino acids, peptides, and analogues |
| 0.996 | Acetyl-leucine | [M-H]− | 172.0980 | C8H15NO3 | Amino acids, peptides, and analogues |
| 1.575 | N-acetylphenylalanine | [M-H]− | 206.0825 | C11H13NO3 | Amino acids, peptides, and analogues |
| 4.260 | 3-Dimethylallyl-4-hydroxybenzoic acid | [M-H]− | 205.0867 | C12H14O3 | Benzoic acids and derivatives |
| 1.851 | Benzoylmalic acid | [M-H-C4H4O4]−/ [M-H]− | 121.0293/ 237.0405 | C11H10O6 | Benzoic acids and derivatives |
| 0.279 | Malate | [M-H]− | 133.0157 | C4H6O5 | Beta hydroxy acids and derivatives |
| 0.631 | Carbohydrate 1 ((2R,3S,4S,5R,6R)-5-[(2S,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxy-2-(hydroxymethyl)-6-[2-(4-hydroxyphenyl)ethoxy]oxane-3,4-diol) | [M+FA-H]− | 477.1605 | C19H28O11 | Carbohydrates and carbohydrate conjugates |
| 0.328 | Carbohydrate 2 (3-[(2S,3R,4S,5S,6R)-6-[[(2R,3R,4R)-3,4-dihydroxy-4-(hydroxymethyl)oxolan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl]oxy-2-methylpyran-4-one) | [M+FA-H]− | 465.1282 | C17H24O12 | Carbohydrates and carbohydrate conjugates |
| 0.252 | D-gluconic acid | [M-H]− | 195.0531 | C6H12O7 | Carbohydrates and carbohydrate conjugates |
| 0.262 | D-glucose | [M-H]− | 179.0583 | C6H12O6 | Carbohydrates and carbohydrate conjugates |
| 0.240 | Maltotriose | [M+FA-H]−/ [M+Cl]− | 549.1705/ 539.1381 | C18H32O16 | Carbohydrates and carbohydrate conjugates |
| 0.243 | Mannitol | [M+Cl]−/ [M-H]−/ [M+FA-H]− | 217.0499/ 181.0737/ 227.0794 | C6H14O6 | Carbohydrates and carbohydrate conjugates |
| 1.146 | Melilotoside | [M+FA-H]− | 371.0993 | C15H18O8 | Carbohydrates and carbohydrate conjugates |
| 0.311 | N-acetylmuramic acid | [M-H]− | 292.1045 | C11H19NO8 | Carbohydrates and carbohydrate conjugates |
| 1.308 | Phenylethyl 2-glucoside | [M+FA-H]− | 329.1236 | C14H20O6 | Carbohydrates and carbohydrate conjugates |
| 1.137 | Prulaurasin | [M+FA-H]−/ [M+Cl]− | 340.1050/ 330.0758 | C14H17NO6 | Carbohydrates and carbohydrate conjugates |
| 1.421 | Sayaendoside | [M+FA-H]−/ [M-H]− | 461.1720/ 415.1613 | C19H28O10 | Carbohydrates and carbohydrate conjugates |
| 0.239 | Trehalose | [M+Cl]−/ [M+FA-H]−/ [M-H]− | 377.0876/ 387.1167/ 341.1112 | C12H22O11 | Carbohydrates and carbohydrate conjugates |
| 1.012 | 3,4-Dihydroxyacetophenone | [M-H]− | 151.0400 | C8H8O3 | Carbonyl compounds |
| 1.220 | 4-Hydroxybenzaldehyde | [M-H]− | 121.0288 | C7H6O2 | Carbonyl compounds |
| 2.175 | Isopeonol | [M-H]− | 165.0414 | C9H10O3 | Carbonyl compounds |
| 7.074 | Cer 18:1;3O/24:0;(2OH) | [M-H]− | 680.6199 | C42H83NO5 | Ceramides |
| 1.401 | Gerberinside | [M-H]− | 337.0793 | C16H18O8 | Coumarin glycosides |
| 0.342 | Methylmalonic acid | [M-H]− | 117.0210 | C4H6O4 | Dicarboxylic acids and derivatives |
| 0.802 | Violaceic acid | [M-H-C8H6O3]− | 137.0099 | C15H12O6 | Diphenylethers |
| 4.629 | (9Z)-5,8,11-Trihydroxyoctadec-9-enoic acid | [M-H]− | 329.2348 | C18H34O5 | Fatty acids and conjugates |
| 0.401 | (2R)-2-(.βeta.-D-glucopyranosyloxy)-2-phenylacetamide | [M+CHO2]− | 358.1162 | C14H19NO7 | Fatty acyl glycosides |
| 1.900 | Hexyl 6-O-pentopyranosylhexopyranoside | [M+CHO2]− | 441.1979 | C17H32O10 | Fatty acyl glycosides |
| 0.247 | Lactobionic acid | [M-H]− | 357.1067 | C12H22O12 | Fatty acyl glycosides |
| 5.512 | 5,7-dihydroxyflavanone | [M-H]− | 255.0665 | C15H12O4 | Flavans |
| 4.379 | Naringenin | [M-H]− | 271.0619 | C15H12O5 | Flavans |
| 4.838 | Isorhamnetin | [M-H]− | 315.0510 | C16H12O7 | Flavones |
| 4.659 | Kaempferol | [M-H]− | 285.0413 | C15H10O6 | Flavones |
| 4.016 | Flavonoid glycoside 1 ([6-[2-(3,4-dihydroxyphenyl)-8-hydroxy-4-oxochromen-7-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methyl (E)-3-(4-hydroxyphenyl)prop-2-enoate) | [M-H]− | 593.1296 | C30H26O13 | Flavonoid glycosides |
| 1.826 | Flavonoid glycoside 2 (2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl 4-O-hexopyranosylhexopyranoside) | [M-H]− | 625.1416 | C27H30O17 | Flavonoid glycosides |
| 2.334 | Eriodictyol-7-O-glucoside | [M-H]− | 449.1093 | C21H22O11 | Flavonoid glycosides |
| 2.371 | Hyperin | [M-H]− | 463.0891 | C21H20O12 | Flavonoid glycosides |
| 2.723 | Ideain | [M-2H]− | 447.0947 | C21H21ClO11 | Flavonoid glycosides |
| 2.862 | Isorhamnetin-3-O-glucoside | [M-H]− | 477.1045 | C22H22O12 | Flavonoid glycosides |
| 2.768 | Isorhamnetin-3-O-rutinoside | [M-H]− | 623.1625 | C28H32O16 | Flavonoid glycosides |
| 2.632 | Kaempferol-3-O-rutinoside | [M-H]− | 593.1521 | C27H30O15 | Flavonoid glycosides |
| 2.786 | Naringenin-7-O-glucoside | [M-H-C6H10O5]−/ [M-H]−/ [M+Cl]− | 271.0606/ 433.1142/ 469.0901 | C21H22O10 | Flavonoid glycosides |
| 2.302 | Quercetin-3-O-rutinoside | [M-H]− | 609.1469 | C27H30O16 | Flavonoid glycosides |
| 2.575 | Quercitrin | [M-H]− | 447.0937 | C21H20O11 | Flavonoid glycosides |
| 2.107 | Rutin | [M-H]− | 609.1474 | C27H30O16 | Flavonoid glycosides |
| 0.480 | 2-(1-hydroxyethyl)-4-(2-hydroxypropyl)-2H-furan-5-one | [M+Cl]− | 221.0566 | C9H14O4 | Furanones |
| 6.049 | 2-Linoleoyllysophosphatidylcholine | [M+FA-H]− | 564.3302 | C26H50NO7P | Glycerophosphocholines |
| 5.948 | LPC 18:3 | [M+FA-H]− | 562.3145 | C26H48NO7P | Glycerophosphocholines |
| 6.802 | Phosphatidylcholine(16:0/18:2w6) | [M+CHO2]− | 802.5610 | C42H80NO8P | Glycerophosphocholines |
| 5.989 | LPG 18:3 | [M-H]− | 505.2558 | C24H43O9P | Glycerophosphoglycerols |
| 5.643 | Glycerophosphoinositol 1 (D-myo-Inositol, 1-[2-hydroxy-3-[(1-oxo-9,12-octadecadienyl)oxy]propyl hydrogen phosphate], [S-(Z,Z)]-) | [M-H]− | 595.2888 | C27H49O12P | Glycerophosphoinositols |
| 5.783 | DGMG 18:3 | [M+FA-H]− | 721.3654 | C33H56O14 | Glycosylglycerols |
| 6.669 | MGDG 18:3/18:3 | [M+HCOO]− | 819.5268 | C45H74O10 | Glycosylglycerols |
| 0.981 | Caffeic acid | [M-H]− | 179.0349 | C9H8O4 | Hydroxycinnamic acids and derivatives |
| 1.857 | Isoferulic acid | [M-H]− | 193.0504 | C10H10O4 | Hydroxycinnamic acids and derivatives |
| 1.724 | 4-Methyldaphnetin | [M-H]− | 191.0340 | C10H8O4 | Hydroxycoumarins |
| 1.105 | Tryptophan | [M-H]− | 203.0827 | C11H12N2O2 | Indolyl carboxylic acids and derivatives |
| 5.976 | 9-Hydroxy-10E,12Z-octadecadienoic acid | [M-H]− | 295.2278 | C18H32O3 | Lineolic acids and derivatives |
| 4.228 | Corchorifatty acid F | [M-H]−/ [M+Cl]− | 327.2181/ 363.1942 | C18H32O5 | Lineolic acids and derivatives |
| 1.576 | Coniferyl aldehyde | [M-H]− | 177.0551 | C10H10O3 | Methoxyphenols |
| 5.460 | Gingerol | [M-H]− | 293.1810 | C17H26O4 | Methoxyphenols |
| 1.377 | DL-3-phenyllactic acid | [M-H]− | 165.0555 | C9H10O3 | Phenylpropanoic acids |
| 0.334 | Adenine | [M-H]− | 134.0488 | C5H5N5 | Purines and purine derivatives |
| 0.390 | 3-Hydroxypicolinic acid | [M-H]− | 138.0202 | C6H5NO3 | Pyridinecarboxylic acids and derivatives |
| 1.443 | Terpene glycoside 1 ((2R,3R,4S,5S,6R)-2-[6-hydroxy-3-[(E)-3-hydroxybut-1-enyl]-2,4,4-trimethylcyclohexyl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol) | [M+Cl]− | 425.2031 | C19H34O8 | Terpene glycosides |
| 0.453 | Theviridoside | [M+FA-H]− | 449.1303 | C17H24O11 | Terpene glycosides |
| 5.685 | Triterpenoid 1 ((1R,2R,4aS,6aS,6bR,10S,12aR,14bS)-1,8,10-trihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid) | [M-H]− | 487.3437 | C30H48O5 | Triterpenoids |
| 5.803 | Triterpenoid 2 ((1S,4aR,6aS,6bR,10R,11R,12aR,14bS)-1,10,11-trihydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid) | [M-H]− | 487.3432 | C30H48O5 | Triterpenoids |
| 5.552 | Triterpenoid 3 ((1S,4aR,6aS,6bR,9S,10R,11R,12aR,14bS)-1,10,11-trihydroxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid) | [M-H]− | 503.3377 | C30H48O6 | Triterpenoids |
| 5.980 | Hederagenin | [M-H]− | 471.3482 | C30H48O4 | Triterpenoids |
| 6.225 | Oleanoic acid | [M-H]− | 455.3534 | C30H48O3 | Triterpenoids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Valdés, A.; Sánchez-Martínez, J.D.; Ibáñez, E.; Bi, J.; Cifuentes, A. Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes. Foods 2022, 11, 2464. https://doi.org/10.3390/foods11162464
Guo C, Valdés A, Sánchez-Martínez JD, Ibáñez E, Bi J, Cifuentes A. Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes. Foods. 2022; 11(16):2464. https://doi.org/10.3390/foods11162464
Chicago/Turabian StyleGuo, Chongting, Alberto Valdés, José David Sánchez-Martínez, Elena Ibáñez, Jinfeng Bi, and Alejandro Cifuentes. 2022. "Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes" Foods 11, no. 16: 2464. https://doi.org/10.3390/foods11162464
APA StyleGuo, C., Valdés, A., Sánchez-Martínez, J. D., Ibáñez, E., Bi, J., & Cifuentes, A. (2022). Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes. Foods, 11(16), 2464. https://doi.org/10.3390/foods11162464








