Incorporation of Mycelium (Pleurotus eryngii) in Pea Protein Based Low Moisture Meat Analogue: Effect on Its Physicochemical, Rehydration and Structural Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
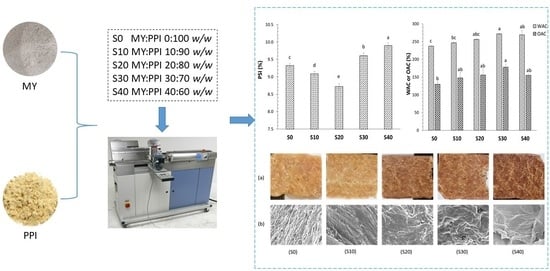
2.2. Preparation of Meat Analogue
2.3. Physicochemical Properties
2.3.1. Color Characteristics
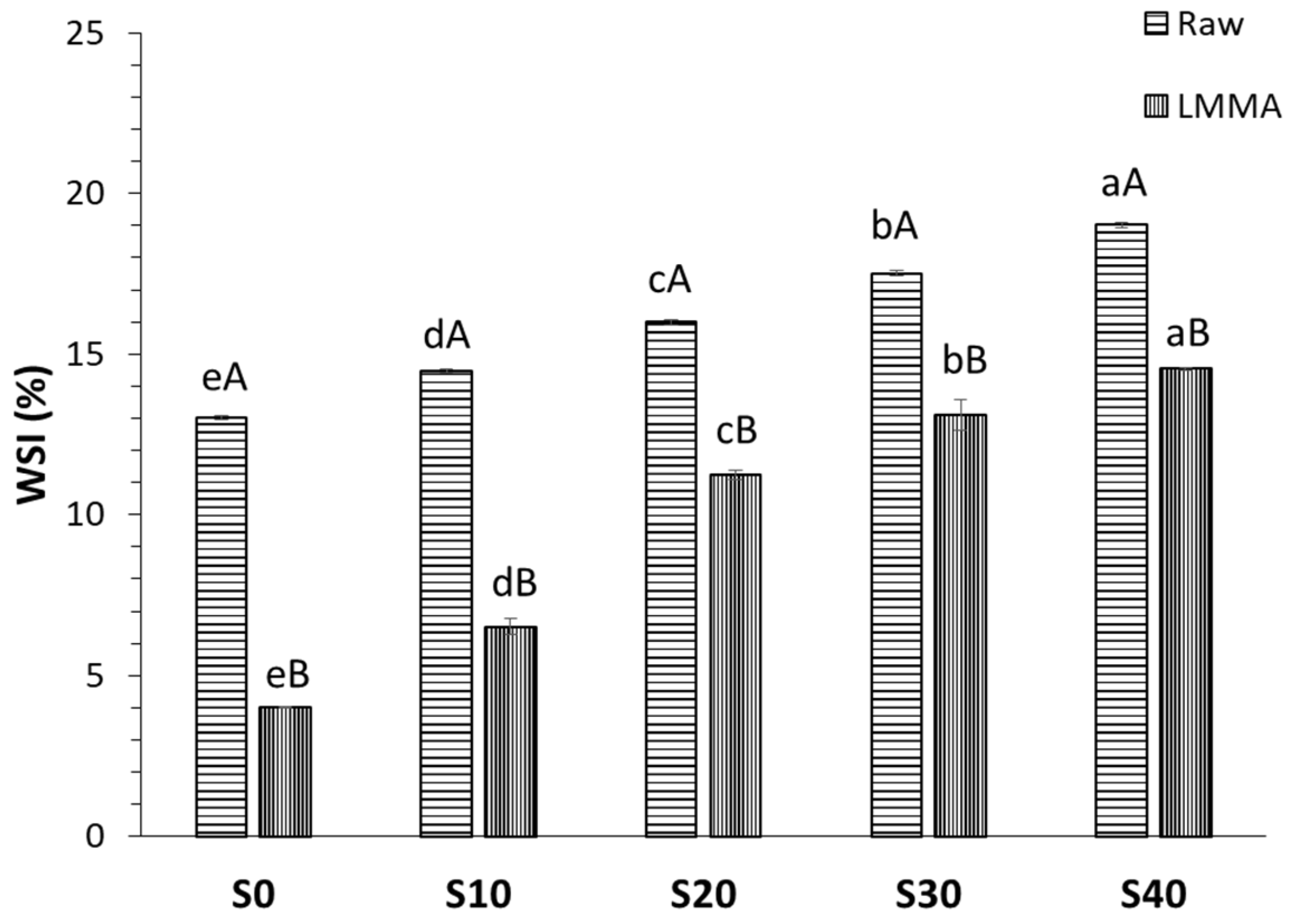
2.3.2. Water Solubility Index (WSI)
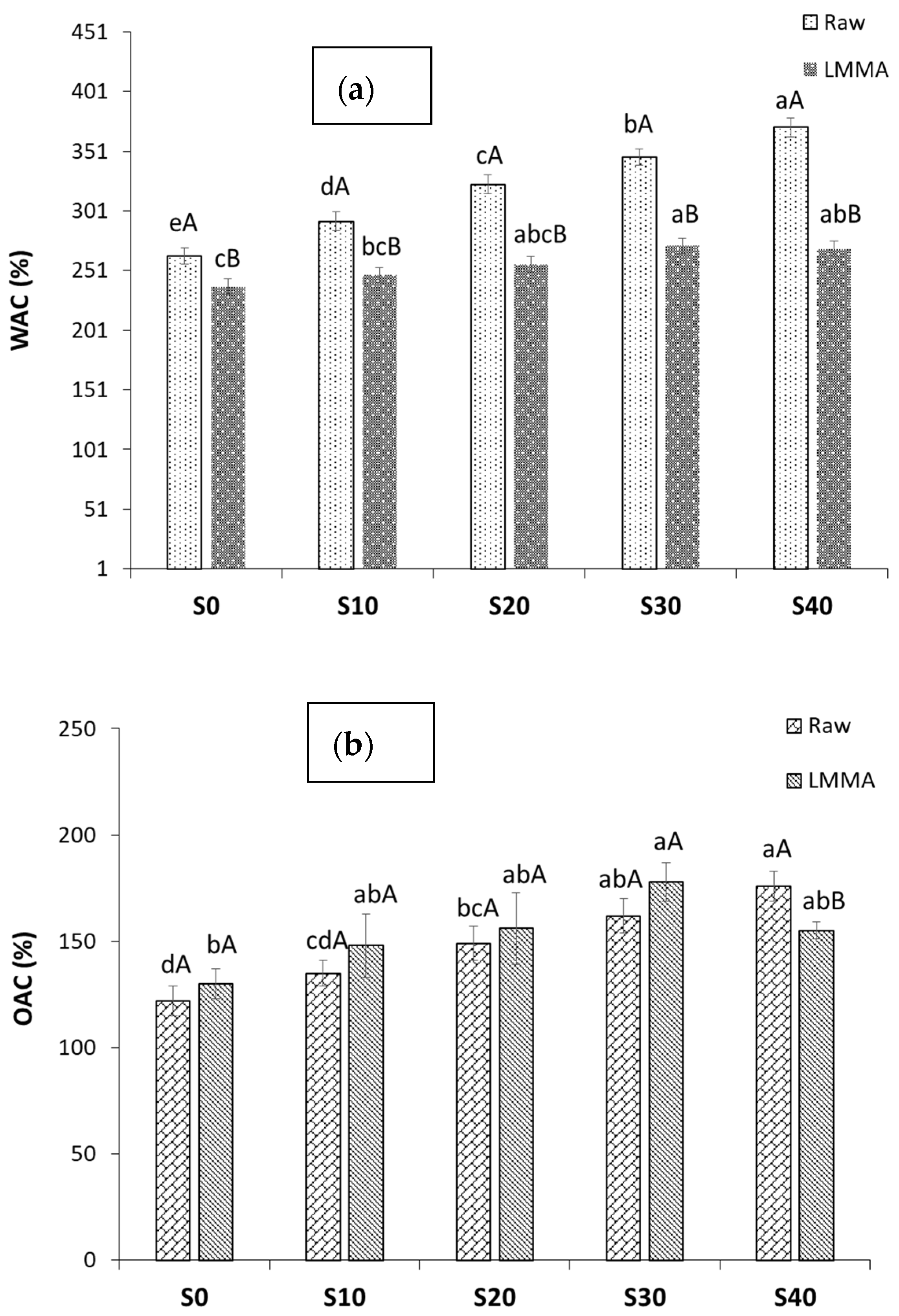
2.3.3. Water Absorption Capacity (WAC) and Oil Absorption Capacity (OAC)
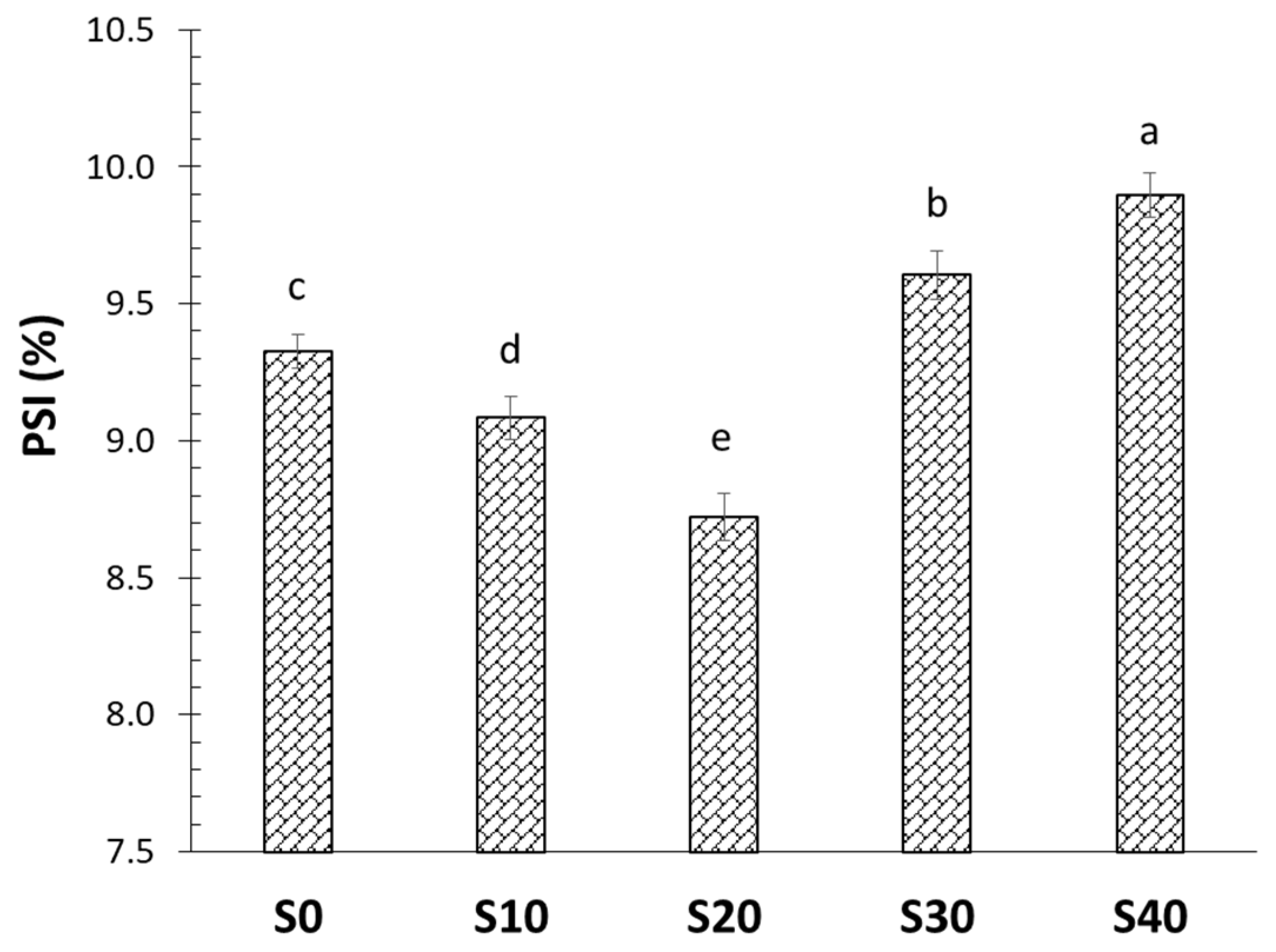
2.4. Protein Solubility Index
2.5. Rehydration Properties
2.5.1. Water Holding Capacity
2.5.2. Volumetric Expansion Ratio
2.6. Microstructure
2.7. Secondary Structure of LMMA
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Raw Blends and Extruded LMMA
3.1.1. Color Characteristics
3.1.2. Water Solubility Index
3.1.3. Water Absorption Capacity and Oil Absorption Capacity
3.2. Protein Solubility Index
3.3. Rehydration Properties
3.3.1. Water Holding Capacity
3.3.2. Volumetric Expansion Ratio
3.4. Effect of Mycelium on Extrusion Process Parameters
3.5. Microstructure
3.6. Secondary Structure of Proteins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sha, L.; Xiong, Y.L. Plant Protein-Based Alternatives of Reconstructed Meat: Science, Technology, and Challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.; Ryu, G. Effects of Extrusion Types, Screw Speed and Addition of Wheat Gluten on Physicochemical Characteristics and Cooking Stability of Meat Analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Kumar, S. Meat Analogues: Plant Based Alternatives to Meat Products—A Review. Int. J. Food Ferment. Technol. 2015, 5, 107–119. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring Processes for Meat Analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; He, S.; Li, X.; Jin, R.; Liu, Q.; Chen, S.; Sun, H. High-Moisture Extrusion Technology Application in the Processing of Textured Plant Protein Meat Analogues: A Review. Food Rev. Int. 2022, 1–36. [Google Scholar] [CrossRef]
- De Angelis, D.; Kaleda, A.; Pasqualone, A.; Vaikma, H.; Tamm, M.; Tammik, M.-L.; Squeo, G.; Summo, C. Physicochemical and Sensorial Evaluation of Meat Analogues Produced from Dry-Fractionated Pea and Oat Proteins. Foods 2020, 9, 1754. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G. A Comparison of Physicochemical Characteristics, Texture, and Structure of Meat Analogue and Meats. J. Sci. Food Agric. 2019, 99, 2708–2715. [Google Scholar] [CrossRef]
- Shi, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Lactic acid fermentation: A novel approach to eliminate unpleasant aroma in pea protein isolates. LWT 2021, 150, 111927. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Kaleda, A.; Talvistu, K.; Vaikma, H.; Tammik, M.L.; Rosenvald, S.; Vilu, R. Physicochemical, Textural, and Sensorial Properties of Fibrous Meat Analogs from Oat-Pea Protein Blends Extruded at Different Moistures, Temperatures, and Screw Speeds. Future Foods 2021, 4, 100092. [Google Scholar] [CrossRef]
- Kim, K.; Choi, B.; Lee, I.; Lee, H.; Kwon, S.; Oh, K.; Kim, A.Y. Bioproduction of Mushroom Mycelium of Agaricus Bisporus by Commercial Submerged Fermentation for the Production of Meat Analogue. J. Sci. Food Agric. 2011, 91, 1561–1568. [Google Scholar] [CrossRef]
- Singh, U.; Gautam, A.; Singha, T.K.; Tiwari, A.; Tiwari, P.; Sahai, V.; Sharma, S. Mass Production of Pleurotus eryngii Mycelia under Submerged Culture Conditions with Improved Minerals and Vitamin D2. LWT 2020, 131, 109665. [Google Scholar] [CrossRef]
- Yang, R.L.; Li, Q.; Hu, Q.P. Physicochemical Properties, Microstructures, Nutritional Components, and Free Amino Acids of Pleurotus eryngii as Affected by Different Drying Methods. Sci. Rep. 2020, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.; Panigrahi, C.; Barua, S.; Sahoo, M.; Mandliya, S. Food Nutrients as Inherent Sources of Immunomodulation during COVID-19 Pandemic. LWT 2022, 158, 113154. [Google Scholar] [CrossRef] [PubMed]
- Trinci, A.P.J. Evolution of the Quorn(R) Myco-Protein Fungus, Fusarium Graminearum A3/5. Microbiology 1994, 140, 2181–2188. [Google Scholar] [CrossRef]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruit and Vegetable Products; Tata McGraw-Hill: New York, NY, USA, 1986; ISBN 9788490225370. [Google Scholar]
- Dalbhagat, C.G.; Mishra, H.N. Effects of Extrusion Process Conditions on System Parameters; Physicochemical Properties and Cooking Characteristics of Extruded Fortified Rice Kernels. J. Cereal Sci. 2019, 89, 102782. [Google Scholar] [CrossRef]
- Dalbhagat, C.G.; Mishra, H.N. Effect of the Drying Process on the Color Change, Fissure Development, and Morphology of Fortified Rice Kernels. J. Food Process Eng. 2021, 44, e13719. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G.H. Physicochemical and Functional Characteristics of Plant Protein-Based Meat Analogs. J. Food Process. Preserv. 2019, 43, e14123. [Google Scholar] [CrossRef]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; van Buren, J.P.; Kilara, A.; Lewis, B.A.; Mangino, M.E. A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Berger, R.G.; Hertel, C. Physico-Chemical and Nutritional Properties of Meat Analogues Based on Spirulina/Lupin Protein Mixtures. Eur. Food Res. Technol. 2019, 245, 1889–1898. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Arcot, J. Effect of Low Moisture Extrusion on a Pea Protein Isolate’s Expansion, Solubility, Molecular Weight Distribution and Secondary Structure as Determined by Fourier Transform Infrared Spectroscopy (FTIR). J. Food Eng. 2017, 214, 166–174. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Schlangen, M.; Kyriakopoulou, K.; Boom, R.M.; van der Goot, A.J. Texture Methods for Evaluating Meat and Meat Analogue Structures: A Review. Food Control 2021, 127, 108103. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Mazlan, M.M.; Talib, R.A.; Chin, N.L.; Shukri, R.; Taip, F.S.; Nor, M.Z.M.; Abdullah, N. Physical and Microstructure Properties of Oyster Mushroom-Soy Protein Meat Analog via Single-Screw Extrusion. Foods 2020, 9, 1023. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Meng, S.; Wang, Q. Rheological Properties of Pea Protein Isolate-Amylose/Amylopectin Mixtures and the Application in the High-Moisture Extruded Meat Substitutes. Food Hydrocoll. 2021, 117, 106732. [Google Scholar] [CrossRef]
- Altmann, B.A.; Gertheiss, J.; Tomasevic, I.; Engelkes, C.; Glaesener, T.; Meyer, J.; Schäfer, A.; Wiesen, R.; Mörlein, D. Human Perception of Color Differences Using Computer Vision System Measurements of Raw Pork Loin. Meat Sci. 2022, 188, 108766. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.N.; Rokey, G.J. Extrusion Problems Solved: Food, Pet Food and Feed; Woodhead Publishing Limited: Sawston, UK, 2012. [Google Scholar] [CrossRef]
- Moisio, T.; Forssell, P.; Partanen, R.; Damerau, A.; Hill, S.E. Reorganisation of Starch, Proteins and Lipids in Extrusion of Oats. J. Cereal Sci. 2015, 64, 48–55. [Google Scholar] [CrossRef]
- Omohimi, C.I.; Sobukola, O.P.; Sarafadeen, K.O.; Sanni, L.O. Effect of Thermo-Extrusion Process Parameters on Selected Quality Attributes of Meat Analogue from Mucuna Bean Seed Flour. Niger. Food J. 2014, 32, 21–30. [Google Scholar] [CrossRef]
- Mandliya, S.; Mishra, H.N. Effect of Different Drying Methods on Quality Attributes and Microstructure of Mycelium (Pleurotus eryngii). Biol. Life Sci. Forum 2021, 6, 10. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea Protein Isolates: Structure, Extraction, and Functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Riaz, M.N. Texturized Soy Protein as an Ingredient. In Proteins in Food Processing; Yada, R.Y., Ed.; Elsevier: Cambridge, UK, 2004; pp. 517–558. [Google Scholar]
- Lin, S.; Huff, H.E.; Hsieh, F. Extrusion Process Parameters, Sensory Characteristics, and Structural Properties of a High Moisture Soy Protein Meat Analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Smetana, S.; Ashtari Larki, N.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure Design of Insect-Based Meat Analogs with High-Moisture Extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- Mandliya, S.; Vishwakarma, S.; Mishra, H.N. Modeling of Vacuum Drying of Pressed Mycelium (Pleurotus eryngii) and Its Microstructure and Physicochemical Properties. J. Food Process Eng. 2022. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein Concentrate: Influence of Thermomechanical Treatment on Protein-Protein Interactions and Rheological Properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Yuliarti, O.; Kiat Kovis, T.J.; Yi, N.J. Structuring the Meat Analogue by Using Plant-Based Derived Composites. J. Food Eng. 2021, 288, 110138. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Structure Formation and Rheological Properties of Pea Protein-Based Gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, L.; Zhao, D.; Liu, H.; Xu, X.; Zhou, G.; Li, C. Real Meat and Plant-Based Meat Analogues Have Different In Vitro Protein Digestibility Properties. Food Chem. 2022, 387, 132917. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Pereira, C.G.; de Almeida Junior, J.C.; Viana, C.C.R.; de Oliveira Neves, L.N.; da Silva, P.H.F.; Bell, M.J.V.; dos Anjos, V.D.C. FTIR-ATR Determination of Protein Content to Evaluate Whey Protein Concentrate Adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]


| Sample | MY (% w/w) | PPI (% w/w) | Extrusion Moisture Content (% wb) | Protein Content (g/100 g) |
|---|---|---|---|---|
| S0 | 0 | 100 | 30 | 55.2 ± 1.2 a |
| S10 | 10 | 90 | 30 | 51.5 ± 1.4 a |
| S20 | 20 | 80 | 30 | 46.0 ± 1.9 b |
| S30 | 30 | 70 | 30 | 41.2 ± 2.1 c |
| S40 | 40 | 60 | 30 | 37.9 ± 0.9 c |
| Sample | L* | a* | b* | ΔE | ||||
|---|---|---|---|---|---|---|---|---|
| Raw | Extruded | Raw | Extruded | Raw | Extruded | Raw | Extruded | |
| S0 | 75.49 ± 0.19 aA | 49.14 ± 0.65 aB | 5.69 ± 0.06 aB | 10.14 ± 0.13 aA | 24.91 ± 0.12 cA | 22.77 ± 0.64 aB | ||
| S10 | 74.31 ± 0.12 bA | 48.79 ± 0.92 aB | 5.62 ± 0.06 aB | 9.75 ± 0.18 aA | 24.82 ± 0.11 cA | 23.54 ± 0.39 aB | 1.19 ± 0.11 dA | 1.21 ± 0.33 dA |
| S20 | 72.48 ± 0.25 cA | 44.13 ± 0.80 bB | 5.61 ± 0.03 aB | 9.95 ± 0.19 aA | 24.98 ± 0.10 bcA | 20.96 ± 0.34 bB | 3.01 ± 0.25 cB | 5.33 ± 0.85 cA |
| S30 | 70.46 ± 0.33 dA | 41.00 ± 0.47 cB | 5.60 ± 0.07 aB | 10.18 ± 0.12 aA | 25.24 ± 0.06 bA | 18.23 ± 0.31 cB | 5.04 ± 0.34 bB | 9.32 ± 0.55 bA |
| S40 | 68.49 ± 0.41 eA | 36.95 ± 1.08 dB | 5.80 ± 0.24 aB | 9.86 ± 0.22 aA | 25.92 ± 0.06 aA | 15.99 ± 1.06 dB | 7.07 ± 0.39 aB | 13.96 ± 1.43 aA |
| Sample | WHC (%) | VER |
|---|---|---|
| S0 | 259.85 ± 19.18 c | 2.81 ± 0.20 c |
| S10 | 385.28 ± 17.83 b | 3.45 ± 0.11 b |
| S20 | 450.24 ± 22.29 a | 3.97 ± 0.22 ab |
| S30 | 469.37 ± 16.14 a | 4.14 ± 0.10 a |
| S40 | 192.51 ± 28.35 d | 2.62 ± 0.27 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandliya, S.; Pratap-Singh, A.; Vishwakarma, S.; Dalbhagat, C.G.; Mishra, H.N. Incorporation of Mycelium (Pleurotus eryngii) in Pea Protein Based Low Moisture Meat Analogue: Effect on Its Physicochemical, Rehydration and Structural Properties. Foods 2022, 11, 2476. https://doi.org/10.3390/foods11162476
Mandliya S, Pratap-Singh A, Vishwakarma S, Dalbhagat CG, Mishra HN. Incorporation of Mycelium (Pleurotus eryngii) in Pea Protein Based Low Moisture Meat Analogue: Effect on Its Physicochemical, Rehydration and Structural Properties. Foods. 2022; 11(16):2476. https://doi.org/10.3390/foods11162476
Chicago/Turabian StyleMandliya, Shubham, Anubhav Pratap-Singh, Siddharth Vishwakarma, Chandrakant Genu Dalbhagat, and Hari Niwas Mishra. 2022. "Incorporation of Mycelium (Pleurotus eryngii) in Pea Protein Based Low Moisture Meat Analogue: Effect on Its Physicochemical, Rehydration and Structural Properties" Foods 11, no. 16: 2476. https://doi.org/10.3390/foods11162476