Characterization of Romanian Bee Pollen—An Important Nutritional Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bee Pollen Samples
2.2. Materials
2.3. Methods
2.3.1. Palynological Analysis
2.3.2. Determination of Routine Physicochemical Parameters: Moisture Content, Water Activity, pH and Free Acidity
- Moisture content
- Water activity
- pH and free acidity
2.3.3. Proximate Composition of Bee Pollen
2.3.4. Determination of Organic Acids
2.3.5. Determination of Free Sugars
2.3.6. Bee Pollen Extracts for the Determination of Phenolic Compounds
2.3.7. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.3.8. Determination of Individual Phenolic Compounds
2.3.9. Determination of Total Free Amino Acids
2.3.10. Fatty Acids Determination Using GC-MS
2.3.11. Determination of FT-IR
2.4. Statistical Analysis
3. Results and Discussion
3.1. Botanical Origin of the Bee Pollen
3.2. Routine Physicochemical Parameters: Moisture Content, Water Activity, pH and Free Aciditiy
3.3. Proximate Composition of Bee Pollen
3.4. Free Sugars of Bee Pollen
3.5. Organic Acids of Bee Pollen
3.6. TPC, TFC and Individual Phenolics Compounds
3.7. Total Free Amino Acid Composition
3.8. Fatty Acids Composition
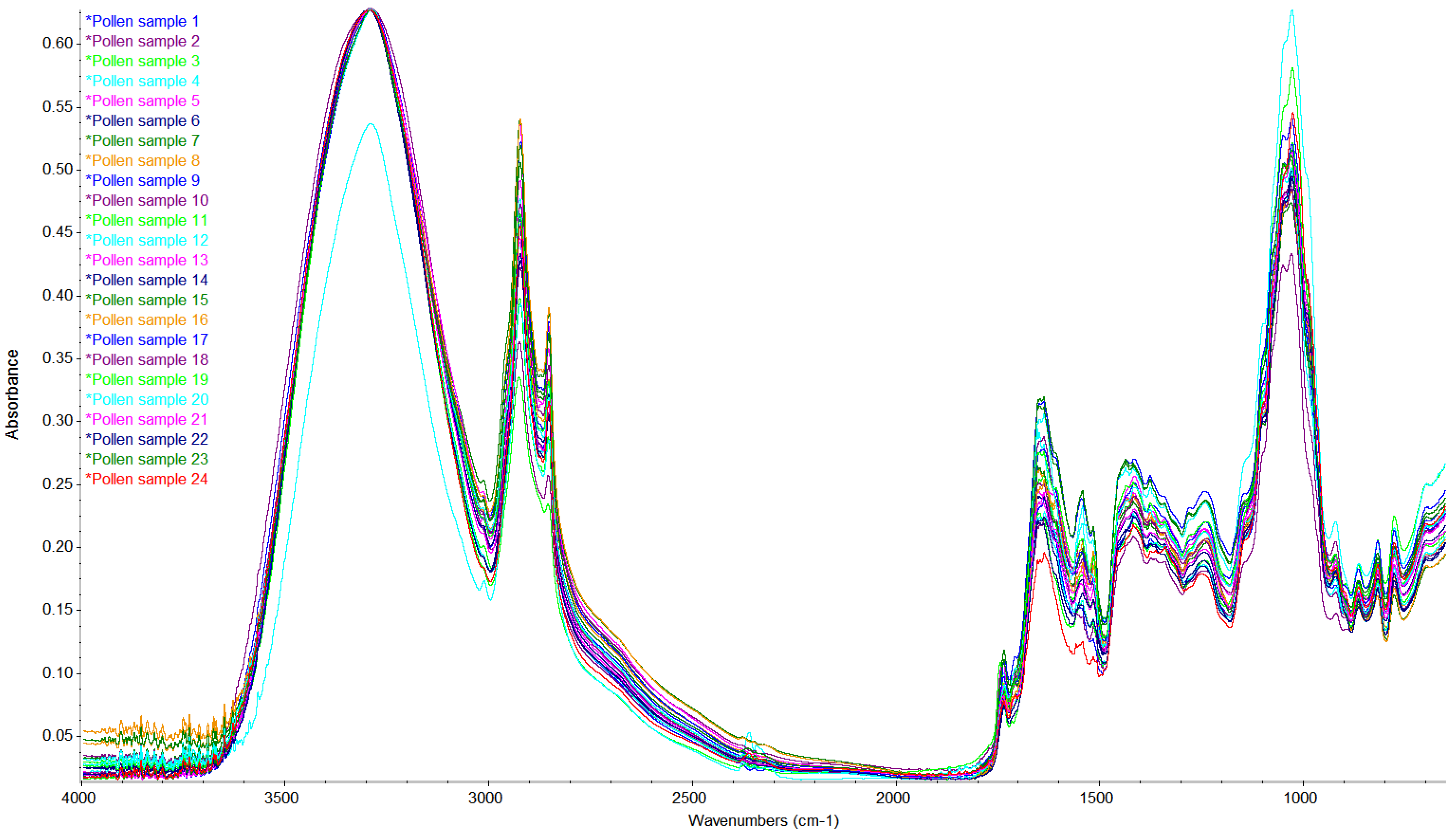
3.9. FTIR-ATR Spectroscopy of Pollen
3.10. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Di Chiacchio, I.M.; Paiva, I.M.; de Abreu, D.J.M.; Carvalho, E.E.N.; Martínez, P.J.; Carvalho, S.M.; Mulero, V.; Murgas, L.D.S. Bee pollen as a dietary supplement for fish: Effect on the reproductive performance of zebrafish and the immunological response of their offspring. Fish Shellfish Immunol. 2021, 119, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Bayram, N.E.; Gercek, Y.C.; Çelik, S.; Mayda, N.; Kostić, A.; Dramićanin, A.M.; Özkök, A. Phenolic and free amino acid profiles of bee bread and bee pollen with the same botanical origin—Similarities and differences. Arab. J. Chem. 2021, 14, 103004. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and polyfloral bee pollens: Comparative evaluation of their phenolics and bioactivity profiles. LWT 2021, 142, 110973. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar]
- Kostić, A.; Milinčić, D.D.; Barać, M.B.; Shariati, M.A.; Tešić, Ž.L.; Pešić, M.B. The application of pollen as a functional food and feed ingredient—The present and perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef]
- Denisow, B.; Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: A review. J. Sci. Food Agric. 2016, 96, 4303–4309. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee collected pollen and bee bread: Bioactive constituents and health benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef]
- Feas, X.; Vazquez-Tato, M.P.; Estevinho, L.; Seijas, J.A.; Iglesias, A. Organic bee pollen: Botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules 2012, 17, 8359–8377. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Ecem Bayram, N.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Screening of Indian bee pollen based on antioxidant properties and polyphenolic composition using UHPLC-DAD-MS/MS: A multivariate analysis and ANN based approach. Food Res. Int. 2021, 140, 110041. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Polak, T.; Pucihar, T.; Lilek, N.; Kandolf Borovšak, A.; Korošec, M. Carbohydrate composition of Slovenian bee pollens. Int. J. Food Sci. Technol. 2018, 53, 1880–1888. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Hang, X.; Wang, H.; Yang, W.; Xu, B. Nutritional effect of alpha-linolenic acid on honey bee colony development (Apis mellifera L.). J. Apic. Sci. 2015, 59, 63–72. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E.; Iriti, M. Polyphenols from Bee Pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Themelis, T.; Gotti, R.; Orlandini, S.; Gatti, R. Quantitative amino acids profile of monofloral bee pollens by microwave hydrolysis and fluorimetric high performance liquid chromatography. J. Pharm. Biomed. Anal. 2019, 173, 144–153. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Human, H. Chemical composition of the “low quality” pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-assisted extraction of polyphenols from crude pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef]
- Urcan, A.C.; Criste, A.D.; Dezmirean, D.S.; Bobiș, O.; Bonta, V.; Dulf, F.V.; Mărgăoan, R.; Cornea-Cipcigan, M.; Campos, M.G. Botanical origin approach for a better understanding of chemical and nutritional composition of beebread as an important value-added food supplement. LWT 2021, 142, 111068. [Google Scholar] [CrossRef]
- Fatrcová-Šramková, K.; Nôžková, J.; Máriássyová, M.; Kačániová, M. Biologically active antimicrobial and antioxidant substances in the Helianthus annuus L. bee pollen. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2016, 51, 176–181. [Google Scholar] [CrossRef]
- Kostić, A.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT 2019, 112, 108244. [Google Scholar] [CrossRef]
- Han, L.; Liu, X.; Yang, N.; Li, J.; Cai, B.; Cheng, S. Simultaneous chromatographic fingerprinting and quantitative analysis of flavonoids in Pollen Typhae by high-performance capillary electrophoresis. Acta Pharm. Sin. B 2012, 2, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Gardana, C.; Del Bo, C.; Quicazán, M.C.; Corrrea, A.R.; Simonetti, P. Nutrients, phytochemicals and botanical origin of commercial bee pollen from different geographical areas. J. Food Compos. Anal. 2018, 29, 29–38. [Google Scholar] [CrossRef]
- Arien, Y.; Dag, A.; Yona, S.; Tietel, Z.; Lapidot Cohen, T.; Shafir, S. Effect of diet lipids and omega-6:3 ratio on honey bee brood development, adult survival and body composition. J. Insect Physiol. 2020, 124, 104074. [Google Scholar] [CrossRef] [PubMed]
- Arien, Y.; Dag, A.; Zarchin, S.; Masci, T.; Shafir, S. Omega-3 deficiency impairs honey bee learning. Proc. Natl. Acad. Sci. USA 2015, 112, 15761–15766. [Google Scholar] [CrossRef]
- Hsu, P.S.; Wu, T.H.; Huang, M.Y.; Wang, D.Y.; Wu, M.C. Nutritive value of 11 bee pollen samples from major floral sources in taiwan. Foods 2021, 10, 2229. [Google Scholar] [CrossRef]
- Kostić, A.; Mačukanović-Jocić, M.P.; Špirović Trifunović, B.D.; Vukašinović, I.; Pavlović, V.B.; Pešić, M.B. Fatty acids of maize pollen—Quantification, nutritional and morphological evaluation. J. Cereal Sci. 2017, 77, 180–185. [Google Scholar] [CrossRef]
- Thakur, M.; Na, V. Assessment of physico-chemical properties, fatty acid, amino acid and mineral profile of bee pollen from India with a multivariate perspective. J. Food Nutr. Res. 2018, 57, 328–340. [Google Scholar]
- Manning, R. Fatty acids in pollen: A review of their importance for honey bees. Bee World 2001, 82, 60–75. [Google Scholar]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar]
- Szczesna, T.; Rybak-Chmielewska, H.; Chmielewski, W. Sugar composition of pollen loads harvested at different periods of the beekeeping season. J. Apic. Sci. 2002, 46, 107–115. [Google Scholar]
- Antonelli, M.; Donelli, D.; Firenzuoli, F. Therapeutic efficacy of orally administered pollen for nonallergic diseases: An umbrella review. Phytother. Res. 2019, 33, 2938–2947. [Google Scholar]
- Li, Q.Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.F.; Wu, L.M.; Hu, F.L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Gaithersbg, MD, USA, 2016. [Google Scholar]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Dranca, F.; Ursachi, F.; Oroian, M. Bee bread: Physicochemical characterization and phenolic content extraction optimization. Foods 2020, 9, 1358. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Gatzias, I.; Riganakos, K.A. Bio-functional properties of bee pollen: The case of “bee pollen yoghurt”. Coatings 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Fuenmayor, B.C.; Zuluaga, D.C.; Díaz, M.C.; Quicazán de C, M.; Cosio, M.; Mannino, S. Evaluation of the physicochemical and functional properties of Colombian bee pollen. Rev. MVZ Córdoba 2014, 19, 4003–4014. [Google Scholar] [CrossRef]
- Martins, M.C.T.; Morgano, M.A.; Vicente, E.; Baggio, S.R.; Rodriguez-Amaya, D.B. Physicochemical composition of bee pollen from eleven Brazilian states. J. Apic. Sci. 2011, 55, 107–116. [Google Scholar]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef]
- Mǎrgǎoan, R.; Al Mǎrghitaş, L.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiş, O. Predominant and secondary pollen botanical origins influence the carotenoid and fatty acid profile in fresh honeybee-collected pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Goras, G.; Karazafiris, E.; Thrasyvoulou, A. Ranking pollen from bee plants according to their protein contribution to honey bees. J. Apic. Res. 2015, 54, 582–592. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Jordà, R.E. Nutrient Composition and Microbiological Quality of Honeybee-Collected Pollen in Spain. J. Agric. Food Chem. 1997, 45, 725–732. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kaygusuz, H.; Döker, S.; Kolaylı, S.; Erim, F.B. Characterization of Turkish honeybee pollens by principal component analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT-Food Sci. Technol. 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Olofsson, T.C.; Vásquez, A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr. Microbiol. 2008, 57, 356–363. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; de Freitas, A.d.S.; Barth, O.M.; de Almeida-Muradian, L.B. Phenolic profile by HPLC-MS, biological potential, and nutritional value of a promising food: Monofloral bee pollen. J. Food Biochem. 2018, 42, e12536. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; de Freitas, A.d.S.; Barth, O.M.; de Almeida-Muradian, L.B. A multivariate approach based on physicochemical parameters and biological potential for the botanical and geographical discrimination of Brazilian bee pollen. Food Biosci. 2018, 25, 91–110. [Google Scholar] [CrossRef]
- Taha, E.K.A.; Al-Kahtani, S.; Taha, R. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci. 2019, 26, 232–237. [Google Scholar] [CrossRef]
- Al-Kahtani, S.N.; Taha, E.K.A.; Farag, S.A.; Taha, R.A.; Abdou, E.A.; Mahfouz, H.M. Harvest Season Significantly Influences the Fatty Acid Composition of Bee Pollen. Biology 2021, 10, 495. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Özkök, A.; Keskin, Ş.; Mayda, N.; Urcan, A.C.; Cornea-Cipcigan, M. Bee collected pollen as a value-added product rich in bioactive compounds and unsaturated fatty acids: A comparative study from Turkey and Romania. LWT 2021, 149, 111925. [Google Scholar] [CrossRef]
- Depciuch, J.; Kasprzyk, I.; Drzymała, E.; Parlinska-Wojtan, M. Identification of birch pollen species using FTIR spectroscopy. Aerobiologia 2018, 34, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Anjos, O.; Santos, A.J.A.; Dias, T.; Estevinho, L.M. Application of FTIR-ATR spectroscopy on the bee pollen characterization. J. Apic. Res. 2017, 56, 210–218. [Google Scholar] [CrossRef]
- Bouknana, D.; Hammouti, B.; Jodeh, S.; Sbaa, M.; Lgaz, H. Extracts of olive inflorescence flower pre-anthesis, at anthesis and grain pollen as eco-friendly corrosion inhibitor for steel in 1M HCl medium. Anal. Bioanal. Electrochem. 2018, 10, 751–777. [Google Scholar]
- Kenđel, A.; Zimmermann, B. Chemical analysis of pollen by FT-Raman and FTIR spectroscopies. Front. Plant Sci. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, I.; Depciuch, J.; Grabek-Lejko, D.; Parlinska-Wojtan, M. FTIR-ATR spectroscopy of pollen and honey as a tool for unifloral honey authentication. The case study of rape honey. Food Control 2018, 84, 33–40. [Google Scholar] [CrossRef]
- Subari, N.; Mohamad Saleh, J.; Md Shakaff, A.; Zakaria, A. A Hybrid Sensing Approach for Pure and Adulterated Honey Classification. Sensors 2012, 12, 14022–14040. [Google Scholar] [CrossRef]


| Sample Code | Dominant Pollen (>45%) | Secondary Pollen (16–44%) | Important Minor Pollen (4–15%) | Minor Pollen (<3%) |
|---|---|---|---|---|
| S1 | Helianthus annuus | - | Robinia pseudoacacia Zea mays Tilia spp. Fagus sylvatica | Quercus spp. Betulus pendula |
| S2 | Pinaceae spp. | - | Corylus spp. Taraxacum spp. Vicia spp. Helianthus annuus | Carduus spp. Ambrosia spp. |
| S3 | Helianthus annuus | - | Robinia pseudoacacia Tilia spp. | Conicum spp. Vicia spp. Pruunus spinosa |
| S4 | Tillia spp. | Allium spp. Helianthus annuus Asteraceae spp. | Brassicaceae spp. Fagopyrum spp. | |
| S5 | - | - | Helianthus annuus Taraxacum spp. Quercus spp. Zea mays | Prunus spp. |
| S6 | - | - | Trifolium spp. Robinia pseudoacacia Helianthus annuus Poaceae spp. Vicia spp. | Fabaceae spp. Conicum spp. Fagus spp. Ulmu spp. |
| S7 | Robinia pseudoacacia | - | Asteraceae spp. Urtisaceae spp. | Rosaceae spp. Prunus spp. |
| S8 | Quercus spp. | - | Pinaceae spp. | Castaneae spp. |
| S9 | Robinia pseudoacacia | - | Cucumber spp. Castaneae spp. Oleaceae spp. | Rosaceae spp. Prunus spp. |
| S10 | - | - | Trifolium spp. Robinia pseudoacacia Urticaceae spp. Castanea spp. | Quercus spp. |
| S11 | Zea mays | Trifolium spp. | Quercus spp. | |
| S12 | Helianthus annuus | Robinia pseudoacacia | - | Vicia spp. Pruunus spinosa Tilia spp. |
| S13 | Crataegus monogyna | - | Helianthus annuus Taraxacum spp Quercus spp. | Robinia pseudoacacia |
| S14 | Helianthus annuus | Tilia spp. | Asteraceae spp. | Taraxacum spp. |
| S15 | Taraxacum spp. | - | Plantago spp. Quercus spp. | |
| S16 | Quercus spp. | - | Taraxacum spp. Castaneae spp. | Ambrosia spp. Helianthus annuus |
| S17 | Robinia pseudoacacia | Helianthus annuus | Taraxacum spp. Castaneae spp. | Tilia spp. |
| S18 | Robinia pseudoacacia | Helianthus annuus | Tilia spp. | Taraxacum spp. Castaneae spp. |
| S19 | Prunus spp. | - | Taraxacum spp. Quercus spp. | Robinia pseudoacacia Helianthus annuus Teucrium spp. |
| S20 | Pinaceae spp. | - | Fagaceae spp. | Prunus spp. Asteraceae spp. Gramineae spp. |
| S21 | - | - | Zea mays Myrcia spp. Helianthus annuus Fireweed spp. Mimosa spp. | Salix spp. Taraxacum spp. |
| S22 | Prunus spp. | Cucumber spp. | Quercus spp. | |
| S23 | Robinia pseudoacacia | - | Salicaceae spp. Sophora spp. Poaceae spp. | Humulus spp. Salicaceae spp. Allium spp. |
| S24 | Helianthus annuus | - | Robinia pseudoacacia Tilia spp. | Quercus spp. Vicia spp. Pruunus spinosa |
| Parameter | Crataegus monogyna | Helianthus annuus | Pinaceae spp. | Polyfloral | Prunus spp. | Quercus spp. | Robinia pseudoacacia | Taraxacum spp. | Tillia spp. | Zea mays | F-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 4.57 (0.07) a,b,c | 4.36 (0.27) a,b | 4.12 (0.30) a | 4.85 (0.23) a,b,c | 5.19 (0.69) c | 4.69 (0.14) a,b,c | 4.24 (0.23) a,b | 4.96 (0.07) b,c | 4.85 (0.07) a,b,c | 4.53 (0.06) a,b,c | 6.4 *** |
| Free acidity (meq/kg) | 233.4 (3.3) a,b | 187.7 (33.0) a | 272.79 (26.90) b | 179.6 (16.5) a | 199.3 (87.8) a,b | 252.0 (59.8) b | 249.13 (24.41) b | 214.2 (3.0) a,b | 194.0 (2.7) a,b | 127.5 (1.8) a | 5.2 *** |
| Moisture content (%) | 4.92 (0.07) a,b | 5.07 (1.62) a,b | 8.84 (3.40) b | 4.74 (1.12) a | 4.86 (2.05) a,b | 4.89 (1.16) a,b | 4.34 (0.57) a | 3.32 (0.05) a | 4.50 (0.06) a | 2.90 (0.04) a | 3.8 ** |
| aw | 0.27 (0.01) a | 0.30 (0.10) a,b | 0.48 (0.08) b | 0.27 (0.06) a | 0.24 (0.08) a | 0.29 (0.09) a,b | 0.28 (0.05) a | 0.17 (0.01) a | 0.28 (0.01) a | 0.20 (0.01) a | 4.2 *** |
| Protein. d.m. (%) | 22.66 (0.32) b,c,d | 18.03 (1.05) a,b | 23.09 (2.48) c,d | 23.06 (1.35) c,d | 24.54 (1.40) c,d | 26.86 (0.95) d | 23.13 (2.82) c,d | 23.71 (0.34) c,d | 21.14 (0.30) b,c | 15.58 (0.22) a | 13.7 *** |
| Lipids d.m. (%) | 2.62 (0.04) a,b | 3.72 (1.11) a,b | 6.05 (1.36) c | 4.31 (1.01) a,b | 5.25 (0.43) b,c | 3.28 (0.87) a,b | 6.02 (0.89) c | 3.51 (0.05) a,b | 3.30 (0.05) a,b | 2.22 (0.03) a | 8.7 *** |
| Ash (%) | 3.23 (0.05) b,c | 2.57 (0.14) a | 3.16 (0.45) b | 3.30 (0.23) b,c | 3.50 (0.13) b,c | 3.83 (0.18) e | 3.32 (0.40) b,c | 3.44 (0.05) b,c | 3.03 (0.04) a,b | 2.27 (0.03) a | 12.4 *** |
| Energy kcal/100 g | 377.0 (5.3) a | 384.8 (8.0) a | 380.30 (18.77) a | 386.2 (5.6) a | 389.9 (7.5) a,b | 378.1 (4.4) a | 396.49 (7.49) b | 387.0 (5.5) a,b | 382.9 (5.4) a | 386.6 (5.3) a,b | 2.6 * |
| Fructose d.m. (%) | 18.82 (0.27) a,b | 19.49 (1.54) a,b,c | 20.00 (1.11) a,b,c | 19.59 (0.64) a,b,c | 21.31 (3.03) c | 20.08 (0.25) a,b,c | 18.46 (0.98) a | 19.44 (0.28) a,b,c | 21.44 (0.31) c | 20.68 (0.30) b,c | 2.4 * |
| Glucose d.m. (%) | 12.78 (0.18) a,b | 14.50 (2.75) a,b | 9.49 (1.03) a | 14.91 (1.78) a,b | 16.48 (5.47) b | 14.52 (0.21) a,b | 11.53 (1.25) a,b | 17.19 (0.25) b | 16.19 (0.23) b | 17.40 (0.25) b | 4.9 *** |
| Sucrose d.m. (%) | 0.13 (0.01) a | 0.48 (0.33) a | 0.73 (0.11) a,b | 0.79 (0.22) a,b | 0.43 (0.29) a | 0.34 (0.39) a | 1.23 (0.43) b | 0.23 (0.01) a | 1.45 (0.02) b | 0.84 (0.01) a,b | 7.2 *** |
| Turanose d.m. (%) | 0 (0) a | 0.10 (0.13) a | 0 (0) a | 0.08 (0.14) a | 0 (0) a | 0.07 (0.09) a | 0.05 (0.01) | 0 (0) a | 0(0) a | 0(0) a | 0.6 ns |
| Maltose d.m. (%) | 0.21 (0.01) a | 0.30 (0.32) a | 0.77 (0.07) | 0.61 (0.14) a | 0.45 (0.09) a | 1.06 (0.97) a | 0.58 (0.25) | 0.46 (0.01) a | 0.92 (0.01) a | 0.50 (0.01) a | 1.3 ns |
| Trehalose d.m. (%) | 0.37 (0.01) a | 1.14 (0.81) a | 1.21 (0.36) | 1.13 (0.04) a | 1.93 (1.27) a | 1.16 (0.60) a | 0.85 (0.23) | 0.96 (0.01) a | 1.03 (0.01) a | 1.03 (0.01) a | 1.5 ns |
| Melesitose d.m. (%) | 2.99 (0.04) a | 2.13 (0.31) a | 0.90 (0.84) | 1.21 (1.30) a | 2.41 (0.73) a | 1.86 (2.14) a | 1.56 (0.89) | 2.29 (0.03) a | 2.60 (0.04) a | 1.53 (0.02) a | 1.7 ns |
| Raffinose d.m. (%) | 0.16 (0.01) a,b | 0.09 (0.09) a | 0.29 (0.19) a,b,c,d | 0.22 (0.14) a,b,c | 0.20 (0.20) a,b,c | 0.56 (0.57) d | 0.07 (0.06) a | 0.44 (0.01) b,c,d | 0.50 (0.01) c,d | 0.06 (0.01) a | 3.4 ** |
| F/G | 1.46 (0.02) b,c | 1.37 (0.23) a,b,c | 2.11 (0.34) d | 1.31 (0.11) a,b,c | 1.35 (0.26) a,b,c | 1.37 (0.02) a,b,c | 1.60 (0.15) c | 1.12 (0.02) a | 1.31 (0.02) a,b,c | 1.18 (0.02) a,b | 8.4 *** |
| Crataegus monogyna | Helianthus annuus | Pinaceae spp. | Polyfloral | Prunus spp. | Quercus spp. | Robinia pseudoacacia | Taraxacum spp. | Tillia spp. | Zea mays | F-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gluconic acid (g/kg) | 33.78 (0.48) a,b,c | 21.68 (1.05) a,b | 34.47 (0.60) b,c | 30.00 (5.04) a,b,c | 29.37 (13.89) a,b,c | 22.22 (4.48) a,b | 36.33 (7.98) c | 24.88 (0.35) a,b,c | 26.24 (0.37) a,b,c | 14.03 (0.20) a | 4.9 *** |
| Lactic acid (g/kg) | 0.67 (0.01) a,b | 0.61 (0.16) a | 1.10 (0.01) b | 0.69 (0.13) a,b | 0.74 (0.41) a,b | 0.67 (0.10) a,b | 0.76 (0.13) a,b | 0.54 (0.01) a | 0.77 (0.01) a,b | 0.47 (0.01) a | 3.8 ** |
| Acetic acid (g/kg) | 0.26 (0.01) a | 0.61 (0.23) a,b | 1.20 (0.01) c | 0.44 (0.09) a,b | 0.71 (0.52) b | 0.28 (0.14) a,b | 0.49 (0.41) a,b | 0.28 (0.01) a,b | 0.58 (0.01) a,b | 0.28 (0.01) a,b | 10.4 *** |
| Propionic acid (g/kg) | 0.43 (0.01) a | 0.11 (0.23) a | 0 (0) a | 0.05 (0.09) a | 0.45 (0.52) a | 0.29 (0.14) a | 0.37 (0.31) a | 0.04 (0.01) a | 0.26 (0.01) a | 0.13 (0.01) a | 1.6 ns |
| TPC (GAE mg/g) | 8.73 (0.12) a,b,c | 7.56 (3.02) a,b | 12.39 (0.17) a,b,c,d | 13.53 (2.16) b,c,d | 15.74 (2.33) d | 15.52 (1.17) d | 14.11 (3.08) c,d | 16.45 (0.24) d | 14.83 (0.21) c,d | 7.10 (0.10) a | 9.3 *** |
| TFC (QE mg/g) | 8.55 (0.12) a,b,c | 5.95 (1.04) a | 11.03 (0.16) b,c,d | 13.97 (1.57) d,e,f | 17.37 (3.33) f | 15.68 (0.42) e,f | 18.81 (2.19) c,d,e | 16.39 (0.23) e,f | 14.79 (0.21) d,e,f | 6.28 (0.09) a,b | 29.1 *** |
| P-A | 0 (0) a | 0 (0) a | 88.93 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 3.8 *** |
| p-H-A | 0 (0) b | 0 (0) b | 21.02 (0) a | 0 (0) b | 0 (0) b | 0 (0) b | 0 (0) b | 0 (0) b | 0 (0) b | 0 (0) b | 3.9 *** |
| V-A (mg/kg) | 0 (0) a | 21.19 (1.20) b | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 4.1 *** |
| C-A | 0 (0) a | 0.78 (0.10) a | 0 (0) a | 3.82 (1.08) a | 0 (0) a | 0 (0) a | 3.63 (4.90) a | 0 (0) a | 0 (0) a | 0 (0) a | 0.8 ns |
| p-C-A (mg/kg) | 2.92 (0.04) a | 24.75 (17.36) a | 139.79 (3.82) a,b | 79.26 (66.99) a | 92.74 (49.07) a | 27.78 (0.33) a | 238.97 (67.93) b | 23.36 (0.33) a | 168.70 (2.41) a,b | 18.58 (0.27) a | 9.5 *** |
| R-A | 0 (0) a | 2.01 (1.25) a,b | 0 (0) a | 11.65 (12.61) a,b | 3.63 (4.19) a,b | 26.08 (14.77) b | 14.92 (12.89) a,b | 14.50 (0.21) a,b | 22.75 (0.32) a,b | 85.14 (1.22) c | 16.8 *** |
| Myricetin (mg/kg) | 397.49 (5.68) b,c,d,e | 33.36 (21.85) a,b | 209.11 (4.54) a,b,c,d | 558.08 (160.75) d,e | 183.92 (22.48) a,b,c | 712.13 (211.67) e | 256.93.16 (206.45) a,b,c,d | 284.01 (4.06) a,b,c,d | 439.01 (6.27) c,d,e | 0 (0) a | 12.7 *** |
| Luteolin (mg/kg) | 0 (0) a | 13.45 (10.73) a,b,c | 10.79 (0.31) a,b | 9.75 (8.05) a,b | 3.08 (3.56) a | 0 (0) a | 26.22 (17.50) b,c | 0 (0) a | 33.46 (0.48) c | 0 (0) a | 3.1 ** |
| Quercitin (mg/kg) | 126.38 (1.81) a | 22.11 (40.39) a | 28.14 (0.80) a | 172.43 (125.23) a | 757.22 (454.34) c | 686.07 (35.27) b,c | 105.063 (100.80) a | 296.34 (4.23) a,b | 381.56 (5.45) a,b,c | 71.36 (1.02) a | 14.2 *** |
| Kaempferol (mg/kg) | 126.45 (1.81) a,b | 52.22 (26.18) a | 186.77 (2.45) a,b | 269.20 (148.03) a,b | 283.42 (56.03) a,b | 269.41 (4.81) a,b | 363.19 (181.96) b,c | 652.38 (9.32) c | 406.47 (5.81) b,c | 323.18 (4.62) a,b | 8.6 *** |
| Crataegus monogyna | Helianthus annuus | Pinaceae spp. | Polyfloral | Prunus spp. | Quercus spp. | Robinia pseudoacacia | Taraxacum spp. | Tillia spp. | Zea mays | F-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acids, μg/mg bee pollen | |||||||||||
| Valine | 0.18 (0.01) a | 0.14 (0.07) a | 0.12 (0.02) a | 0.19 (0.08) a | 0.22 (0.03) a | 0.24 (0.09) a,b | 0.20 (0.03) a | 0.41 (0.01) b | 0.21 (0.01) a | 0.18 (0.01) a | 5.1 *** |
| Leucine | 0.08 (0.01) a,b | 0.08 (0.01) a,b | 0.08 (0.03) a,b | 0.09 (0.01) a,b | 0.06 (0.03) a | 0.08 (0.01) a,b | 0.11 (0.02) b | 0.09 (0.01) a,b | 0.09 (0.01) a,b | 0.09 (0.01) a,b | 3.5 ** |
| Isoleucine | 0.12 (0.01) a | 0.16 (0.06) a | 0.13 (0.03) a | 0.17 (0.04) a | 0.16 (0.05) a | 0.20 (0.04) a | 0.16 (0.03) a | 0.24 (0.01) a | 0.21 (0.01) a | 0.16 (0.01) a | 1.8 ns |
| Threonine | 0 (0) a | 0.10 (0.03) b | 0.12 (0.04) b,c | 0.21 (0.05) d,e | 0.16 (0.06) b,c,d | 0.20 (0.03) c,d,e | 0.15 (0.02) b,c,d | 0.27 (0.01) e | 0.09 (0.01) b | 0.22 (0.01) d,e | 15.2 *** |
| Phenylalanine | 0.25 (0.01) a,b | 0.23 (0.08) a,b | 0.18 (0.05) a | 0.29 (0.09) a,b | 0.21 (0.08) a | 0.28 (0.02) a,b | 0.19 (0.04) a | 0.40 (0.01) b | 0.30 (0.01) a,b | 0.21 (0.01) a | 3.2 ** |
| Histidine | 1.18 (0.02) a,b,c | 1.28 (0.71) a,b,c | 0.45 (0.18) a | 0.97 (0.13) a,b,c | 0.84 (0.44) a,b,c | 1.83 (0.52) c | 0.62 (0.26) a,b | 1.64 (0.02) b,c | 1.09 (0.02) a,b,c | 0.50 (0.01) a | 4.8 *** |
| Lysine | 0.40 (0.01) a,b,c | 0.35 (0.23) a,b,c | 0.01 (0.00) a | 0.32 (0.20) a,b,c | 0.10 (0.11) a,b | 0.47 (0.20) b,c | 0.29 (0.23) a,b,c | 0.25 (0.01) a,b,c | 0.73 (0.01) c | 0.46 (0.01) a,b,c | 3.3 ** |
| Tryptophan | 0.18 (0.01) a | 0.12 (0.07) a | 0.11 (0.01) a | 0.18 (0.02) a | 0.14 (0.01) a | 0.16 (0.01) a | 0.16 (0.04) a | 0.18 (0.01) a | 0.17 (0.01) a | 0.16 (0.01) a | 1.5 ns |
| Alanine | 0.31 (0.01) a,b | 0.26 (0.07) a,b | 0.21 (0.03) a | 0.26 (0.04) a,b | 0.22 (0.01) a | 0.31 (0.04) a,b | 0.38 (0.07) b | 0.26 (0.01) a,b | 0.31 (0.01) ab | 0.32 (0.01) a,b | 5.6 *** |
| Sarcosine | 0.04 (0.01) a | 0.03 (0.01) a | 0.03 (0.00) a | 0.03 (0.01) a | 0.03 (0.01) a | 0.03 (0.01) a | 0.03 (0.01) a | 0.04 (0.01) a | 0.04 (0.01) a | 0.04 (0.01) a | 1.6 ns |
| Glycine | 0.08 (0.01) a | 0.05 (0.03) a | 0.06 (0.03) a | 0.07 (0.02) a | 0.03 (0.01) a | 0.06 (0.02) a | 0.06 (0.02) a | 0.04 (0.01) a | 0.04 (0.01) a | 0.04 (0.01) a | 1.9 ns |
| Serine | 1.32 (0.02) d | 0.71 (0.29) a,b,c | 0.33 (0.11) a | 0.76 (0.16) a,b,c | 0.49 (0.28) a,b | 0.85 (0.10) a,b,c,d | 0.85 (0.22) a,b,c | 0.67 (0.01) a,b,c | 0.97 (0.01) b,c,d | 1.12 (0.02) c,d | 5.4 *** |
| Proline | 7.12 (0.10) c | 3.66 (0.46) a,b | 3.32 (0.64) a,b | 3.74 (0.37) a,b | 3.78 (0.86) a,b | 4.92 (0.27) b | 4.92 (0.94) a,b | 4.88 (0.07) b | 4.25 (0.06) a,b | 3.20 (0.05) a | 8.7 *** |
| Asparagine | 1.34 (0.02) a,b,c | 0.74 (0.53) a,b | 0.50 (0.01) a | 1.22 (0.50) a,b,c | 1.03 (0.29) a,b,c | 1.64 (0.03) b,c | 1.64 (0.18) a | 1.71 (0.01) c | 0.99 (0.01) a,b,c | 0.77 (0.01) a,b | 5.9 *** |
| Aspartic acid | 6.94 (0.10) a,b,c | 5.62 (4.92) a,b,c | 10.77 (0.86) b,c | 3.32 (3.55) a,b,c | 1.02 (0.89) a | 2.57 (2.51) a,b | 2.57 (2.99) a,b,c | 3.08 (0.04) a,b,c | 11.64 (0.17) c | 0.38 (0.01) a | 4.2 ** |
| Glutamic acid | 11.50 (0.16) a,b,c | 8.35 (6.17) a,b,c | 14.82 (1.49) b,c | 5.12 (5.08) a,b | 1.31 (0.96) a | 4.20 (2.16) a,b | 4.20 (4.01) a,b | 3.06 (0.04) a | 17.83 (0.25) c | 3.88 (0.06) a,b | 4.9 *** |
| Tyrosine | 0.25 (0.01) a,b | 0.21 (0.05) a,b | 0.18 (0.01) a | 0.21 (0.04) a,b | 0.15 (0.01) a | 0.20 (0.05) a,b | 0.15 (0.03) a,b | 0.18 (0.01) a,b | 0.22 (0.01) b | 0.16 (0.01) a,b | 2.5 * |
| Total AA content | 31.26 (0.45) b,c | 22.11 (9.49) a,b,c | 31.40 (2.24) b,c | 17.13 (8.14) a,b | 9.94 (1.28) a | 18.23 (4.67) a,b | 8.84 (7.22) a,b | 17.38 (0.25) a,b | 28.41 (0.56) c | 11.87 (0.17) a | 5.4 *** |
| Fatty acids, μg/g bee pollen | |||||||||||
| (C6:0) | 0.04 (0.01) a | 0.29 (0.21) a,b | 0.75 (0.35) d | 0.44 (0.30) b,c,d | 0.42 (0.35) a,b,c,d | 0.09 (0.03) a,b | 0.75 (0.23) c,d | 0.09 (0.01) a,b | 0.52 (0.01) c,d | 0.11 (0.01) a,b | 3.8 ** |
| (C8:0) | 0.29 (0.01) a | 1.38 (1.27) a,b | 2.73 (1.56) b | 1.65 (1.05) a,b | 1.18 (0.31) a,b | 0.33 (0.19) a,b | 1.05 (0.39)ab | 0.23 (0.01)a | 1.81 (0.03) a,b | 0.30 (0.01) a | 3.1 ** |
| (C10:0) | 0.26 (0.01) a | 0.99 (0.71) a | 1.47 (1.08) a | 0.66 (0.42) a | 0.57 (0.36) a | 0.44 (0.20) a | 1.09 (0.61)a | 0.26 (0.01)a | 0.76 (0.01) a | 0.45 (0.01) a | 1.5 ns |
| (C12:0) | 0.33 (0.01) a | 3.64 (2.92) a | 4.42 (3.99) a | 2.43 (1.51) a | 1.81 (0.53) a | 0.85 (0.21) a | 2.37 (0.84)a | 0.74 (0.01)a | 3.09 (0.04) a | 1.29 (0.02) a | 1.9 ns |
| (C14:0) | 0.61 (0.01) a | 4.12 (5.43) a | 5.46 (3.86) a | 2.63 (1.16) a | 3.71 (2.10) a | 0.93 (0.07) a | 6.56 (1.83)a | 0.49 (0.01)a | 3.31 (0.05) a | 0.55 (0.01) a | 1.5 ns |
| (C15:0) | 0 (0) a | 0.12 (0.16) a | 0.24 (0.28) a | 0.23 (0.19) a | 0 (0) a | 0.16 (0.08) a | 0.08 (0.03)a | 0 (0)a | 0.23 (0.01) a | 0 (0) a | 1.2 ns |
| (C16:0) | 9.14 (0.13) a | 48.96 (50.24) a | 149.07 (137.91) a | 46.56 (39.24) a | 115.20 (25.43) a | 13.57 (2.94) a | 120.34 (57.35)a | 12.98 (0.19)a | 23.85 (0.34) a | 12.78 (0.18) a | 2.4 ns |
| (C16:1 [cis-9]) | 0.59 (0.01) a | 1.77 (1.95) a | 10.81 (11.80) b | 0.68 (0.38) a | 1.24 (0.09) a | 0.25 (0.10) a | 1.62 (0.82)a | 0.33 (0.01)a | 0.21 (0.01) a | 0.32 (0.01) a | 3.5 ** |
| (C17:0) | |||||||||||
| (C17:1 [cis-10]) | 0 (0) a | 1.95 (3.87) a | 1.60 (1.05) a | 2.34 (1.11) a | 0 (0) a | 5.18 (4.99) a | 3.21 (3.06)a | 0 (0)a | 2.58 (0.34) a | 0 (0) a | 0.8 ns |
| (C18:0) | 0 (0) a | 0.02 (0.05) a | 0 (0) a | 1.00 (0.16) a | 0 (0) a | 1.43 (0.65) a | 2.09 (1.22)a | 0 (0)a | 0 (0) a | 0 (0) a | 1.6 ns |
| (C18:1 [trans-9]) + (C18:1 [cis-9]) | 10.07 (0.14) a,b | 19.58 (9.55) a,b | 32.28 (22.78) b | 14.76 (7.53) a,b | 24.78 (15.43) a,b | 7.03 (3.73) a | 33.11 (11.29) a,b | 14.34 (0.20)ab | 9.15 (0.13) a,b | 13.85 (0.20) a,b | 2.6 * |
| (C18:2 [trans-9,12]) | 15.84 (0.23) a | 21.62 (17.91) a | 33.39 (24.03) a | 19.59 (12.89) a | 35.24 (0.09) a | 8.52 (3.58) a | 42.93 (13.75)a | 18.74 (0.27)a | 10.56 (0.15) a | 9.65 (0.14) a | 2.3 ns |
| (C18:2 [cis-9,12]) | 0 (0) a | 2.82 (3.74) a | 9.83 (11.35) a | 29.06 (18.53) a | 0 (0) a | 0 (0) a | 0 (0)a | 0 (0)a | 14.75 (0.21) a | 0 (0) a | 1.7 ns |
| (C18:3 [cis-6,9,12]) | 15.36 (0.22) a | 44.26 (35.23) a,b | 93.06 (64.14) a,b | 46.94 (38.53) a,b | 121.73 (14.53) b | 23.31 (1.15) a | 117.91 (32.98)ab | 28.00 (0.40)a | 21.49 (0.31) a | 24.08 (0.34) a | 5.0 *** |
| (C20:1 [cis-11]) | 19.39 (0.28) a | 85.87 (74.06) a | 283.61 (253.73) a | 90.81 (83.28) a | 207.81 (21.02) a | 35.64 (6.99) a | 232.90 (98.51)a | 34.74 (0.50)a | 28.13 (0.40) a | 36.38 (0.52) a | 2.7 ns |
| (C18:3 [cis-9,12,15]) | 2.35 (0.03) a | 7.33 (3.13) a,b,c,d | 7.52 (5.84) c,d | 3.83 (1.59) a,b,c,d | 2.59 (1.04) a,b | 2.39 (0.64) a | 10.24 (3.23) d | 3.16 (0.05) a,b,c | 3.28 (0.05) a,b,c | 3.25 (0.05) a,b,c | 3.6 ** |
| (C20:3 [cis-11,14,17]) | 0 (0) a | 7.44 (5.68) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 0 (0) a | 2.10 (0.01) a | 0.8 ns |
| (C22:2 [cis-13,16]) | 0 (0) a | 0.95 (2.00) a | 2.79 (2.22) a | 0 (0) a | 0 (0) a | 5.57 (0.01) a | 0 (0)a | 0 (0)a | 0 (0) a | 0 (0) a | 1.9 ns |
| Total FA content | 12.74 (0.18) a | 19.27 (12.47) a | 106.36 (83.32) b | 36.54 (29.33) a,b | 92.96 (32.59) a,b | 34.21 (4.99) a | 18.67 (9.98) a,b | 65.01 (0.33) a,b | 23.15 (0.12) a | 15.61 (0.26) a | 3.8 ** |
| MUFA | 87.01 (1.24) a | 272.35 (200.87) a,b | 745.39 (585.93) b | 300.15 (221.09) a,b | 609.33 (111.84) a,b | 238.04 (9.36) a,b | 118.79 (35.87) a,b | 641.26 (1.96) a,b | 137.23 (1.94) a,b | 136.66 (1.73) a,b | 3.1 * |
| PUFA | 18.78 (0.27) a | 30.71 (19.30) a | 51.71 (41.68) a | 24.10 (6.45) a | 39.08 (9.94) a | 15.62 (4.31) a | 11.17 (6.75) a | 54.79 (0.32) a | 22.23 (0.20) a | 18.10 (0.19) a | 2.5 * |
| UFA | 47.49 (0.68) a | 160.60 (116.14) a,b | 495.65 (386.63) b | 203.35 (160.92) a,b | 422.49 (67.82) a,b | 160.88 (10.88) a,b | 77.62 (58.37) a,b | 415.82 (1.23) a,b | 85.89 (1.10) a,b | 81.27 (1.12) a,b | 3.3 ** |
| SFA | 66.27 (0.95) a | 191.31 (135.42) a,b | 547.36 (428.31) b | 227.45 (167.06) a,b | 461.57 (77.76) a,b | 176.50 (6.61) a,b | 88.78 (73.60) a,b | 470.61 (1.54) a,b | 108.12 (1.30) a,b | 99.37 (1.31) a,b | 3.2 ** |
| UFA/SFA | 20.73 (0.30) a | 81.02 (66.27) a | 198.02 (157.62) a | 71.70 (54.96) a | 147.77 (34.19) a | 61.54 (14.13) a | 28.58 (7.75)a | 168.56 (0.42)a | 29.12 (0.65) a | 37.30 (0.42) a | 2.7 * |
| (C6:0) | 3.17 (0.05) a | 2.51 (0.64) a | 2.78 (0.64) | 3.22 (0.54) a | 3.09 (0.39) a | 2.84 (0.14) a | 3.87 (0.61) a | 3.50 (0.05) a | 3.67 (0.03) a | 2.82 (0.04) a | 1.8 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oroian, M.; Dranca, F.; Ursachi, F. Characterization of Romanian Bee Pollen—An Important Nutritional Source. Foods 2022, 11, 2633. https://doi.org/10.3390/foods11172633
Oroian M, Dranca F, Ursachi F. Characterization of Romanian Bee Pollen—An Important Nutritional Source. Foods. 2022; 11(17):2633. https://doi.org/10.3390/foods11172633
Chicago/Turabian StyleOroian, Mircea, Florina Dranca, and Florin Ursachi. 2022. "Characterization of Romanian Bee Pollen—An Important Nutritional Source" Foods 11, no. 17: 2633. https://doi.org/10.3390/foods11172633





