Effect of Different Thawing Methods on the Physicochemical Properties and Microstructure of Frozen Instant Sea Cucumber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Determination of Water Retention and Moisture Migration
2.2.1. Thawing Loss
2.2.2. Cooking Loss
2.2.3. Water-Holding Capacity (WHC)
2.2.4. Moisture Migration
2.3. Determination of Mechanical Properties
2.3.1. Texture Profile Analysis (TPA)
2.3.2. Rheological Properties
2.4. Determination of Protein Properties
2.4.1. Protein Thermal Stability
2.4.2. Protein Secondary Structure
2.4.3. Protein Tertiary Structure
2.4.4. Surface Hydrophobicity
2.5. Determination of Scanning Electron Microscopy (SEM)
2.6. Principal Component Analysis (PCA)
2.7. Statistical Analysis
3. Results and Discussions
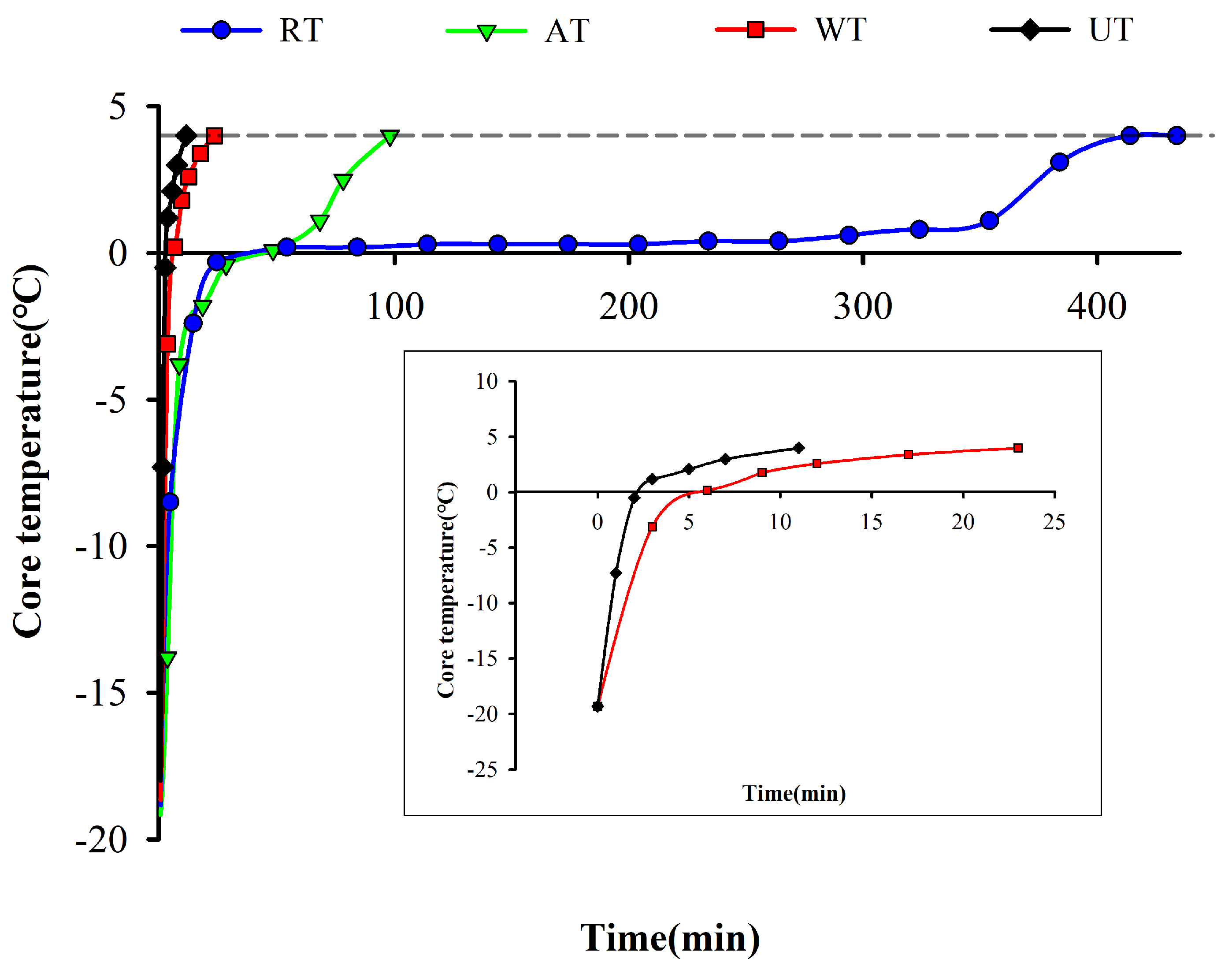
3.1. Thawing Curve
3.2. Water Retention and Moisture Migration
3.2.1. Water Retention
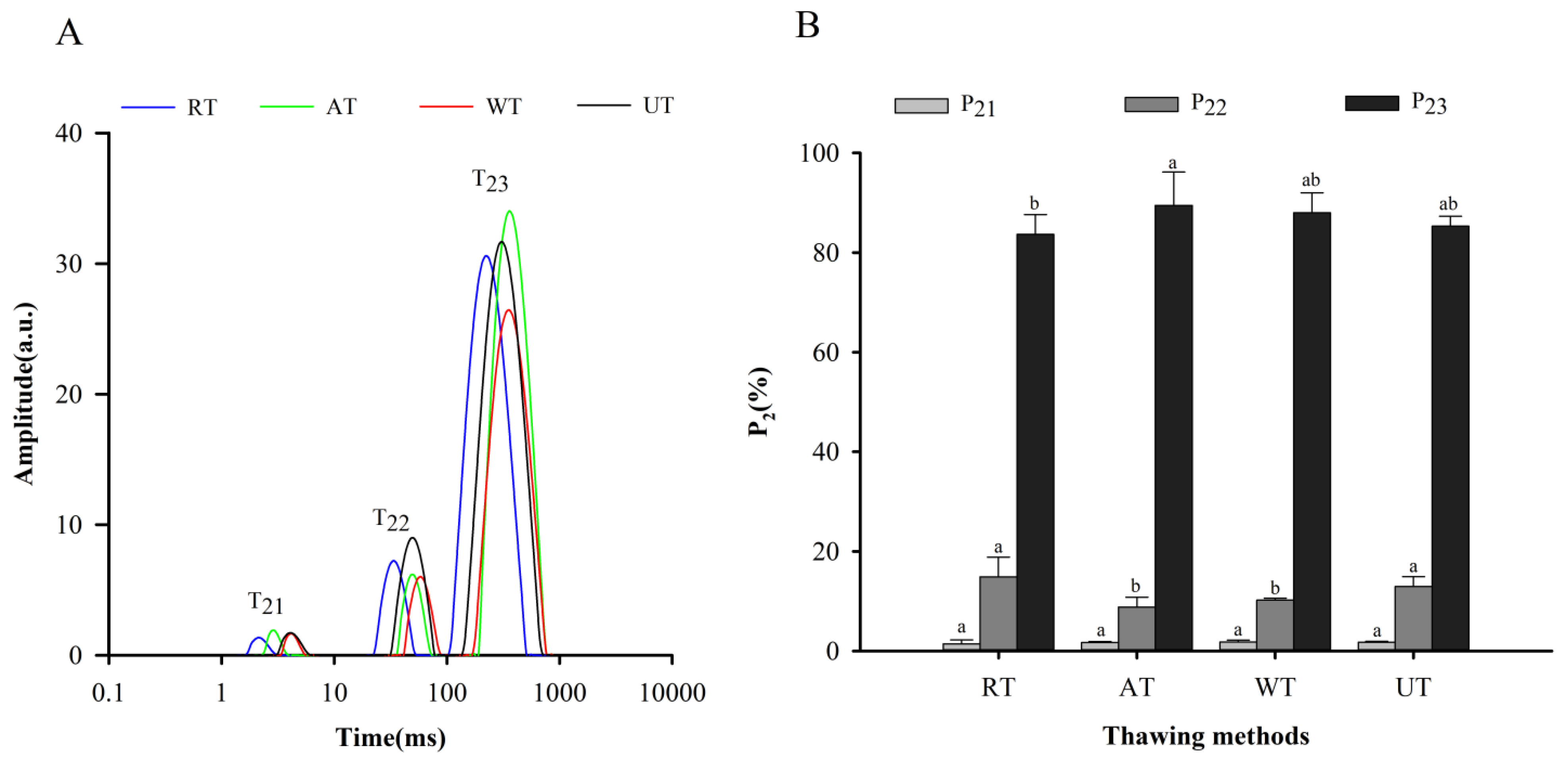
3.2.2. Moisture Migration
3.3. Mechanical Properties
3.3.1. TPA
3.3.2. Rheological Properties
3.4. Protein Properties
3.4.1. Thermal Stability of Proteins
3.4.2. Secondary Protein Structure
3.4.3. Tertiary Protein Structure
3.4.4. Surface Hydrophobicity
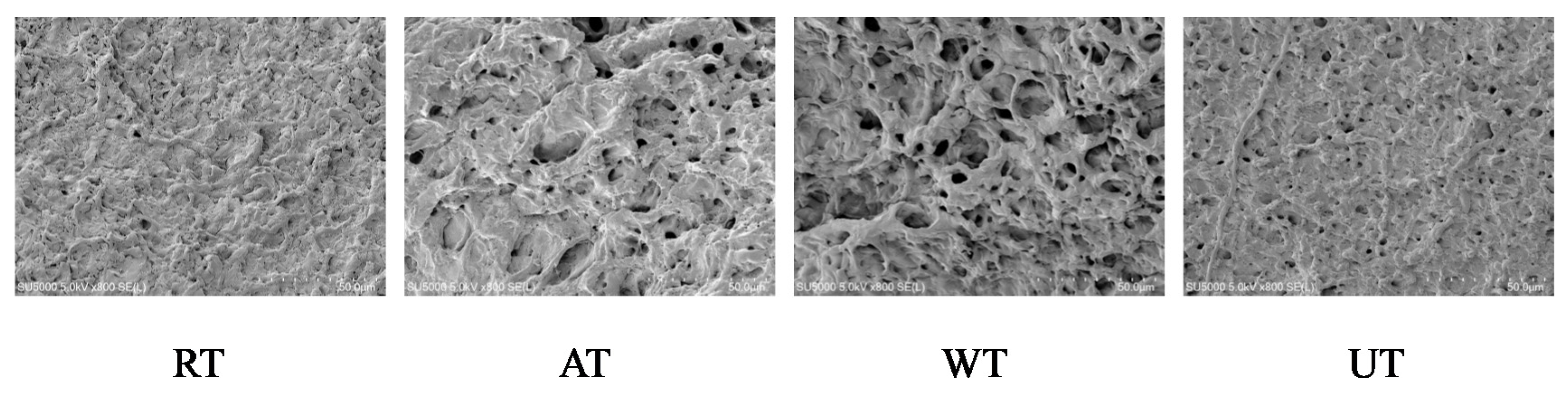
3.5. Microstructure
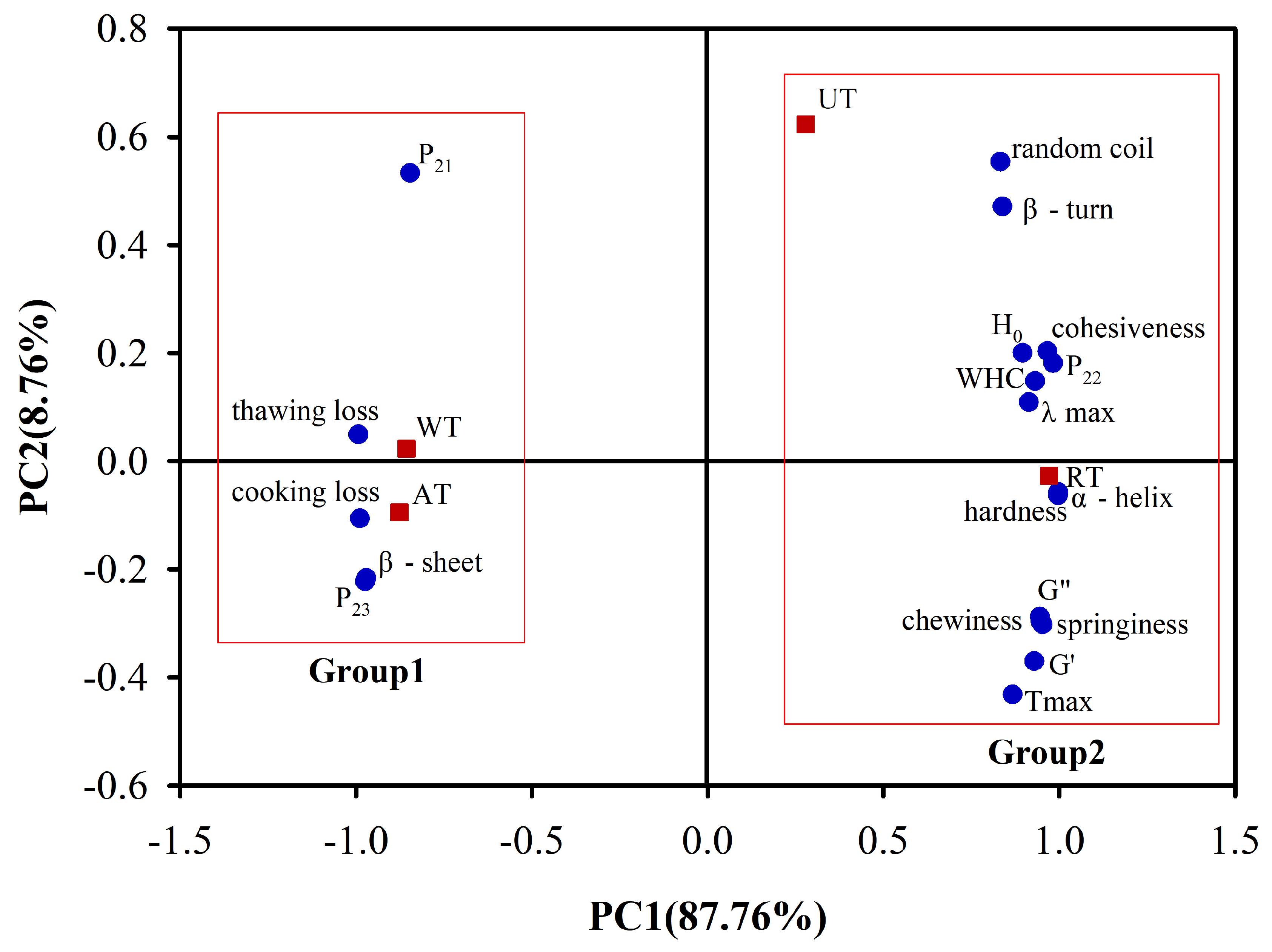
3.6. Principal Component Analysis
3.7. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Li, D.Y.; Song, L.; Liu, Y.X.; Shahidi, F. Effects of proteolysis and oxidation on mechanical properties of sea cucumber (Stichopus japonicus) during thermal processing and storage and their control. Food Chem. 2020, 330, 127248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Chang, X.N.; Bao, S.S.; Song, L.; Zhu, B.W.; Dong, X.P. Purification and partial characterisation of a cathepsin L-like proteinase from sea cucumber (Stichopus japonicus) and its tissue distribution in body wall. Food Chem. 2014, 158, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Gianasi, B.L.; Hamel, J.F.; Mercier, A. Experimental test of optimal holding conditions for live transport of temperate sea cucumbers. Fish. Res. 2016, 174, 298–308. [Google Scholar] [CrossRef]
- Ji, H.M.; Yoon, W.B. Size dependence of the salting process for dry salted sea cucumber (Stichopus japonicus). J. Food Eng. 2015, 170, 170–178. [Google Scholar]
- Matser, A.M.; Krebbers, B.; Berg, R.W.; Bartels, P.V. Advantages of high pressure sterilisation on quality of food products. Trends Food Sci. Technol. 2004, 15, 79–85. [Google Scholar] [CrossRef]
- Qian, S.Y.; Li, X.; Wang, H.; Mehmood, W.; Zhong, M.; Zhang, C.H.; Blecker, C. Effects of low voltage electrostatic field thawing on the changes in physicochemical properties of myofibrillar proteins of bovine Longissimus dorsi muscle. J. Food Eng. 2019, 261, 140–149. [Google Scholar] [CrossRef]
- Okamoto, A.A.; Suzuki, B.A. Effects of high hydrostatic pressure-thawing on pork meat. Biotechnol. Prog. 2002, 19, 571–576. [Google Scholar]
- Li, F.F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.W.; Xia, X.F. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef]
- Choi, E.J.; Park, H.W.; Chung, Y.B.; Park, S.H.; Kim, J.S.; Chun, H.H. Effect of tempering methods on quality changes of pork loin frozen by cryogenic immersion. Meat Sci. 2017, 124, 69–76. [Google Scholar] [CrossRef]
- Shi, Z.J.; Zhong, S.Y.; Yan, W.J.; Liu, M.; Yang, Z.; Qiao, X.L. The effects of ultrasonic treatment on the freezing rate, physicochemical quality, and microstructure of the back muscle of grass carp (Ctenopharyngodon idella). Food Sci. Technol. 2019, 111, 301–308. [Google Scholar] [CrossRef]
- Wang, X.S.; Xie, J. Effects of different thawing methods on the quality of frozen horse mackerel. Food Sci. 2020, 41, 137–143. [Google Scholar]
- Gambuteanu, C.; Alexe, P. Comparison of thawing assisted by low-intensity ultrasound on technological properties of pork Longissimus dorsi muscle. J. Food Sci. Technol. 2015, 52, 2130–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.; Zhang, H.; Sun, J.; Qian, L. Study on Nutritional Value of Instant Sea Cucumber Processed by the New Processing Approach. Am. J. Biochem. Biotechnol. 2017, 13, 51–57. [Google Scholar] [CrossRef]
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018, 109, 65–71. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Wang, H.L.; Zheng, Y.; Qiu, Z.H.; Wang, X.C. Effects of Ultrasound-Assisted Vacuum Impregnation Antifreeze Protein on the Water-Holding Capacity and Texture Properties of the Yesso Scallop Adductor Muscle during Freeze–Thaw Cycles. Foods 2022, 11, 320. [Google Scholar] [CrossRef]
- Torres, J.A.; Saraiva, J.A.; Guerra-Rodríguez, E.; Aubourg, S.P.; Vázquez, M. Effect of combining high-pressure processing and frozen storage on the functional and sensory properties of horse mackerel (Trachurus trachurus). Innov. Food Sci. Emerg. Technol. 2014, 21, 2–11. [Google Scholar] [CrossRef]
- Bi, J.; Yong, L.; Cheng, S.; Dong, X.; Kamal, T.; Zhou, D. Changes in Body Wall of Sea Cucumber ( Stichopus japonicus ) during a two-Step Heating Process Assessed by Rheology, LF-NMR, and Texture Profile Analysis. Food Biophys. 2016, 11, 257–265. [Google Scholar] [CrossRef]
- Xiong, X.; Xie, W.; Xie, J.; Qi, H.; Dong, X. Protein oxidation results in textural changes in sea cucumber (Apostichopus japonicus) during tenderization. LWT 2021, 144, 111231. [Google Scholar] [CrossRef]
- Dong, X.; Yang, X.; Li, H.; Che, H.; Qi, H. Effects of oxidation on the structure of collagen fibers of sea cucumber (Apostichopus japonicus) body wall during thermal processing. LWT 2020, 138, 110528. [Google Scholar] [CrossRef]
- Kong, J.; Shaoning, Y.U. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2010, 39, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, Y.; Zhao, X.; Wan, W. Effects of different ultrasound powers on the structure and stability of protein from sea cucumber gonad. LWT 2020, 137, 110403. [Google Scholar] [CrossRef]
- Po, H.I.; Wen, C.L.; Yung, J.C.; Wen, C.K. Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture 2019, 515, 734590. [Google Scholar]
- Liu, Z.Q.; Liu, Y.X.; Zhou, D.Y.; Liu, X.Y.; Dong, X.P.; Li, D.M.; Shahidi, F. The role of matrix metalloprotease (MMP) to the autolysis of sea cucumber (Stichopus japonicus). J. Sci. Food Agric. 2019, 99, 5752–5759. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.; Zhang, Z.; Sun, D.W. Experimental analysis and modeling of ultrasound assisted freezing of potato spheres. Ultrason. Sonochem. 2015, 26, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cheng, T.; Wang, J. Effect of Ultrasonic Thawing on the Quality Characteristics of Frozen Pork. Food Res. 2017, 31, 14–19. [Google Scholar]
- Miles, C.A.; Morley, M.J.; Rendell, M. High power ultrasonic thawing of frozen foods. J. Food Eng. 1999, 39, 151–159. [Google Scholar] [CrossRef]
- Ma, L.K.; Zhang, B.; Deng, S.G.; Xie, C. Comparison of the Cryoprotective Effects of Trehalose, Alginate, and Its Oligosaccharides on Peeled Shrimp (Litopenaeus Vannamei) During Frozen Storage. J. Food Sci. 2015, 80, 540–546. [Google Scholar] [CrossRef]
- Kong, C.H.Z.; Hamid, N.; Ma, Q.; Lu, J.; Wang, B.; Sarojini, V. Antifreeze peptide pretreatment minimizes freeze-thaw damage to cherries: An in-depth investigation. LWT 2017, 84, 441–448. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi-ScienceDirect. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, K.; Ye, T.; Wan, S.; Wang, Y.; Dan, W. Influence of konjac glucomannan on gelling properties and water state in egg white protein gel. Food Res. Int. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Ducel, V.; Pouliquen, D.; Richard, J.; Boury, F. 1H NMR relaxation studies of protein-polysaccharide mixtures. J. Biol. Macromol. 2008, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, F.; Sun, D.W.; Zhao, M. Effect of Oxidation on the Emulsifying Properties of Myofibrillar Proteins. Food Bioproc. Tech. 2013, 6, 1703–1712. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, W.; Cao, A. Effects of ultrasonics combined with far infrared or microwave thawing on protein denaturation and moisture migration of Sciaenops ocellatus (red drum). Ultrason. Sonochem. 2019, 55, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Renou, J.P.; Foucat, L.; Bonny, J.M. Magnetic resonance imaging studies of water interactions in meat. Food Chem. 2003, 82, 35–39. [Google Scholar] [CrossRef]
- Alakhrash, F.; Anyanwu, U.; Tahergorabi, R. Physicochemical properties of Alaska pollock (Theragra chalcograma) surimi gels with oat bran. LWT 2016, 66, 41–47. [Google Scholar] [CrossRef]
- Mousakhani, G.A.; Hamdami, N.; Soltanizadeh, N. Effect of high voltage electrostatic field thawing on the lipid oxidation of frozen tuna fish (Thunnus albacares). Innov. Food Sci. Emerg. Technol. 2016, 156, 42–47. [Google Scholar] [CrossRef]
- Wu, Q.; Li, D.; Pan, J.; Wang, N.; Dong, X. Effect of cooking methods on the processing characteristics of muscle from turbot (Scophthalmus maximus). Zhongguo Shipin Xuebao 2016, 16, 129–135. [Google Scholar]
- Zhu, B.W.; Zhao, L.L.; Sun, L.M.; Dong, L.I.; Murata, Y.; Lei, Y.U. Purification and Characterization of a Cathepsin L-Like Enzyme from the Body Wall of the Sea Cucumber Stichopus japonicus. Biosci. Biotechnol. Biochem. 2008, 72, 1430–1437. [Google Scholar] [CrossRef]
- El-Garawany, G.A.; Salam, M. Preparation and rheological properties of a dairy dessert based on whey protein/potato starch. Food Chem. 2005, 91, 261–267. [Google Scholar] [CrossRef]
- Rabin, Y.; Taylor, M.J.; Wolmark, N. Thermal expansion measurements of frozen biological tissues at cryogenic temperatures. J. Biomech. Eng. 1998, 120, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Xue, Y.; Dong, J.; Li, Z.J.; Xue, C.H. Effect of two plant extracts on the stability of the body wall collagen in instant sea cucumber. Xiandai Shipin Keji 2015, 11, 113–119. [Google Scholar]
- Kamińska, A.; Sionkowska, A. Effect of UV radiation on the infrared spectra of collagen. Polym. Degrad. Stab. 1996, 51, 19–26. [Google Scholar] [CrossRef]
- Min, Z.; Liu, W.; Li, G. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar]
- Berisio, R.; Vitagliano, L.; Mazzarella, L.; Zagari, A. Crystal structure of the collagen triple helix model [(Pro-Pro-Gly)(10)](3). Protein Sci. 2002, 11, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Brauner, J.W.; Mendelsohn, R. A Quantitative Reconstruction of the Amide I Contour in the IR Spectra of Peptides and Proteins: From Structure to Spectrum. J. Am. Chem. Soc. 2005, 127, 100–109. [Google Scholar] [CrossRef]
- Hao, H.; Wu, J.; Li, E.; Le, Z.; Fang, Z.; Xu, X. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar]
- Liang, M.; Cai, L.; Yi, L.; Li, J. Effect of combined ultrasonic and alkali pretreatment on enzymatic preparation of angiotensin converting enzyme (ACE) inhibitory peptides from native collagenous materials. Ultrason. Sonochem. 2017, 36, 88–94. [Google Scholar]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Zhang, M.M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, P.P.; Zhang, K.; Wang, L.; Zhang, M.H.; Sun, Z.L. Effects of ultrasound-assisted alkaline extraction on the physiochemical and functional characteristics of chicken liver protein isolate. Poult. Sci. 2017, 96, 2975–2985. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Wang, L.; Shao, J.; Liu, D.; Kong, B. Changes in the structural and gel properties of pork myofibrillar protein induced by catechin modification. Meat Sci. 2017, 127, 45–50. [Google Scholar]
- Yu, L.; He, Z.; Li, H.; Wang, Z.M.; Huang, H.; Xu, M.; Wang, S. Effect of Different Thawing Methods on Quality Characteristics of Hyra Rabbit Meat. J. Food Sci. 2015, 36, 258–264. [Google Scholar]
- Xia, X.; Kong, B.; Jing, L.; Diao, X.; Qian, L. Influence of different thawing methods on physicochemical changes and protein oxidation of porcine longissimus muscle. LWT 2012, 46, 280–286. [Google Scholar] [CrossRef]
- Sun, Q.X.; Sun, F.D.; Xia, X.F.; Xu, H.H.; Kong, B.H. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019, 51, 281–291. [Google Scholar] [CrossRef]


| Thawing Methods | Thawing Loss (%) | Cooking Loss (%) | Water-Holding Capacity (%) |
|---|---|---|---|
| RT | 17.11 ± 1.30 c | 9.59 ± 1.03 b | 74.87 ± 0.60 a |
| AT | 22.68 ± 1.00 a | 13.44 ± 0.64 a | 65.42 ± 2.82 c |
| WT | 21.81 ± 1.73 ab | 13.24 ± 0.50 a | 69.56 ± 1.52 b |
| UT | 20.15 ± 0.73 b | 10.90 ± 0.00 b | 70.84 ± 0.88 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, X.; Wang, H.; Yin, M.; Wang, X. Effect of Different Thawing Methods on the Physicochemical Properties and Microstructure of Frozen Instant Sea Cucumber. Foods 2022, 11, 2616. https://doi.org/10.3390/foods11172616
Ge X, Wang H, Yin M, Wang X. Effect of Different Thawing Methods on the Physicochemical Properties and Microstructure of Frozen Instant Sea Cucumber. Foods. 2022; 11(17):2616. https://doi.org/10.3390/foods11172616
Chicago/Turabian StyleGe, Xiaotong, Hongli Wang, Mingyu Yin, and Xichang Wang. 2022. "Effect of Different Thawing Methods on the Physicochemical Properties and Microstructure of Frozen Instant Sea Cucumber" Foods 11, no. 17: 2616. https://doi.org/10.3390/foods11172616






