The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops
Abstract
:1. Introduction
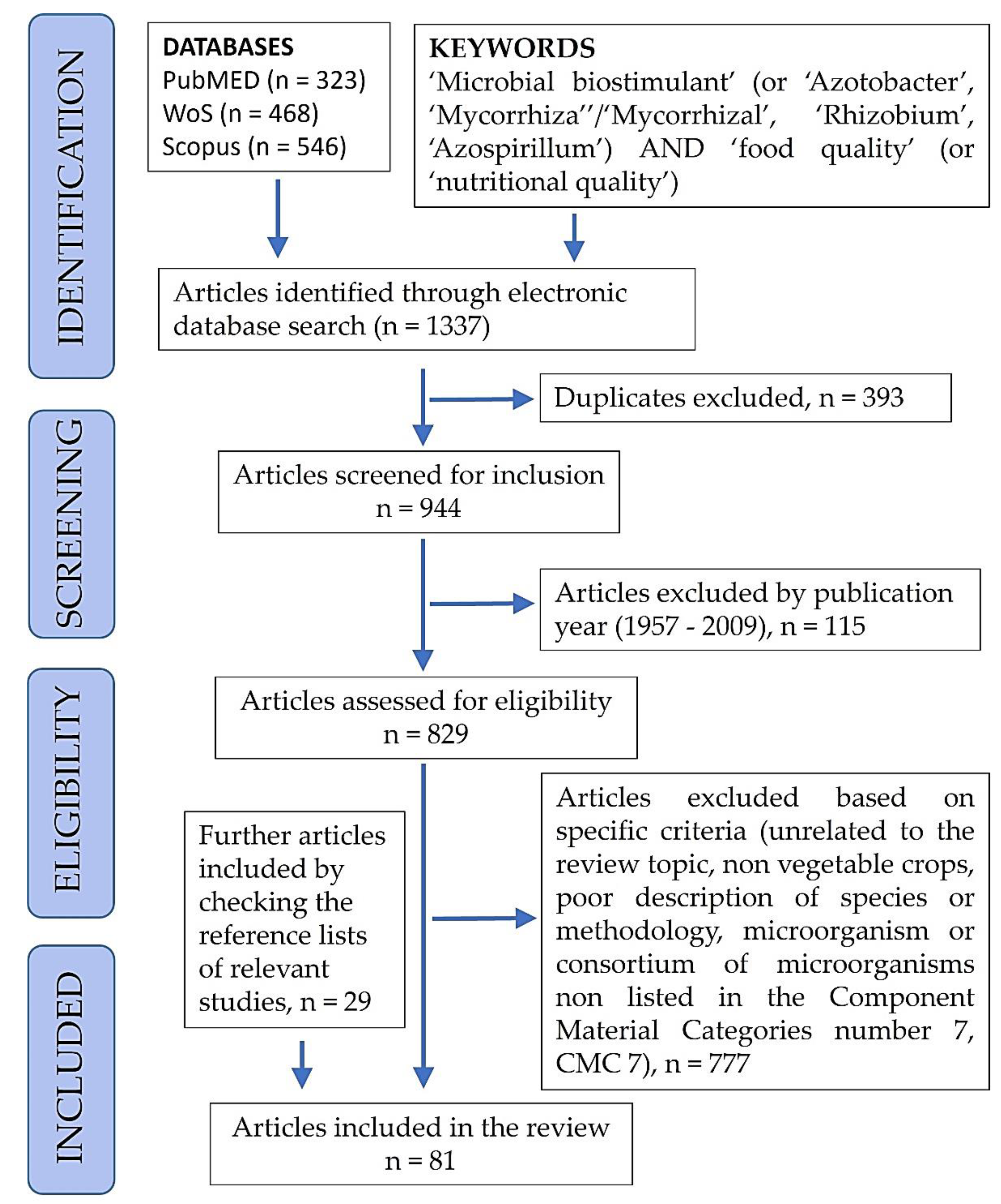
2. Review Method
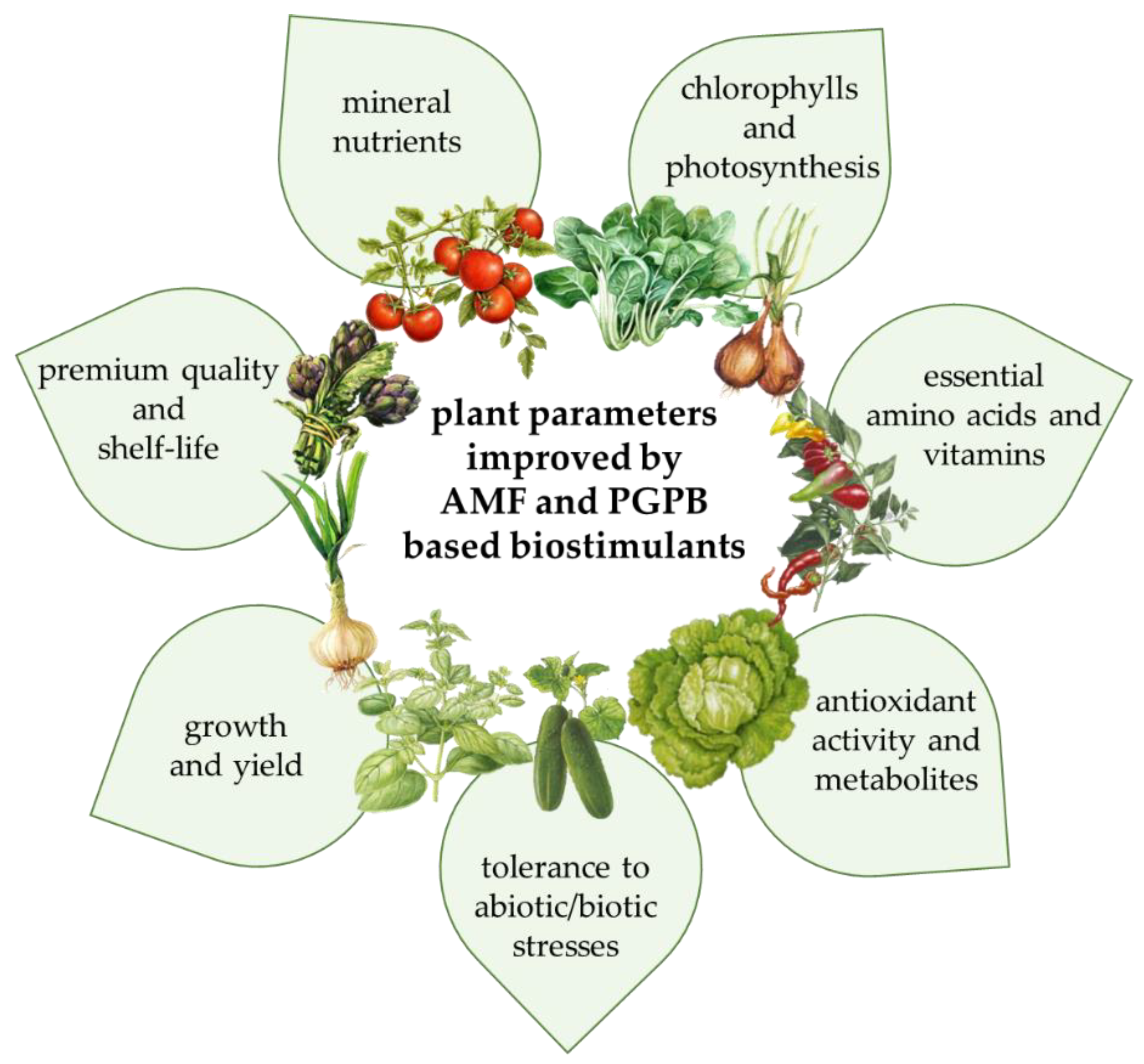
3. Literature Review
4. Arbuscular Mycorrhizal Fungi (AMF)
| AMF | Plant Species | Treatments | Observed Effects | Refs |
|---|---|---|---|---|
| Funneliformis mosseae Claroideoglomus claroideum | C. cardunculus L. cv. romanesco C3 Italy and Violetto Tema | Pots inoculated with crude inoculum of C. claroideum 22W3 and/or F. mosseae (2W3, IMA1, IN101C) | C. claroideum increased total phenols. C. claroideum and F. mosseae IMA1 increased antioxidant activity. | [64] |
| Funneliformis mosseae Diversispora versiformis Rhizophagus intraradices Glomus sp. (G. versiforme, G. intraradices and G. mosseae) | Allium cepa L. Iranian genotypes Red Azar-shahr, White Kashan, Yellow Gholi Ghesse, Pink Horand, and a commercial cultivar Red Rosita | Pots inoculated with 50 g AMF crude inoculum mixed into 1 kg of soil, or 50 g sterilized inoculum (control) | AMF increased total phenolics, pyruvic acid, ascorbic acid, flavonol glucosides and antioxidant enzymes, with the highest beneficial effect caused by D. versiformis. | [57] |
| Funneliformis mosseae Rhizoglomus irregulare | Lactuca sativa var. capitata cv. Bolla | Inoculation at transplant with one tablet (225 spores of each AMF, Trichoderma koningii TK7 strain at 1 × 106 UFC g−1) per pot, under sufficient, moderate or severe deficit irrigation | AMF mix increased P, Mg, Fe, Mn, Zn and phenolic acids independently of water availability. Under well-watered and moderate irrigation, AMF increased plant yield, Ca, Cu and isochlorogenic acid. | [46] |
| Funneliformis mosseae Rhizoglomus irregulare | Ocimum basilicum L. Gecom | Inoculation at transplant with one tablet (225 spores of each AMF, T. koningii TK7 strain at 1 × 106 UFC g−1, Bacillus megaterium MHBM77 1 × 10⁶ UFC g−1 and B. megaterium MHBM06 1 × 10⁶ UFC g−1) per pot below the basil roots under NaCl 1 or 40 mM NaCl | AMF enhanced the marketable fresh yield, chlorophylls and phenols but decreased nitrate content. | [38] |
| Funneliformis mosseae Rhizoglomus irregulare | Ocimum basilicum L. Gecom | At transplant with one tablet (225 spores of each AMF, T. koningii TK7 strain at 1 × 106 UFC g−1, B. megaterium MHBM77 1 × 10⁶ UFC g−1 and B. megaterium MHBM06 1 × 10⁶ UFC g−1) per pot below the basil roots | Under low stress, AMF increased photosynthesis, Fe, Mn and caffeic and rosmarinic acids. Under salinity, enhanced Na compartmentalization and P availability, accumulation of polyphenols (i.e., ferulic and chicoric acids and quercetin-rutinoside) but did not change VOC composition. | [37] |
| Funneliformis mosseae Rhizoglomus irregulare | Solanum lycopersicum L. var. Moneymaker | Pots inoculated with 5% (v/v) F. mosseae and/or R. irregularis | AMF increased mineral content (e.g., N, P and Cu), carotenoids, antioxidant capacity and volatile compounds but not vitamins. | [42] |
| Funneliformis mosseae AZ225C Rhizoglomus irregulare IMA6 | Lactuca sativa L. var. crispa | Transplant in peat mixed with crude inoculum (1:5 v/v) | R. irregulare more than F. mosseae enhanced concentration of phenolics and antioxidant activity. | [71] |
| Funneliformis mosseae and Rhizoglomus irregulare (commercial mix) | Allium cepa L. cv. Stuttgarter Riesen | AMF commercial inoculum equally mixed with the quartz sand before seeding or at the start of bulbing | Inoculation before seeding (highest amount) and colonization at late development stages (bulb growth) increased quercetin compounds if plants were additionally supplied with ammonium. | [69] |
| Funneliformis mosseae BEG234 and Rhizoglomus irregulare BEG72 (commercial mix) | Solanum lycopersicum L. var. “Pomodorino del Piennolo del Vesuvio” landraces Giagiù (yellow) and Lucariello (red) | Planting holes inoculated with 2 g of commercial microgranular inoculum containing 25 spores g−1 of each AM fungus | AMF increased lycopene, total ascorbic acid, alanine GABA and branched-chain amino acids in the red cherry tomato, and Ca, Zn, GABA and the essential amino acids arginine and lysine in the yellow one. In both landraces, AMF improved the antioxidant activity related to the shelf life of tomato fruits. | [43] |
| Funneliformis mosseae BEG 234 and Rhizoglomus irregulare BEG72 and Trichoderma koningii (commercial mix) | C. cardunculus subsp. scolymus L. Hayek cv. cultivars Romolo and Istar | Seed priming with consortium of endophytic fungi containing arbuscular mycorrhizal fungi and Trichoderma koningii in a 6:1 ratio | Both microbial-inoculated cultivars showed higher primary and total fresh marketable yields, higher total phenols and higher antioxidant activity. | [66] |
| Funneliformis mosseae and Rhizophagus intraradices (commercial mix) Rhizophagus intraradices | Crocus sativus L. | 10 g of each inoculum were placed under corms before planting | AMF mix increased saffron flower production and yield. R. intraradices alone enhanced the content of active metabolites (picrocrocin, crocin II and quercitrin) and antioxidant activity. | [68] |
| Funneliformis mosseae BEG234 and Rhizoglomus irregulare BEG72 and (commercial mix) Streptomyces roseocinereus MS1B15 | Solanum lycopersicum L. var. cerasiforme | Pots inoculated with 5 g commercial mix (25 spores g−1) in the planting holes and/or by fertigation with 3.33 g of bacterial suspension (5.8 × 106 CFU g−1) in 100 mL sterile ddH2O | AMF and S. roseocinereus solubilized P sources, improved P uptake and content, vegetative growth, total fruit, red fruit number and fruit quality (i.e., colour, shape and Vitamin C). | [44] |
| Funneliformis mosseae, Rhizophagus intraradices, Claroideoglomus claroideum, Claroideoglomus etunicatum, G. microaggregatum, Funneliformis geosperum (2:2:1:1:1:1 mix 1) Funneliformis mosseae, Rhizophagus inraradices, G. aggregatum, Claroideoglomus etunicatum, G.deserticola, Rhizophagus clarus, G. monosporum (3:3:3:3:1:1:1:1:1 mix 2) | Solanum lycopersicum L. var. Admiro F1 | Inoculation of plastic covering (before planting) with 10 g of mix 1 (720 propagules g−1), and of growing substrate at two-week intervals (14 and 28 DAT) with mix 2 (2.5 g inoculum dm−3 water, 160 propagules g−1). Each plant received 60 cm3 per inoculation. Cultivation with P 15 or 50 mg dm−3 and two substrates (i.e., rockwool or coconut coir) | Increase of ascorbic acid and total soluble sugars in fruits of AMF-inoculated plants (particularly with high P) grown on rockwool. | [76] |
| Funneliformis sp., Claroideoglomus sp., Diversispora sp., Glomus sp. and Rhizophagus sp. (commercial mix) Glomus intraradices, G. microageregatum BEG and G. Claroideum BEG 210 (commercial mix) Funneliformis mosseae (Fm) | Cucumis sativus L. cv. Zhongnong No. 106 | Pots inoculated with 10 g of crude inoculum of commercial mixes or F. mossaeae containing about 2200 infective propagules g−1 from infected cultures | AMF increased plant growth, photosynthetic activity and macro- and micronutrient use efficiency. | [51] |
| Glomus etunicatum Glomus fasciculatum Glomus intraradices | Ocimum basilicum L. | Holes inoculated with 5 g per seed of AMF before planting | Increase of root dry weight, leaf area, plant height and number of lateral branches, minerals (N, P, K, Ca, Fe, Cu and Mn). G. fasciculatum increased yield and essential oils (particularly linalool) more than other AM fungi. | [39] |
| Glomus etunicatum, G. microaggregatum, G. intraradices, G. claroideum, G. mosseae and G. geosporum (commercial mix) | Capsicum annuum L. cv. SLAVY F1 | Inoculation of seedling substrate with commercial mix at 10% concentration with two levels of irrigation (optimum and stress) | AMF increased yield under water stress and increased total antioxidant capacity in control plants. | [79] |
| Glomus etunicatum, G. microaggregatum, G. intraradices, G. claroideum, G. mosseae and G. geosporum (commercial mix) G. intraradices BEG140 | Allium cepa L. cv. Alice | Planting holes inoculated with 120 g of crude inoculum of commercial AMF mix or G. intraradices BEG140, originated from infected maize plants in presence of bark chips preinoculated with saprotrophic fungi | AMF mix increased growth (100%) more than G. intraradices alone (50%). G. intraradices alone increased Mg and K in bulb tissue, and S in presence of saprotrophic fungi. | [53] |
| Glomus fasciculatum | Ocimum basilicum L. cv. Cinnamon, Siam Queen, Sweet Dani and Red Rubin | Pots inoculated with 50 g of crude inoculum (15 propagules g−1 soil substrate) before sowing seeds with 64 or 128 mg·L−1 P | AMF inoculation enhanced N, K, S, B, Fe and Zn uptake, and the content of phenolics (chicoric acid and a caffeic acid derivative). AMF and high P increased biomass and P content. | [40] |
| Glomus fasciculatum Commercial mix of Glomus intraradices and Glomus mosseae | Lactuca sativa L. var. capitata or longifolia | Pots inoculated with 2 g each of G. fasciculatum infected alfalfa soil (mycorrhizal roots and soil containing spores and extraradical mycelium) or commercial mix. | Increase in minerals, chlorophylls, carotenoids, starch and soluble sugars, proteins, ascorbate and tocopherol, phenolics and growth. | [47,48,82] |
| Glomus intraradices | Solanum lycopersicum L. var. Moneymaker | Pots inoculated with 30 g of crude inoculum from infected pot cultures and 50 mL of a filtrate of mycorrhizal inoculum (50 μm pore ø) to all treatments, included controls, to ensure common microflora | AMF increased growth, mineral nutrients of plants and lycopene in fruits. The extracts from these tomatoes did not contain mutagenic compounds; both the hydrophilic and lipophilic fractions of these extracts showed anti-estrogenic power. | [41] |
| Glomus intraradices and Glomus mosseae (commercial mix) | Lactuca sativa L. var. capitata | Pots inoculated under 100%, 75% or 50% water field capacity (FC) | Normal plant growth under 75% FC, increase of carotenoids, anthocyanins and to a lesser extent chlorophylls and phenolics | [72] |
| Glomus intraradices Glomus mosseae | C. cardunculus L. var. scolymus | Offshoot inoculated with 50 g of crude inoculum originated from infected pot cultures of each or both AMF species | Each species, but even more so both species together, were able to increase total phenolic content and antioxidant activity. | [65] |
| Glomus intraradices with low concentrations of Trichoderma harzianum and Bacillus subtilis (commercial mix) | Allium sativum L. cultivar Maysky Allium cepa L. cultivar Kaba | AMF double inoculation before planting and at beginning of bulb formation and/or foliar supply of sodium selenate | AMF + Se increased (i) yield, monosaccharides, P, K and Se in both garlic and onion bulbs; (ii) ascorbic acid, flavonoids, Mg and microelements (B, Cu, Fe, Mn, Si and Zn) in onion; (iii) flavonoids in garlic. | [54,55] |
| Glomus mosseae | Coriander sativum L. | Pots inoculated with 100 g of G. mosseae crude inoculum from infected Sudan grass with 0/100 mg kg−1 KH2PO4 | AMF increased growth, P and N in shoot and root tissues, total soluble proteins in root tissues, fruit yield and essential oil contents. Addition of P reduced AMF colonization and its beneficial effects. | [52] |
| Glomus mosseae | Solanum lycopersicum L. | Inoculation with 10 g G. mosseae commercial granulate kg−1 peat before sowing and/or Trichoderma harzianum applied at sowing or two weeks later as wettable powder to reach a population of 1.8 × 107 conidia g−1 peat | AMF increased the percentage of extra-large fruit, while T. harzianum inoculated two weeks after sowing decreased Ca and Mg in tomato fruit. AMF and T. harzianum increased total and marketable yield and lycopene of tomato fruits, but not other antioxidant metabolites or antioxidant activity. | [45] |
| Glomus mosseae | Solanum lycopersicum cv. Micro Tom | Inoculation by mixing AMF commercial inoculum and sand (30:70, v/v) | Increase of fruit yield and free amino acid content (i.e., glutamine and asparagine). Upregulation of transcription of genes involved in N and C metabolism. | [75] |
| Rhizophagus irregularis | Allium cepa cv. Karmen, Kuba, Sochaczewska and Wolska | Inoculation of upper layer of substrate (15 g of commercial inoculum per pot) | AMF improved onion photosynthesis, growth and yield, and increased vitamin B1 and organic acids. | [70] |
| Rhizophagus intraradices | Solanum lycopersicum L. cv. Rio Fuego | Priming with (3 g L−1 (~ 100 spores g−1) AMF and/or 50 mL of 0.8% seaweed extract (SE; Padina gymnospora) and/or watering with nutritive solution. Control groups treated only with water. | AMF increased polyphenol content. SE favoured protein content. AMF + SE accelerated flowering and AMF colonization and increased root and shoot growth, protein and carbohydrate content. | [74] |
| Rhizophagus intraradices | Lactuca sativa cv. Meraviglia d’Inverno | Inoculation at transplant under roots with one tablet (containing 200 spores of R. intraradices BEG72 and 4.5 × 107 CFU of T. atroviride MUCL45632) and/or 2.5 mL L−1 legume-derived PH foliar spray (4 times at weekly intervals from 6 DAT) and standard, saline (25 mM NaCl) or alkaline (10 mM NaHCO3 + 0.5 g L−1 CaCO3) solution | AMF tablet, especially with PH, improved fresh marketable yield, dry weight, SPAD index, antioxidant activities (CAT and GPX), proline, P, K and Fe via an increase of total root length and surface. | [50] |
| Rhizophagus intraradices | Lactuca sativa cv. Valeska | Peat substrate inoculated within AMF (4.25 g L−1, 720 propagules g−1), medium with P 70 or 140 mg dm−3 and Se in the substrate 0, 6 or 12 mg dm−3 | AMF in plants under low P concentration improved yield but did not affect Se or sugar accumulation. | [49] |
| Rhizophagus intraradices BEG72, Funneliformis mosseae and Trichoderma atroviride (commercial mix) | C. cardunculus subsp. scolymus L. Hayek cv. cultivars Romolo and Istar | Seed coating at 6:1 ratio with commercial mix (300 spores g−1 R. intraradices, 200 spores g−1 Funneli- formis mosseae, and 3 × 108 CFU Trichoderma atroviride) planted in September or October | AMF mix increased the content of 3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid and apigenin 7-O-glucuronide in primary heads as well 1,5-di-O-caffeoylquinic acid in secondary heads especially in Romolo cv. | [67] |
| Rhizophagus intraradices, G. aggregatum, G. viscosum, Claroideoglomus etunicatum and Claroideoglomus claroideum (commercial mix) | Solanum lycopersicum var. TC 2000 cultivated in real industrial tomato farm | Alveolar boxes inoculated with 20 mL of commercial mix inoculum (85,000 infective propagules/l or 10 mL of two Pseudomonas bacterial suspension (108 CFU mL−1)) | AMF mix increased citric acid concentration, while bacteria positively modulated the sugar production and the sweetness of the tomatoes. Both treatments allowed the reduction of chemical inputs and positively influenced tomato quality. | [77] |
| Rhizophagus irregularis strain K8/QS69 | Solanum lycopersicum cv. Micro Tom, Brioso | Inoculation of one-week-old seedlings or cuttings with AMF after enrichment by previous co-cultivation with leek, with 2.7, 6.7 and 10.7 mM phosphate | AMF enhanced the nutritional value of tomatoes in industrialized production by increasing BRIX values, carotenoids and free amino acids (up to fourfold). | [78] |
| Rhizophagus irregularis WFVAM10 | Solanum lycopersicum L. cv. 76R, Lactuca sativa L., Daucus carota L., Cucumis sativus L., Allium ampeloprasum L. var. porrum and other legumes and cereals | Soil mixed with AMF crude inoculum (9:1 w/w) after enrichment by previous co-cultivation with clover | AMF increased the content of P, Cu, Zn and S in shoots and edible parts (where present) of plants, and in particular in leek, whose biomass was also enhanced. The mineral content (N, P, S and Cu) of carrot was also highly increased. Plant ionome was more affected by plant species than by inoculation with AMF. | [36] |
5. Plant Growth-Promoting Bacteria (PGPB)
| PGPB | Plant Species | Treatments | Observed Effects | Refs |
|---|---|---|---|---|
| Azospirillum brasilense Sp245 | Lactuca sativa L. cv. Elisa | Seeds inoculated with 109 CFU per seed or phosphate buffer, and plants grown under salinity (0–40 mM NaCl) | Increase of plant survival of transplantation at 40 mM NaCl and enhancement of fresh and dry leaf weight, leaf area, chlorophyll and ascorbic acid content | [101] |
| Azospirillum brasilense Cd (DSM-1843) | Ocimum basilicum L. cv. Genovese and Red Rubin | Plants inoculated twice with bacteria 106 CFU mL−1 in the nutrient solution and/or with additional 20 mM NO3– or 8 mM SO42− | Additional nutrients but not A. brasilense enhanced fresh biomass. Inoculation increased root growth, unsaturated fatty acids, flavonoids, alkaloids and several terpene derivatives, particularly in Red Rubin. | [104] |
| Azospirillum brasilense and Azotobacter chroococcum (commercial mix) | Ocimum basilicum L. | Inoculation of soil or seed soaking and application to soil (2 l ha−1 with 108 CFU mL−1), bacteria + 50% N or 100% N with and without intercropping with maize | Bacteria application increased fresh and dry yield independently of cropping system. 100% N and bacteria + 50% N were both effective in increasing essential oil (methyl chavicol). | [105] |
| Azotobacter sp., Azospirillum sp., Bacillus licheniformis, B. megatheriumstrain Herbaspirillum sp. and Chlorella vulgaris (commercial mix) | Lactuca sativa var. crispa L. cv. Santoro and var. longifolia Lam. cv. Quintus | Application of 0.4 l of bacterial and algal mix per plant every 14 days, for a total of four treatments | Bacterial–algal mix increased the weight of both lettuce varieties but increased total carotenoid and antioxidant activity only in the cv. Quintus (romaine lettuce). | [102,103] |
| Azospirillum lipoferum DO12 and Brevibacillus parabrevis B50 | Lycoperson esculentum Mill. cv. Menhir F1 | Rhizosphere inoculation with 25 g m−2 A. lipoferum (2 × 108 CFU g−1), or B. parabrevis (3 × 109 CFU g−1) | Both bacteria increased tomato marketable yield. A. lipoferum enhanced lycopene, Vitamin C and total polyphenols; B. parabrevis increased mainly polyphenols. | [106] |
| Azospirillum sp. and Azotobacter sp. (commercial mix) | Solanum lycopersicum L. var. cerasiforme | Plastic bag inoculation with 1.4 l of solution prepared with 1 mL L−1 of commercial mix (1.3 × 107 CFU mL−1 of Azospirillum and 5.9 × 107 CFU mL−1 of Azotobacter) with different levels of NaCl (0, 50, 100, 150 mM) | Bacterial mix improved plant growth and yield, fruit dry matter content, pH 4.52, and TSS even under salinity. | [107] |
| Azospirillum sp.
G. intraradices | Capsicum annuum L. (Chile Morrón, Pimiento) | Inoculation with Azospirillum sp. 104 and 106 CFU mL−1 in the nutrient solution at transplant and twice every 30 days, and 25 or 50 spores of G. intraradices at transplant with 50%/100% N and P | Higher concentration of spores and bacteria increased Vitamin C, carotenoids, total soluble solids and acidity; moreover, they improved N and P uptake at reduced N rate. | [108] |
| Azospirillum strains (lipoferum, brasilense, irakense and strain 21) | Foeniculum vulgare cv. Isfahan | Seed-priming with Azospirillum solution (4 mL g−1) × 12 h or microelements | Priming increased seed weight uniformly, essential oil yield, in particular α-pinene and limonene, and in strain 21 also β-pinene but not limonene. | [109] |
| Rhizobium laguerreae strain HUTR05 | Lactuca sativa L. var. romaine | Seedling inoculation with 150 µL of bacterial suspension with 108 CFU mL−1 | It increased N and P content, phenolic acids (e.g., dicaffeoyl quinic and cichoric acids) and quercetin 3-O-glucoside flavonoid. | [91] |
| Rhizobium laguerreae strain PEPV40 | Spinacia oleracea L. | Inoculation of each seedling at the intersection between roots cotyledons with 250 μL of suspension (108 CFU mL−1) | Increase of spinach leaf number, size and weight, as well as chlorophyll and nitrogen contents. | [84] |
| Rhizobium laguerreae strain PEPV40 and Bacillus halotolerans SCCPVE07 | Cichorium endivia L. | Plants inoculated with 2 mL of bacterial suspension (108 CFU mL−1) and irrigated with water containing 0 or 100 mM NaCl | Bacteria promoted plant development even under salinity. They increased K, Fe, Mg, N, phenolic acids (cichoric acid and caffeoyl-tartaric acid) and flavonoids (kaempferol 3-O-glucuronide). | [110] |
| Rhizobium legiminosarum strain TPV08 Rhizobium sp. strain PETP01 | Solanum lycopersicum L. var. Cherry Capsicum annuum L. var. Verde Italiano | Seedlings inoculated with 108 CFU of each strain | TPV08 and PETP01 promoted growth of both tomato and pepper, but particularly pepper fresh weight production and tomato quality (higher N, P, K or Mg). | [90] |
| Rhizobium etli CE-3, R. leguminosarum SCR R. leguminosarum Semia—4088. | Solanum lycopersicum L. | Seed priming with 4 mL of each inoculum (108 CFU mL−1) kg−1 seeds + inoculation at 30 DAS with 10% of the covering of the root balls in each treatment | Rhizobia (particularly etli CE-3 and Rl SCR) improved tomato yield, probably by a more efficient acquisition of N, P and K. There were no monetary losses despite the different effects. | [111] |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein Goldewijk, K.; Beusen, A.; van Drecht, G.; de Vos, M. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 2011, 20, 73–86. [Google Scholar] [CrossRef]
- Worldmeter. World Population Clock. Available online: https://www.worldometers.info/world-population/ (accessed on 23 July 2022).
- AsiaNews. About a Billion People Are Invisible, One Third of Them Children. AsiaNews Agencies. 2017. Available online: https://www.asianews.it/news-en/About-a-billion-people-are-invisible,-one-third-of-them-children-42131.html (accessed on 23 July 2022).
- Simson, K. The Value of Early Action on Energy Efficiency. Speech by Commissioner Simson at the IEA’s 7th Annual Global Conference on Energy Efficiency Opening Plenary. SPEECH/22/3546. 2022. Available online: https://ec.europa.eu/commission/presscorner/detail/en/SPEECH_22_3546 (accessed on 23 July 2022).
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.I.; Kyriacou, M.C.; et al. Biostimulant Application with a Tropical Plant Extract Enhances Corchorus olitorius Adaptation to Sub-Optimal Nutrient Regimens by Improving Physiological Parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing climate change: Application of microbial biostimulants to mitigate stress in horticultural crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Scagliola, M.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Crecchio, C.; Pii, Y. Bioinoculants as Promising Complement of Chemical Fertilizers for a More Sustainable Agricultural Practice. Front. Sustain. Food Syst. 2021, 4, 622169. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar] [CrossRef]
- Singh, S.K.; Wu, X.; Shao, C.; Zhang, H. Microbial enhancement of plant nutrient acquisition. Stress Biol. 2022, 2, 3. [Google Scholar] [CrossRef]
- Dell’Aversana, E.; D’Amelia, L.; De Pascale, S.; Carillo, P. Use of Biostimulants to Improve Salinity Tolerance in Agronomic Crops. In Agronomic Crops: Volume 3: Stress Responses and Tolerance; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 423–441. [Google Scholar]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of Plant Defense System in Response to Microbial Interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J.A. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate-Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef] [Green Version]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- EC. REGULATION (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 24 July 2022).
- Barros-Rodriguez, A.; Rangseekaew, P.; Lasudee, K.; Pathom-aree, W.; Manzanera, M. Regulatory risks associated with bacteria as biostimulants and biofertilizers in the frame of the European Regulation (EU) 2019/1009. Sci. Total Environ. 2020, 740, 140239. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, N.B. Microbial biostimulants—The need for clarification in EU regulation. Trends Microbiol. 2022, 30, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Abdelgani, M.E.; Elsheikh, E.A.E.; Mukhtar, N.O. The effect of Rhizobium inoculation and chemical fertilization on seed quality of fenugreek. Food Chem. 1999, 64, 289–293. [Google Scholar] [CrossRef]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Mycorrhization of coriander (Coriandrum sativum L.) to enhance the concentration and quality of essential oil. J. Sci. Food Agric. 2002, 82, 339–342. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.D.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Tran, B.T.T.; Watts-Williams, S.J.; Cavagnaro, T.R. Impact of an arbuscular mycorrhizal fungus on the growth and nutrition of fifteen crop and pasture plant species. Funct. Plant Biol. 2019, 46, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.; Corrado, G.; Vitaglione, P.; Colla, G.; Bonini, P.; Giordano, M.; Di Stasio, E.; Raimondi, G.; Sacchi, R.; Rouphael, Y. An Endophytic Fungi-Based Biostimulant Modulates Volatile and Non-Volatile Secondary Metabolites and Yield of Greenhouse Basil (Ocimum basilicum L.) through Variable Mechanisms Dependent on Salinity Stress Level. Pathogens 2021, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G.; Giordano, M.; Raimondi, G.; Pannico, A.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; de Pascale, S. Endophytic fungi induce salt stress tolerance in greenhouse-grown basil. Acta Hortic. 2020, 1268, 125–131. [Google Scholar] [CrossRef]
- Rasouli-Sadaghiani, M.; Hassani, A.; Barin, M.; Rezaee Danesh, Y.; Sefidkon, F. Effects of arbuscular mycorrhizal (AM) fungi on growth, essential oil production and nutrients uptake in basil. J. Med. Plants Res. 2010, 4, 2222–2228. [Google Scholar]
- Scagel, C.F.; Lee, J. Phenolic Composition of Basil Plants Is Differentially Altered by Plant Nutrient Status and Inoculation with Mycorrhizal Fungi. HortScience Horts 2012, 47, 660–671. [Google Scholar] [CrossRef]
- Giovannetti, M.; Avio, L.; Barale, R.; Ceccarelli, N.; Cristofani, R.; Iezzi, A.; Mignolli, F.; Picciarelli, P.; Pinto, B.; Reali, D.; et al. Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br. J. Nutr. 2012, 107, 242–251. [Google Scholar] [CrossRef]
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef]
- Carillo, P.; Kyratzis, A.; Kyriacou, M.C.; Dell’Aversana, E.; Fusco, G.M.; Corrado, G.; Rouphael, Y. Biostimulatory Action of Arbuscular Mycorrhizal Fungi Enhances Productivity, Functional and Sensory Quality in ‘Piennolo del Vesuvio’ Cherry Tomato Landraces. Agronomy 2020, 10, 911. [Google Scholar] [CrossRef]
- Chouyia, F.E.; Fiorentino, N.; Rouphael, Y.; Ventorino, V.; Fechtali, T.; Visconti, D.; Cozzolino, E.; Idbella, M.; Giordano, M.; Fagnano, M. Assessing the effect of P-solubilizing bacteria and mycorrhizal fungi on tomato yield and quality under different crop rotations. Sci. Hortic. 2022, 293, 110740. [Google Scholar] [CrossRef]
- Nzanza, B.; Marais, D.; Soundy, P. Yield and nutrient content of tomato (Solanum lycopersicum L.) as influenced by Trichoderma harzianum and Glomus mosseae inoculation. Sci. Hortic. 2012, 144, 55–59. [Google Scholar] [CrossRef]
- Saia, S.; Colla, G.; Raimondi, G.; Di Stasio, E.; Cardarelli, M.; Bonini, P.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. An endophytic fungi-based biostimulant modulated lettuce yield, physiological and functional quality responses to both moderate and severe water limitation. Sci. Hortic. 2019, 256, 108595. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Enhanced Accumulation of Vitamins, Nutraceuticals and Minerals in Lettuces Associated with Arbuscular Mycorrhizal Fungi (AMF): A Question of Interest for Both Vegetables and Humans. Agriculture 2013, 3, 188–209. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, I.; Konieczny, A. Effect of mycorrhiza on yield and quality of lettuce grown on medium with different levels of phosphorus and selenium. Agric. Food Sci. 2019, 28, 84–92. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined Inoculation with Multiple Arbuscular Mycorrhizal Fungi Improves Growth, Nutrient Uptake and Photosynthesis in Cucumber Seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef]
- Al-Amri, S.M.; Elhindi, K.M.; El-Din, A.F.S. Effects of arbuscular mycorrhizal fungus Glomus mosseae and phosphorus application on plant growth rate, essential oil content and composition of coriander (Coriander sativum L.). Prog. Nutr. 2016, 18, 443–454. [Google Scholar]
- Albrechtova, J.; Latr, A.; Nedorost, L.; Pokluda, R.; Posta, K.; Vosatka, M. Dual inoculation with mycorrhizal and saprotrophic fungi applicable in sustainable cultivation improves the yield and nutritive value of onion. Sci. World J. 2012, 2012, 374091. [Google Scholar] [CrossRef]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of Arbuscular Mycorrhizal Fungi on Yield, Biochemical Characteristics, and Elemental Composition of Garlic and Onion under Selenium Supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of Selenium Biofortification and Beneficial Microorganism Inoculation on Yield, Quality and Antioxidant Properties of Shallot Bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef]
- Castellanos-Morales, V.; Villegas, J.; Wendelin, S.; Vierheilig, H.; Eder, R.; Cárdenas-Navarro, R. Root colonisation by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruits (Fragaria × ananassa Duch.) at different nitrogen levels. J. Sci. Food Agric. 2010, 90, 1774–1782. [Google Scholar] [CrossRef]
- Mollavali, M.; Bolandnazar, S.A.; Schwarz, D.; Rohn, S.; Riehle, P.; Zaare Nahandi, F. Flavonol Glucoside and Antioxidant Enzyme Biosynthesis Affected by Mycorrhizal Fungi in Various Cultivars of Onion (Allium cepa L.). J. Agric. Food Chem. 2016, 64, 71–77. [Google Scholar] [CrossRef]
- Ahmad, H.; Hayat, S.; Ali, M.; Liu, T.; Cheng, Z. The combination of arbuscular mycorrhizal fungi inoculation (Glomus versiforme) and 28-homobrassinolide spraying intervals improves growth by enhancing photosynthesis, nutrient absorption, and antioxidant system in cucumber (Cucumis sativus L.) under salinity. Ecol. Evol. 2018, 8, 5724–5740. [Google Scholar] [CrossRef]
- Ganugi, P.; Martinelli, E.; Lucini, L. Microbial biostimulants as a sustainable approach to improve the functional quality in plant-based foods: A review. Curr. Opin. Food Sci. 2021, 41, 217–223. [Google Scholar] [CrossRef]
- Fernández, I.; Merlos, M.; López-Ráez, J.A.; Martínez-Medina, A.; Ferrol, N.; Azcón, C.; Bonfante, P.; Flors, V.; Pozo, M.J. Defense Related Phytohormones Regulation in Arbuscular Mycorrhizal Symbioses Depends on the Partner Genotypes. J. Chem. Ecol. 2014, 40, 791–803. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Noceto, P.A.; Bettenfeld, P.; Boussageon, R.; Hériché, M.; Sportes, A.; van Tuinen, D.; Courty, P.E.; Wipf, D. Arbuscular mycorrhizal fungi, a key symbiosis in the development of quality traits in crop production, alone or combined with plant growth-promoting bacteria. Mycorrhiza 2021, 31, 655–669. [Google Scholar] [CrossRef]
- Avio, L.; Maggini, R.; Ujvári, G.; Incrocci, L.; Giovannetti, M.; Turrini, A. Phenolics content and antioxidant activity in the leaves of two artichoke cultivars are differentially affected by six mycorrhizal symbionts. Sci. Hortic. 2020, 264, 109153. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Martelloni, L.; Sbrana, C.; Picciarelli, P.; Giovannetti, M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 2010, 335, 311–323. [Google Scholar] [CrossRef]
- Cardarelli, M.; Rouphael, Y.; De Pascale, S.; Bonini, P.; Colla, G. Seed treatment with endophytic fungi enhances yield and nutritional quality of seed-propagated artichokes. In Proceedings of the 10th International Symposium on Artichoke, Cardoon and Their Wild Relatives, Orihuela, Spain, 12–15 March 2019; pp. 57–63. [Google Scholar]
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef]
- Caser, M.; Victorino, Í.M.M.; Demasi, S.; Berruti, A.; Donno, D.; Lumini, E.; Bianciotto, V.; Scariot, V. Saffron Cultivation in Marginal Alpine Environments: How AMF Inoculation Modulates Yield and Bioactive Compounds. Agronomy 2019, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Mollavali, M.; Perner, H.; Rohn, S.; Riehle, P.; Hanschen, F.S.; Schwarz, D. Nitrogen form and mycorrhizal inoculation amount and timing affect flavonol biosynthesis in onion (Allium cepa L.). Mycorrhiza 2018, 28, 59–70. [Google Scholar] [CrossRef]
- Rozpądek, P.; Rąpała-Kozik, M.; Wężowicz, K.; Grandin, A.; Karlsson, S.; Ważny, R.; Anielska, T.; Turnau, K. Arbuscular mycorrhiza improves yield and nutritional properties of onion (Allium cepa). Plant Physiol. Biochem. 2016, 107, 264–272. [Google Scholar] [CrossRef]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Baslam, M.; Goicoechea, N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 2012, 22, 347–359. [Google Scholar] [CrossRef]
- Gholami, R.; Fahadi Hoveizeh, N.; Zahedi, S.M.; Gholami, H.; Carillo, P. Melatonin alleviates the adverse effects of water stress in adult olive cultivars (Olea europea cv. Sevillana & Roughani) in field condition. Agric. Water Manag. 2022, 269, 107681. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, Ecological, and Biochemical Implications in Tomato Plants of Two Plant Biostimulants: Arbuscular Mycorrhizal Fungi and Seaweed Extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Salvioli, A.; Zouari, I.; Chalot, M.; Bonfante, P. The arbuscular mycorrhizal status has an impact on the transcriptome profile and amino acid composition of tomato fruit. BMC Plant Biol. 2012, 12, 44. [Google Scholar] [CrossRef]
- Kowalska, I.; Konieczny, A.; Gastol, M.; Sady, W.; Hanus-Fajerska, E. Effect of mycorrhiza and phosphorus content in nutrient solution on the yield and nutritional status of tomato plants grown on rockwool or coconut coir. Agric. Food Sci. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Werner, S.; Cirka, H.; Rödel, P.; Tandron Moya, Y.; Mock, H.P.; Hutter, I.; Kunze, G.; Hause, B. Effects of Arbuscular Mycorrhization on Fruit Quality in Industrialized Tomato Production. Int. J. Mol. Sci. 2020, 21, 7029. [Google Scholar] [CrossRef] [PubMed]
- Nedorost, L.; Vojtíšková, J.; Pokluda, R. Influence of watering regime and mycorrhizal inoculation on growth and nutrient uptake of pepper (Capsicum annuum L.). Acta Hortic. 2014, 1038, 559–564. [Google Scholar] [CrossRef]
- Avio, L.; Turrini, A.; Giovannetti, M.; Sbrana, C. Designing the Ideotype Mycorrhizal Symbionts for the Production of Healthy Food. Front. Plant Sci. 2018, 9, 1089. [Google Scholar] [CrossRef]
- Agnihotri, R.; Sharma, M.P.; Prakash, A.; Ramesh, A.; Bhattacharjya, S.; Patra, A.K.; Manna, M.C.; Kurganova, I.; Kuzyakov, Y. Glycoproteins of arbuscular mycorrhiza for soil carbon sequestration: Review of mechanisms and controls. Sci. Total Environ. 2022, 806, 150571. [Google Scholar] [CrossRef]
- Baslam, M.; Pascual, I.; Sánchez-Díaz, M.; Erro, J.; García-Mina, J.M.; Goicoechea, N. Improvement of nutritional quality of greenhouse-grown lettuce by arbuscular mycorrhizal fungi is conditioned by the source of phosphorus nutrition. J. Agric. Food Chem. 2011, 59, 11129–11140. [Google Scholar] [CrossRef]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef]
- Jimenez-Gomez, A.; Flores-Felix, J.D.; Garcia-Fraile, P.; Mateos, P.F.; Menendez, E.; Velazquez, E.; Rivas, R. Probiotic activities of Rhizobium laguerreae on growth and quality of spinach. Sci. Rep. 2018, 8, 295. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator—Siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- Höfte, M.; Bakker, P. Competition for Iron and Induced Systemic Resistance by Siderophores of Plant Growth Promoting Rhizobacteria. In Microbial Siderophores; Springer: Berlin/Heidelberg, Germany, 2007; pp. 121–133. [Google Scholar]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef]
- Lucena, C.; Romera, F.J.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene Participates in the Regulation of Fe Deficiency Responses in Strategy I Plants and in Rice. Front. Plant Sci. 2015, 6, 1056. [Google Scholar] [CrossRef]
- Camelo, M.; Vera, S.P.; Bonilla, R.R. Mechanisms of Action of Plant Growth Promoting Rhizobacteria. Cienc. Y Tecnol. Agropecu. 2011, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- García-Fraile, P.; Carro, L.; Robledo, M.; Ramírez-Bahena, M.H.; Flores-Félix, J.D.; Fernández, M.T.; Mateos, P.F.; Rivas, R.; Igual, J.M.; Martínez-Molina, E.; et al. Rhizobium promotes non-legumes growth and quality in several production steps: Towards a biofertilization of edible raw vegetables healthy for humans. PLoS ONE 2012, 7, e38122. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; Garcia-Estevez, I.; Jimenez-Gomez, A.; Flores-Felix, J.D.; Escribano-Bailon, M.T.; Rivas, R. Rhizobium laguerreae Improves Productivity and Phenolic Compound Content of Lettuce (Lactuca sativa L.) under Saline Stress Conditions. Foods 2020, 9, 1166. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; García-Estévez, I.; Escribano-Bailón, M.T.; García-Fraile, P.; Rivas, R. Bacterial Fertilizers Based on Rhizobium laguerreae and Bacillus halotolerans Enhance Cichorium endivia L. Phenolic Compound and Mineral Contents and Plant Development. Foods 2021, 10, 424. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Cassán, F.; Coniglio, A.; López, G.; Molina, R.; Nievas, S.; de Carlan, C.L.N.; Donadio, F.; Torres, D.; Rosas, S.; Pedrosa, F.O.; et al. Everything you must know about Azospirillum and its impact on agriculture and beyond. Biol. Fertil. Soils 2020, 56, 461–479. [Google Scholar] [CrossRef]
- Xu, Z.; Pehlivan, N.; Ghorbani, A.; Wu, C. Effects of Azorhizobium caulinodans and Piriformospora indica Co-Inoculation on Growth and Fruit Quality of Tomato (Solanum lycopersicum L.) under Salt Stress. Horticulturae 2022, 8, 302. [Google Scholar] [CrossRef]
- Chanratana, M.; Joe, M.M.; Roy Choudhury, A.; Anandham, R.; Krishnamoorthy, R.; Kim, K.; Jeon, S.; Choi, J.; Choi, J.; Sa, T. Physiological response of tomato plant to chitosan-immobilized aggregated Methylobacterium oryzae CBMB20 inoculation under salinity stress. 3 Biotech 2019, 9, 397. [Google Scholar] [CrossRef]
- Pérez-Rodriguez, M.M.; Piccoli, P.; Anzuay, M.S.; Baraldi, R.; Neri, L.; Taurian, T.; Lobato Ureche, M.A.; Segura, D.M.; Cohen, A.C. Native bacteria isolated from roots and rhizosphere of Solanum lycopersicum L. increase tomato seedling growth under a reduced fertilization regime. Sci. Rep. 2020, 10, 15642. [Google Scholar] [CrossRef]
- Galleguillos, C.; Aguirre, C.; Miguel Barea, J.; Azcón, R. Growth promoting effect of two Sinorhizobium meliloti strains (a wild type and its genetically modified derivative) on a non-legume plant species in specific interaction with two arbuscular mycorrhizal fungi. Plant Sci. 2000, 159, 57–63. [Google Scholar] [CrossRef]
- Young, J.M. The genus name Ensifer Casida 1982 takes priority over Sinorhizobium Chen et al. 1988, and Sinorhizobium morelense Wang et al. 2002 is a later synonym of Ensifer adhaerens Casida 1982. Is the combination ‘Sinorhizobium adhaerens’ (Casida 1982) Willems et al. 2003 legitimate? Request for an Opinion. Int. J. Syst. Evol. Microbiol. 2003, 53, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Didonato Floro, R.; Lee, J.; Bogosian, G.; Bryant, D. Compositions and methods for improving tomato production. U.S. Patent 11147276B2, 17 March 2014. [Google Scholar]
- Fasciglione, G.; Casanovas, E.M.; Yommi, A.; Sueldo, R.J.; Barassi, C.A. Azospirillum improves lettuce growth and transplant under saline conditions. J. Sci. Food Agric. 2012, 92, 2518–2523. [Google Scholar] [CrossRef] [PubMed]
- Kopta, T.; Pavlikova, M.; Sekara, A.; Pokluda, R.; Marsalek, B. Effect of Bacterial-algal Biostimulant on the Yield and Internal Quality of Lettuce (Lactuca sativa L.) Produced for Spring and Summer Crop. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Kopta, T.; Pokluda, R.; Marsalek, B. Effect of algae and bacteria application on nutritional value of selected leafy vegetables. Acta Hortic. 2016, 1123, 47–52. [Google Scholar] [CrossRef]
- Kolega, S.; Miras-Moreno, B.; Buffagni, V.; Lucini, L.; Valentinuzzi, F.; Maver, M.; Mimmo, T.; Trevisan, M.; Pii, Y.; Cesco, S. Nutraceutical Profiles of Two Hydroponically Grown Sweet Basil Cultivars as Affected by the Composition of the Nutrient Solution and the Inoculation With Azospirillum brasilense. Front. Plant Sci. 2020, 11, 596000. [Google Scholar] [CrossRef]
- Kordi, S.; Salmasi, S.Z.; Kolvanagh, J.S.; Weisany, W.; Shannon, D.A. Intercropping System and N-2 Fixing Bacteria Can Increase Land Use Efficiency and Improve the Essential Oil Quantity and Quality of Sweet Basil (Ocimum basilicum L.). Front. Plant Sci. 2020, 11, 610026. [Google Scholar] [CrossRef] [PubMed]
- Oancea, F.; Raut, I.; Zamfiropol Cristea, V. Influence of soil treatment with microbial plant biostimulant on tomato yield and quality. Agric. Food 2017, 5, 156–165. [Google Scholar]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Abd El-Lateef, H.M.; Yasir, M.; Shah, S.T.; Ullah, I.; Mohamed, M.E.M.; Ali, I.; Ali, F.; et al. Effect of Azospirillum and Azotobacter Species on the Performance of Cherry Tomato under Different Salinity Levels. Gesunde Pflanz. 2022, 74, 487–499. [Google Scholar] [CrossRef]
- Pérez-Velasco, E.; Mendoza-Villarreal, R.; Sandoval Rangel, A.; De la Fuente, M.; Robledo-Torres, V.; Valdez-Aguilar, L. Evaluación del uso de endomicorrizas y Azospirillum sp. en la productividad y calidad nutracéutica de chile morrón (Capsicum annuum) en invernadero. Inf. Técnica Económica Agrar. 2019, 115, 18–30. [Google Scholar] [CrossRef]
- Mirshekari, B.; Valizadeh, N.; Roudsari, A.M.; Maleki, S.H.; Farahvash, F.; Kouchebagh, S.B. Improved growth and essential oil quality of Foeniculum vulgare by Azospirillum inoculation and nutrient seed priming. J. Food Agric. Environ. 2010, 8, 403–406. [Google Scholar]
- Jimenez-Gomez, A.; Garcia-Estevez, I.; Garcia-Fraile, P.; Escribano-Bailon, M.T.; Rivas, R. Increase in phenolic compounds of Coriandrum sativum L. after the application of a Bacillus halotolerans biofertilizer. J. Sci. Food Agric. 2020, 100, 2742–2749. [Google Scholar] [CrossRef]
- Toledo Cabrera, B. Effect of Rhizobium Inoculation on Tomato (Solanum lycopersicum L.) Yield in Protected Crops. Biol. Life Sci. Forum 2021, 3, 52. [Google Scholar]
- Giovannetti, M.; Avio, L.; Sbrana, C. Improvement of nutraceutical value of food by plant symbionts. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2641–2662. [Google Scholar] [CrossRef]
- Agnolucci, M.; Avio, L.; Palla, M.; Sbrana, C.; Turrini, A.; Giovannetti, M. Health-promoting properties of plant products: The role of mycorrhizal fungi and associated bacteria. Agronomy 2020, 10, 1864. [Google Scholar] [CrossRef]
| Criterion | Keywords |
|---|---|
| Microbe or microbial biostimulant | ‘microbial biostimulant’ (and/or ‘Azotobacter’, ‘Mycorrhiza’/‘Mycorrhizal’, ‘Rhizobium’, ‘Azospirillum’) |
| Parameter | ‘food quality’ and/or ‘nutritional quality’ and ‘yield’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods 2022, 11, 2656. https://doi.org/10.3390/foods11172656
Fusco GM, Nicastro R, Rouphael Y, Carillo P. The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods. 2022; 11(17):2656. https://doi.org/10.3390/foods11172656
Chicago/Turabian StyleFusco, Giovanna Marta, Rosalinda Nicastro, Youssef Rouphael, and Petronia Carillo. 2022. "The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops" Foods 11, no. 17: 2656. https://doi.org/10.3390/foods11172656
APA StyleFusco, G. M., Nicastro, R., Rouphael, Y., & Carillo, P. (2022). The Effects of the Microbial Biostimulants Approved by EU Regulation 2019/1009 on Yield and Quality of Vegetable Crops. Foods, 11(17), 2656. https://doi.org/10.3390/foods11172656









