Insight into the Characterization of Volatile Compounds in Smoke-Flavored Sea Bass (Lateolabrax maculatus) during Processing via HS-SPME-GC-MS and HS-GC-IMS
Abstract
:1. Introduction
2. Materials and Methods
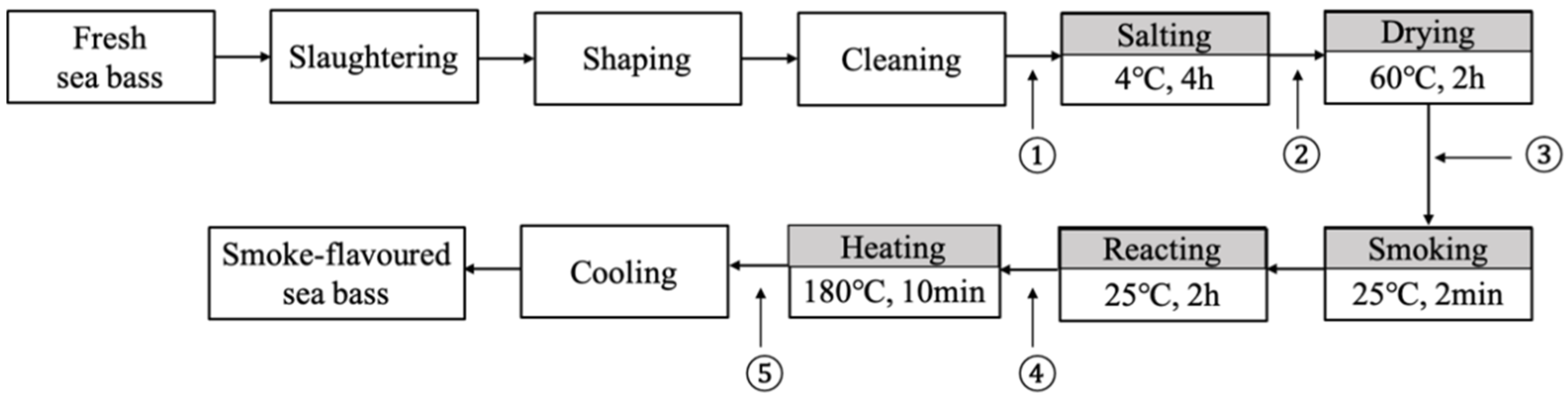
2.1. Smoke-Flavored Sea Bass Preparation
2.2. HS-SPME-GC-MS Analysis
2.2.1. Extraction of Volatile Compounds
2.2.2. HS-SPME-GC-MS Analysis of Volatile Compounds
2.3. HS-GC-IMS Analysis
2.4. Statistical Analysis
3. Results
3.1. HS-SPME-GC-MS Analysis of Smoke-Flavored Sea Bass in Different Production Steps
3.1.1. Identification of Volatile Compounds in Different Production Steps
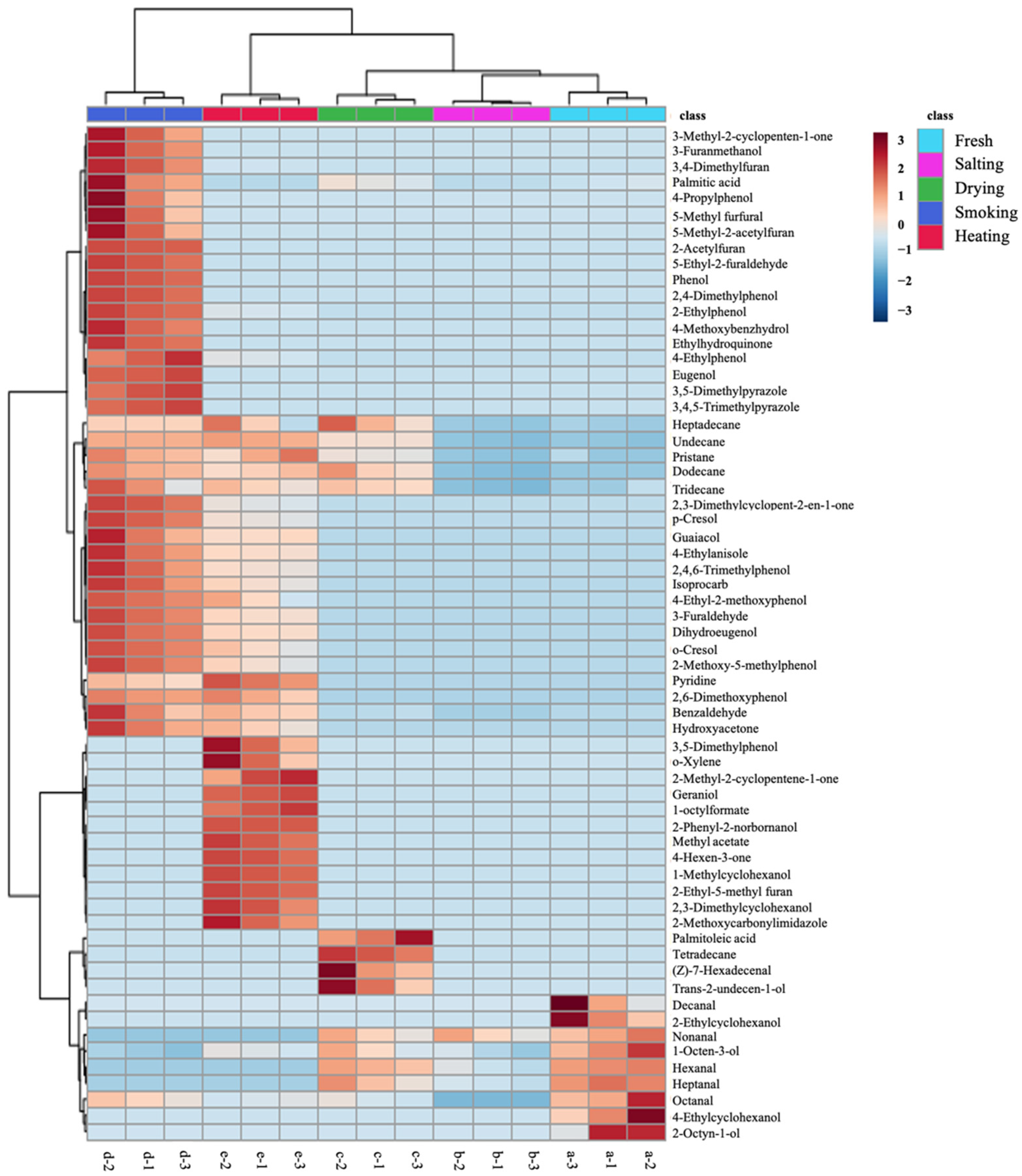
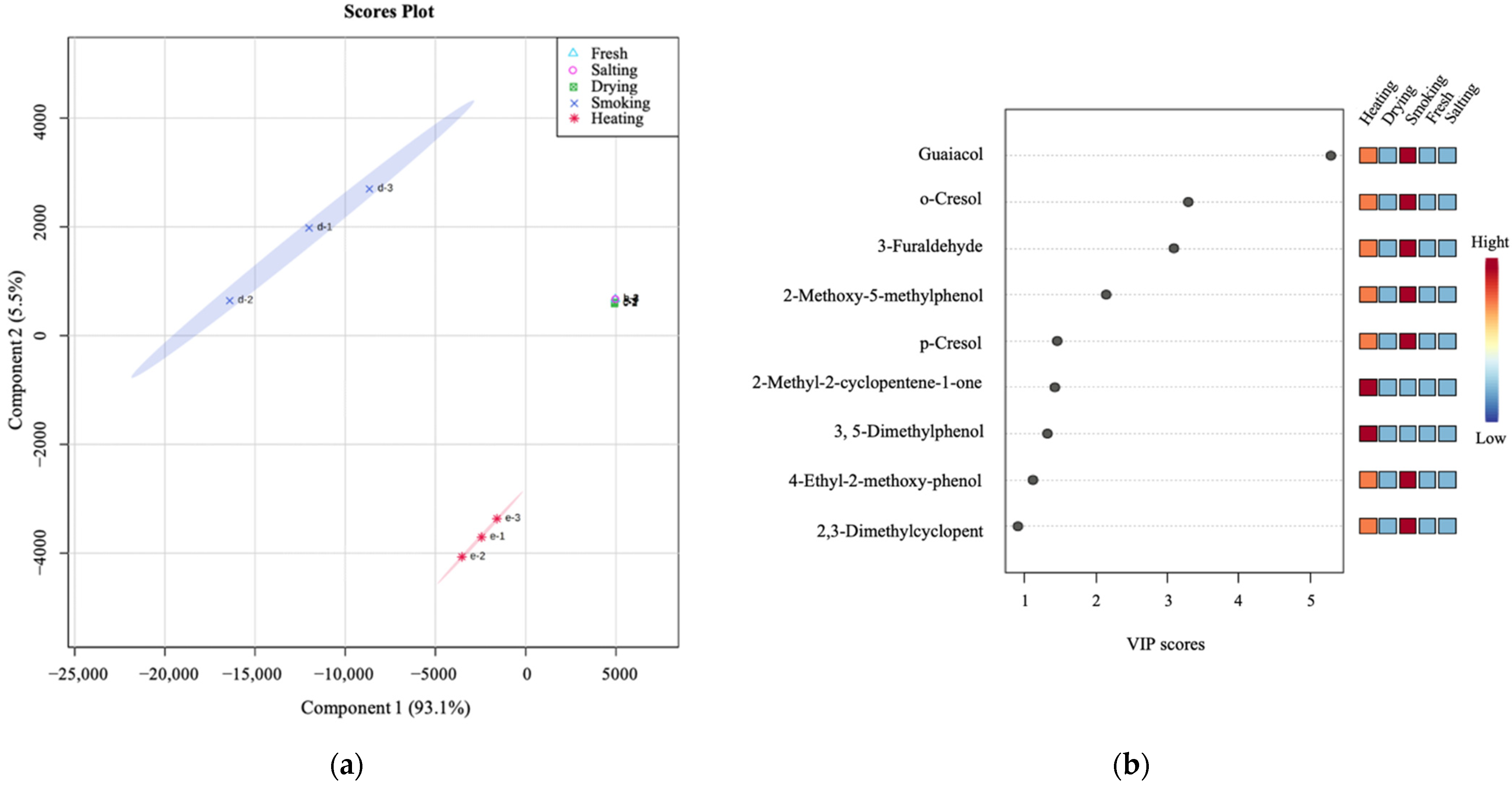
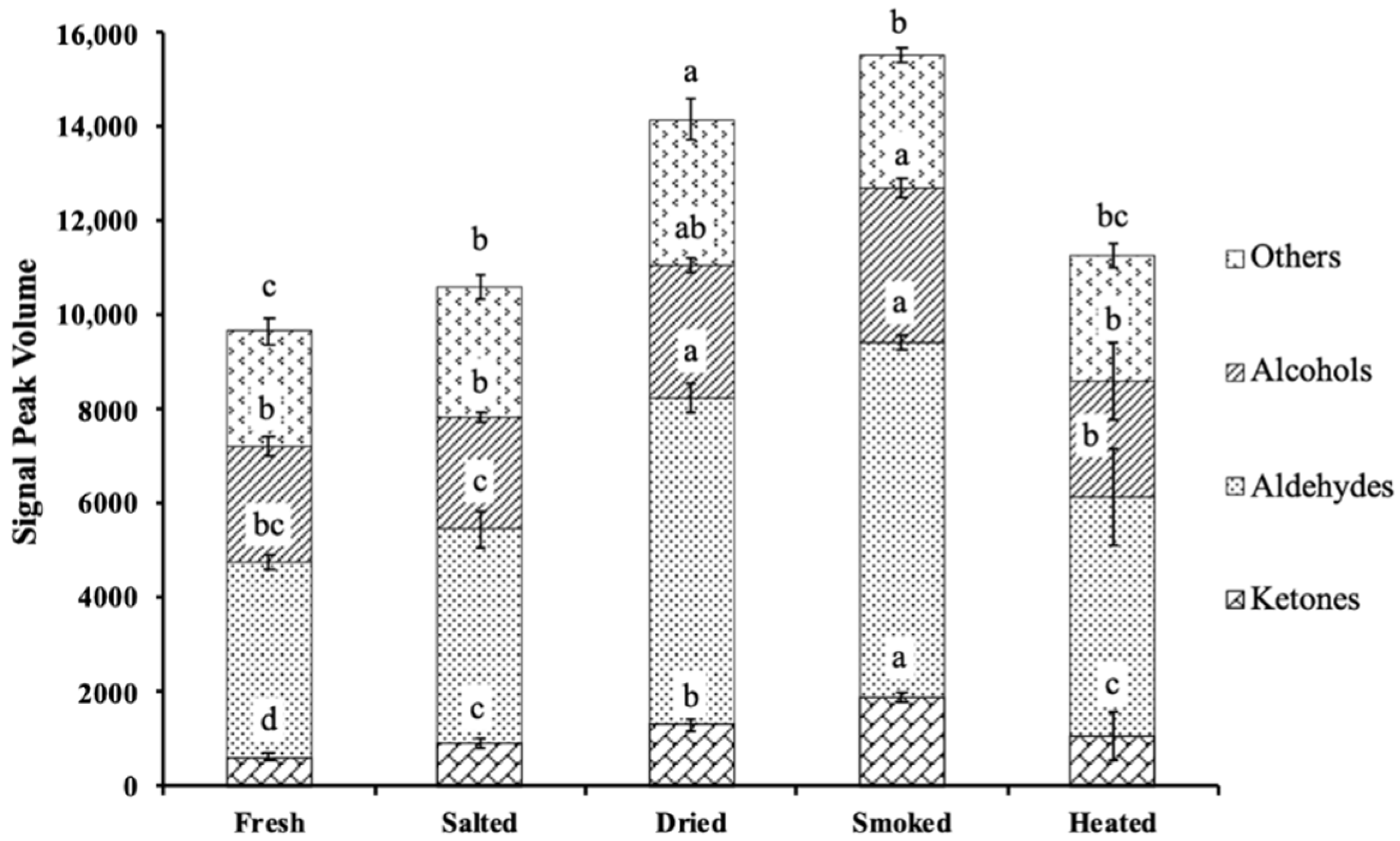
3.1.2. Changes in the Volatile Compounds in Different Production Steps
3.2. HS-GC-IMS Analysis of Smoke-Flavored Sea Bass in Different Production Steps
3.2.1. Identification of Volatile Compounds
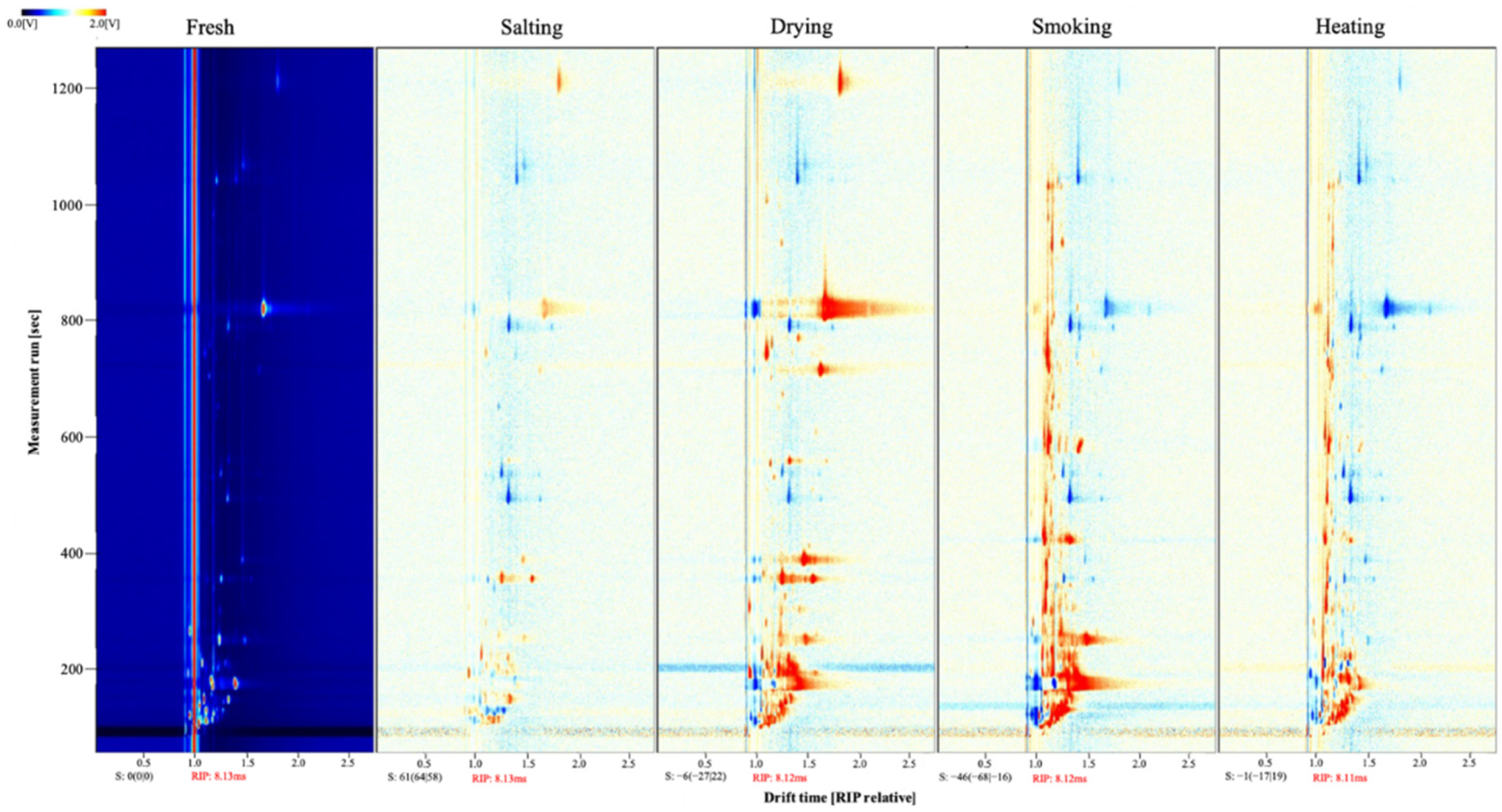
3.2.2. Changes in the Volatile Compounds of Different Smoke-Flavored Sea Bass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, H.; Jiang, J.; Li, D. Potential hazards in smoke-flavored fish. J. Ocean. Univ. China 2008, 7, 294–298. [Google Scholar] [CrossRef]
- Nieva-Echevarria, B.; Goicoechea, E.; Guillen, M.D. Effect of liquid smoking on lipid hydrolysis and oxidation reactions during in vitro gastrointestinal digestion of European sea bass. Food Res. Int. 2017, 97, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Alçiçek, Z.; Balaban, M.Ö. Characterization of Green Lipped Mussel Meat. Part II: Changes in Physical Characteristics as a Result of Brining and Liquid Smoke Application. J. Aquat. Food Prod. Technol. 2015, 24, 15–30. [Google Scholar] [CrossRef]
- Xin, X.; Dell, K.; Udugama, I.A.; Young, B.R.; Baroutian, S. Transforming biomass pyrolysis technologies to produce liquid smoke food flavouring. J. Clean. Prod. 2021, 294, 125368. [Google Scholar] [CrossRef]
- Ntzimani, A.; Angelakopoulos, R.; Semenoglou, I.; Dermesonlouoglou, E.; Taoukis, P. Slurry ice as an alternative cooling medium for fish harvesting and transportation: Study of the effect on seabass flesh quality and shelf life. Aquac. Fish. 2021. [Google Scholar] [CrossRef]
- Fan, H.; Wang, L.; Wen, H.; Wang, K.; Qi, X.; Li, J.; He, F.; Li, Y. Genome-wide identification and characterization of toll-like receptor genes in spotted sea bass (Lateolabrax maculatus) and their involvement in the host immune response to Vibrio harveyi infection. Fish Shellfish. Immunol. 2019, 92, 782–791. [Google Scholar] [CrossRef]
- Martinez, O.; Salmeron, J.; Epelde, L.; Vicente, M.S.; de Vega, C. Quality enhancement of smoked sea bass (Dicentrarchus labrax) fillets by adding resveratrol and coating with chitosan and alginate edible films. Food Control 2018, 85, 168–176. [Google Scholar] [CrossRef]
- Cardinal, M.; Knockaert, C.; Torrissen, O.; Sigurgisladottir, S.; Mørkøre, T.; Thomassen, M.; Luc Vallet, J. Relation of smoking parameters to the yield, colour and sensory quality of smoked Atlantic salmon (Salmo salar). Food Res. Int. 2001, 34, 537–550. [Google Scholar] [CrossRef]
- Kostyra, E.; Baryłko-Pikielna, N. Volatiles composition and flavour profile identity of smoke flavourings. Food Qual. Prefer. 2006, 17, 85–95. [Google Scholar] [CrossRef]
- Horner, W.F.A. Preservation of fish by curing (drying, salting and smoking). In Fish Processing Technology; Hall, G.M., Ed.; Chapman & Hall: New York, NY, USA, 1997; pp. 32–73. [Google Scholar]
- Mireille, C.; Berdagué, J.L.; Dinel, V.; Knockaert, C.; Vallet, J.L. Effect of various smoking techniques on the nature of volatile compounds and on the sensory characteristics of salmon meat. Sci. Des Aliment. 1997, 17, 679–696. [Google Scholar]
- Varlet, V.; Serot, T.; Knockaert, C.; Cornet, J.; Cardinal, M.; Monteau, F.; Le Bizec, B.; Prost, C. Organoleptic characterization and PAH content of salmon (Salmo salar) fillets smoked according to four industrial smoking techniques. J. Sci. Food Agric. 2007, 87, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Nithin, C.T.; Joshy, C.G.; Chatterjee, N.S.; Panda, S.K.; Yathavamoorthi, R.; Ananthanarayanan, T.R.; Mathew, S.; Bindu, J.; Gopal, T.K.S. Liquid smoking—A safe and convenient alternative for traditional fish smoked products. Food Control 2020, 113, 107186. [Google Scholar] [CrossRef]
- Ruiz-Alonso, S.A.; Giron-Hernandez, L.J.; Lopez-Vargas, J.H.; Munoz-Ramirez, A.P.; Simal-Gandara, J. Optimizing salting and smoking conditions for the production and preservation of smoked-flavoured tilapia fillets. Lwt Food Sci. Technol. 2021, 138, 110733. [Google Scholar] [CrossRef]
- Val, T.; Jakobsen, A.N.; Lerfall, J. The use of atomized purified condensed smoke (PCS) in cold-smoke processing of Atlantic salmon—Effects on quality and microbiological stability of a lightly salted product. Food Control 2020, 112, 107155. [Google Scholar] [CrossRef]
- Rizo, A.; Fuentes, A.; Fernandez-Segovia, I.; Barat, J.M. Development of a novel smoke-flavoured trout product: An approach to sodium reduction and shelf life assessment. J. Food Eng. 2017, 211, 22–29. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Z.; Chen, X.; Weng, P.; Zhang, X. Characterization of flavour and volatile compounds of fermented squid using electronic nose and HPMS in combination with GC-MS. Int. J. Food Prop. 2018, 21, 760–770. [Google Scholar] [CrossRef]
- Shi, J.; Nian, Y.; Da, D.; Xu, X.; Zhou, G.; Zhao, D.; Li, C. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography–mass spectrometry and electronic nose. LWT 2020, 124, 109182. [Google Scholar] [CrossRef]
- Yui, O.; Yutaka, I.; Yusuke, K.; Tadayoshi, K. Odor-active compounds contributing to the characteristic aroma of shrimp cooked whole, including shells and viscera. Eur. Food Res. Technol. 2018, 245, 233–241. [Google Scholar] [CrossRef]
- Huang, X.H.; Qi, L.B.; Fu, B.S.; Chen, Z.H.; Zhang, Y.Y.; Du, M.; Dong, X.P.; Zhu, B.W.; Qin, L. Flavor formation in different production steps during the processing of cold-smoked Spanish mackerel. Food Chem. 2019, 286, 241–249. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Sun, L.; Dong, L.; Du, M. Dynamics of Microbial Communities, Texture and Flavor in Suan zuo yu during Fermentation. Food Chem. 2020, 332, 127364. [Google Scholar] [CrossRef]
- Tian, X.; Li, Z.J.; Chao, Y.Z.; Wu, Z.Q.; Zhou, M.X.; Xiao, X.T.; Zeng, J.; Zhe, J. Evaluation by electronic tongue and headspace-GC-IMS analyses of the flavor compounds in dry-cured pork with different salt content. Food Res. Int. 2020, 137, 109456. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, K.; Yang, R.; Dong, Y.; Lin, S. Mechanism of aroma compounds changes from sea cucumber peptide powders (SCPPs) under different storage conditions. Food Res. Int. 2020, 128, 108757. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Hu, X.; Wang, R.R.; Guo, J.J.; Ge, K.D.; Li, Y.; Fu, F.H.; Ding, S.H.; Shan, Y. Changes in the Volatile Components of Candied Kumquats in Different Processing Methodologies with Headspace–Gas Chromatography–Ion Mobility Spectrometry. Molecules 2019, 24, 3053. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.; Liu, T.; Gong, P.; Wang, Y.; Wang, H.; Tian, X.; Liu, Q.; Cui, Q.; Xie, X.; et al. Aroma classification and flavor characterization of Streptococcus thermophilus fermented milk by HS-GC-IMS and HS-SPME-GC-TOF/MS. Food Biosci. 2022, 49, 101832. [Google Scholar] [CrossRef]
- Lin, H.; Yan, Y.; Zhao, T.; Peng, L.; Zou, H.; Li, J.; Yang, X.; Xiong, Y.; Wang, M.; Wu, H. Rapid discrimination of Apiaceae plants by electronic nose coupled with multivariate statistical analyses. J. Pharm. Biomed. Anal. 2013, 84, 1–4. [Google Scholar] [CrossRef]
- Radulović, N.S.; Blagojević, P.D. Average mass scan of the total ion chromatograms: A new gas chromatography–mass spectrometry derived variable for fast and reliable multivariate statistical treatment of essential oil compositional data. J. Chromatogr. A 2013, 1301, 190–199. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.; Lee, S.; Oh, J.Y.; Jeon, E.J.; Ryu, H.S.; Lee, C.H. Primary and secondary metabolite profiling of doenjang, a fermented soybean paste during industrial processing. Food Chem. 2014, 165, 157–166. [Google Scholar] [CrossRef]
- Chen, X.A.; Chen, H.; Xiao, J.; Liu, J.Y.; Tang, N.; Zhou, A.M. Variations of volatile flavour compounds in finger citron (Citrus medica L. var. sarcodactylis) pickling process revealed by E-nose, HS-SPME-GC-MS and HS-GC-IMS. Food Res. Int. 2020, 138, 109717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Niu, C.; Yang, X.; Xu, X.; Zheng, F.; Liu, C.; Wang, J.; Li, Q. Roles of sunlight exposure on chemosensory characteristic of broad bean paste by untargeted profiling of volatile flavors and multivariate statistical analysis. Food Chem. 2022, 381, 132115. [Google Scholar] [CrossRef] [PubMed]
- Çakir, F.; Ayvaz, Z. Investigation of the Effect of Different Immersion Times of Anchovy Fillets in Liquid Smoke Flavoring on Color by Image Analysis. J. Aquat. Food Prod. Technol. 2020, 29, 865–870. [Google Scholar] [CrossRef]
- Belichovska, K.; Belichovska, D.; Pejkovski, Z. Smoke and Smoked Fish Production. Meat Technol. 2019, 60, 37–43. [Google Scholar] [CrossRef]
- Chen, J.; Tao, L.; Zhang, T.; Zhang, J.; Wu, T.; Luan, D.; Ni, L.; Wang, X.; Zhong, J. Effect of four types of thermal processing methods on the aroma profiles of acidity regulator-treated tilapia muscles using E-nose, HS-SPME-GC-MS, and HS-GC-IMS. LWT 2021, 147, 111585. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, Y.; Gu, S.; Zhu, S.; Zhou, X.; Ding, Y. Identification of changes in volatile compounds in dry-cured fish during storage using HS-GC-IMS. Food Res. Int. 2020, 137, 109339. [Google Scholar] [CrossRef]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid discrimination of Citrus reticulata ‘Chachi’ by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z.; Fan, X.; Liu, M.; Ma, J.; Shang, W.; Liu, J.; Strappe, P.; Blanchard, C.; Zhou, Z. A study on volatile metabolites screening by HS-SPME-GC-MS and HS-GC-IMS for discrimination and characterization of white and yellowed rice. Cereal Chem. 2020, 97, 496–504. [Google Scholar] [CrossRef]
- Hu, M.Y.; Wang, S.Y.; Liu, Q.; Cao, R.; Xue, Y. Flavor profile of dried shrimp at different processing stages. Lwt Food Sci. Technol. 2021, 146, 111403. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Martin-Gomez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace-gas chromatography-ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef]
- Vasconi, M.; Caprino, F.; Bellagamba, F.; Busetto, M.L.; Bernardi, C.; Puzzi, C.; Moretti, V.M. Fatty Acid Composition of Freshwater Wild Fish in Subalpine Lakes: A Comparative Study. Lipids 2015, 50, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.J.; Schilling, M.W.; Mikel, W.B.; Williams, J.B.; Martin, J.M.; Coggins, P.C. Relationships between sensory descriptors, consumer acceptability and volatile flavor compounds of American dry-cured ham. Meat Sci. 2008, 80, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Purriños, L.; Franco, D.; Carballo, J.; Lorenzo, J.M. Influence of the salting time on volatile compounds during the manufacture of dry-cured pork shoulder “lacón”. Meat Sci. 2012, 92, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, Y.; Pan, D.; Wang, Y.; Cao, J. Effects of high pressure treatment on lipolysis-oxidation and volatiles of marinated pork meat in soy sauce. Meat Sci. 2018, 145, 186–194. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C. c4-Heptenal: An Influential Volatile Compound in Boiled Potato Flavor. J. Food Sci. 1987, 52, 328–331. [Google Scholar] [CrossRef]
- Guillén, M.D.; Errecalde, M.C. Volatile components of raw and smoked black bream (Brama raii) and rainbow trout (Oncorhynchus mykiss) studied by means of solid phase microextraction and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2002, 82, 945–952. [Google Scholar] [CrossRef]
- Alfonzo, A.; Gaglio, R.; Francesca, N.; Barbera, M.; Saiano, F.; Santulli, A.; Matraxia, M.; Rallo, F.; Moschetti, G. Influence of salt of different origin on the microbiological characteristics, histamine generation and volatile profile of salted anchovies (Engraulis encrasicolus L.). Food Control 2018, 92, 301–311. [Google Scholar] [CrossRef]
- Joffraud, J.J.; Leroi, F.; Roy, C.; Berdagué, J.L. Characterisation of volatile compounds produced by bacteria isolated from the spoilage flora of cold-smoked salmon. Int. J. Food Microbiol. 2001, 66, 175–184. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Huss, H.H.; Dalgaard, P. Significance of Volatile Compounds Produced by Spoilage Bacteria in Vacuum-Packed Cold-Smoked Salmon (Salmo salar) Analyzed by GC-MS and Multivariate Regression. J. Agric. Food Chem. 2001, 49, 2376–2381. [Google Scholar] [CrossRef]
- Sérot, T.; Baron, R.; Knockaert, C.; Vallet, J.L. Effect of smoking processes on the contents of 10 major phenolic compounds in smoked fillets of herring (Cuplea harengus). Food Chem. 2004, 85, 111–120. [Google Scholar] [CrossRef]
- Salum, P.; Guclu, G.; Selli, S. Comparative Evaluation of Key Aroma-Active Compounds in Raw and Cooked Red Mullet (Mullus barbatus) by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2017, 65, 8402–8408. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, E.; Kristensson, A.; Ohlsson, M.; Johansson, C.; Johansson, P.-Å.; Swietlicki, E.; Vesely, V.; Wideqvist, U.; Westerholm, R. Chemical and physical characterization of emissions from birch wood combustion in a wood stove. Atmos. Environ. 2002, 36, 4823–4837. [Google Scholar] [CrossRef]
- Song, S.; Tang, Q.; Fan, L.; Xu, X.; Song, Z.; Hayat, K.; Feng, T.; Wang, Y. Identification of pork flavour precursors from enzyme-treated lard using Maillard model system assessed by GC–MS and partial least squares regression. Meat Sci. 2017, 124, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wen, R.; Sun, F.; Wang, Y.; Kong, B.; Chen, Q. Collaborative analysis on differences in volatile compounds of Harbin red sausages smoked with different types of woodchips based on gas chromatography–mass spectrometry combined with electronic nose. LWT 2021, 143, 111144. [Google Scholar] [CrossRef]
- Saldaña, E.; Saldarriaga, L.; Cabrera, J.; Siche, R.; Behrens, J.H.; Selani, M.M.; de Almeida, M.A.; Silva, L.D.; Silva Pinto, J.S.; Contreras-Castillo, C.J. Relationship between volatile compounds and consumer-based sensory characteristics of bacon smoked with different Brazilian woods. Food Res. Int. 2019, 119, 839–849. [Google Scholar] [CrossRef]
- Varlet, V.; Knockaert, C.; Prost, C.; Serot, T. Comparison of Odor-Active Volatile Compounds of Fresh and Smoked Salmon. J. Agric. Food Chem. 2006, 54, 3391–3401. [Google Scholar] [CrossRef]
- Varlet, V.; Prost, C.; Serot, T. Volatile aldehydes in smoked fish: Analysis methods, occurence and mechanisms of formation. Food Chem. 2007, 105, 1536–1556. [Google Scholar] [CrossRef]
- Grosch, W. Reactions of hydroperoxides—Products of low molecular weight. In Autooxidation of Unsaturated Lipids; Chan, H.W.S., Ed.; Academic Press: London, UK, 1987; pp. 95–139. [Google Scholar]
- Ren, S.; Li, P.P.; Geng, Z.M.; Sun, C.; Song, H.; Wang, D.Y.; Zhang, M.H.; Liu, F.; Xu, W.M. Lipolysis and Lipid Oxidation during Processing of Chinese Traditional Dry-Cured White Amur Bream (Parabramis pekinensis). J. Aquat. Food Prod. Technol. 2017, 26, 719–730. [Google Scholar] [CrossRef]
- Ramalingam, V.; Song, Z.; Hwang, I. The potential role of secondary metabolites in modulating the flavor and taste of the meat. Food Res. Int. 2019, 122, 174–182. [Google Scholar] [CrossRef]
- Arief, I.I.; Afiyah, D.N.; Wulandari, Z.; Budiman, C. Physicochemical Properties, Fatty Acid Profiles, and Sensory Characteristics of Fermented Beef Sausage by Probiotics Lactobacillus plantarum IIA-2C12 or Lactobacillus acidophilus IIA-2B4. J. Food Sci. 2016, 81, 2761–2769. [Google Scholar] [CrossRef]
- Purrinos, L.; Bermudez, R.; Franco, D.; Carballo, J.; Lorenzo, J.M. Development of Volatile Compounds during the Manufacture of Dry-Cured “Lacon”, a Spanish Traditional Meat Product. J. Food Sci. 2011, 76, C89–C97. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.P.; Li, D.Y.; Huang, Y.; Wu, Q.; Liu, W.T.; Qin, L.; Zhou, D.Y.; Prakash, S.; Yu, C.X. Nutritional value and flavor of turbot (Scophthalmus maximus) muscle as affected by cooking methods. Int. J. Food Prop. 2018, 21, 1972–1985. [Google Scholar] [CrossRef]
- Zzaman, W.; Bhat, R.; Yang, T.A.; Easa, A.M. Influences of superheated steam roasting on changes in sugar, amino acid and flavour active components of cocoa bean (Theobroma cacao). J. Sci. Food Agric. 2017, 97, 4429–4437. [Google Scholar] [CrossRef]
- Cantalejo, M.J. Effects of Roasting Temperature on the Aroma Components of Carob (Ceratonia siliqua L.). J. Agric. Food Chem. 1997, 45, 1345–1350. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Hui, T.; Ma, J. Generation of key aroma compounds in Beijing roasted duck induced via Maillard reaction and lipid pyrolysis reaction. Food Res. Int. 2020, 136, 109328. [Google Scholar] [CrossRef] [PubMed]


| Gas Phase-Ion Mobility Spectrometry Unit | |
|---|---|
| Analysis time | 30 min |
| Column type | MXT-5 (15 m × 0.53 mm) |
| Column temperature | 60 °C |
| Carrier gas/drift gas | N2 (99.99%) |
| IMS temperature | 45 °C |
| Automatic headspace sampling unit | |
| Injection volume | 500 μL |
| Incubation time | 20 min |
| Incubation temperature | 40 °C |
| Syringe temperature | 85 °C |
| Incubation speed | 500 rpm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Timira, V.; Zhao, J.; Lin, H.; Wang, H.; Li, Z. Insight into the Characterization of Volatile Compounds in Smoke-Flavored Sea Bass (Lateolabrax maculatus) during Processing via HS-SPME-GC-MS and HS-GC-IMS. Foods 2022, 11, 2614. https://doi.org/10.3390/foods11172614
Feng H, Timira V, Zhao J, Lin H, Wang H, Li Z. Insight into the Characterization of Volatile Compounds in Smoke-Flavored Sea Bass (Lateolabrax maculatus) during Processing via HS-SPME-GC-MS and HS-GC-IMS. Foods. 2022; 11(17):2614. https://doi.org/10.3390/foods11172614
Chicago/Turabian StyleFeng, Hua, Vaileth Timira, Jinlong Zhao, Hong Lin, Hao Wang, and Zhenxing Li. 2022. "Insight into the Characterization of Volatile Compounds in Smoke-Flavored Sea Bass (Lateolabrax maculatus) during Processing via HS-SPME-GC-MS and HS-GC-IMS" Foods 11, no. 17: 2614. https://doi.org/10.3390/foods11172614






