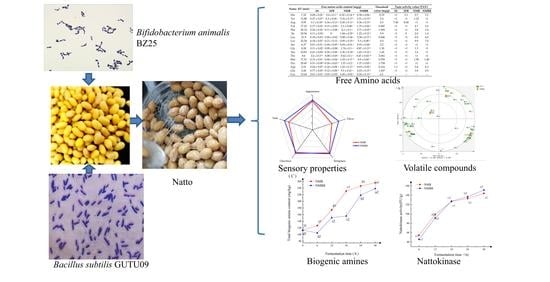
Effect of Adding Bifidobacterium animalis BZ25 on the Flavor, Functional Components and Biogenic Amines of Natto by Bacillus subtilis GUTU09
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Natto
Seed Culture
2.3. Determination of Total Viable Count (TVC)
2.4. Determination of Titratable Acidity (TA) and the pH Value
2.5. Solid-Phase Microextraction–GC–MS Analysis
2.5.1. Sample Treatment
2.5.2. Separation of Volatiles
2.5.3. Qualitative and Quantitative Analyses of Samples
2.5.4. Odor Activity Value (OAV)
2.6. Sensory Properties
2.7. Determination of FAA Content
2.8. Determination of NK Activity
2.9. Determination of BAs
2.10. Statistics and Analysis
3. Results and Discussion
3.1. TVC of Soybean Fermented by BZ25 (SFB), Natto Made by GUTU09 (NMB), or Natto Made by Two Mixed Strains (NMBB)
3.2. pH and Titratable Acid (TA) Analysis
3.3. Effect of BZ25 Addition on the VOCs of Natto
Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
3.4. Sensory Properties
3.5. Effect of BZ25 Addition on FAAs and Bitter Amino Acids
| Name | RT (min) | Free Amino Acids Content (mg/g) | Threshold Value (mg/g) | Taste Activity Value (TAV) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SS | SFB | NMB | NMBB | SS | SFB | NMB | NMBB | |||
| His | 7.32 | 0.08 ± 0.02 c | 0.4 ± 0.1 b | 0.52 ± 0.14 ab | 0.58 ± 0.06 a | 0.19 | <1 | 2.1 | 2.7 | 3.1 |
| Tyr | 12.88 | 0.27 ± 0.07 c | 0.3 ± 0.04 c | 3.16 ± 0.17 a | 2.51 ± 0.12 b | 2.6 | <1 | <1 | 1.22 | <1 |
| Arg | 9.95 | 3.7 ± 0.18 b | 4.34 ± 0.13 a | 0.45 ± 0.11 c | 0.36 ± 0.15 c | 0.5 | 7.40 | 8.68 | <1 | <1 |
| Val | 17.23 | 0.17 ± 0.02 c | 0.15 ± 0.03 c | 2.3 ± 0.08 a | 1.75 ± 0.06 b | 0.487 | <1 | <1 | 4.7 | 3.6 |
| Phe | 20.14 | 0.24 ± 0.02 c | 0.11 ± 0.08 c | 4.1 ± 0.3 a | 3.71 ± 0.03 b | 1.092 | <1 | <1 | 3.8 | 3.4 |
| Ile | 20.54 | 0.11 ± 0.02 c | 0 c | 1.84 ± 0.25 a | 1.22 ± 0.12 b | 0.9 | <1 | 0 | 2.0 | 1.4 |
| Leu | 21.9 | 0.18 ± 0.03 c | 0.04 ± 0.02 c | 5.08 ± 0.36 a | 3.38 ± 0.15 b | 0.846 | <1 | <1 | 6.0 | 4.0 |
| Lys | 22.54 | 0.18 ± 0.07 c | 0.21 ± 0.12 c | 4.95 ± 0.25 a | 3.5 ± 0.08 b | 0.5 | <1 | <1 | 9.9 | 7.0 |
| Ser | 6.37 | 0.01 ± 0.01 a | 0.04 ± 0.05 a | 0.04 ± 0.01 a | 0.33 ± 0.48 a | 2.2 | <1 | <1 | <1 | <1 |
| Gly | 8.28 | 0.11 ± 0.02 c | 0.09 ± 0.08 c | 1.74 ± 0.1 a | 0.87 ± 0.13 b | 1.30 | <1 | <1 | 1.3 | <1 |
| Ala | 10.83 | 0.41 ± 0.04 c | 0.24 ± 0.06 c | 2.36 ± 0.18 a | 1.43 ± 0.12 b | 1.44 | <1 | <1 | 1.6 | <1 |
| Thr | 8.6 | 0.2 ± 0.3 bc | 0.08 ± 0.05 c | 0.62 ± 0.1 a | 0.47 ± 0.02 ab | 3.061 | <1 | <1 | <1 | <1 |
| Met | 17.51 | 0.13 ± 0.01 c | 0.06 ± 0.04 c | 1.03 ± 0.17 a | 0.8 ± 0.04 b | 0.555 | <1 | <1 | 1.90 | 1.40 |
| Pro | 29.45 | 0.31 ± 0.09 b | 0.24 ± 0.03 b | 1.57 ± 0.2 a | 1.37 ± 0.05 a | 1.738 | <1 | <1 | <1 | <1 |
| Asp | 2.91 | 0.24 ± 0.07 c | 0.18 ± 0.08 c | 1.25 ± 0.17 a | 0.93 ± 0.02 b | 0.216 | 1.1 | <1 | 5.8 | 4.3 |
| Glu | 3.26 | 0.77 ± 0.05 c | 0.12 ± 0.08 d | 5.5 ± 0.21 a | 4.23 ± 0.15 b | 1.437 | <1 | <1 | 3.8 | 2.9 |
| Cys | 15.64 | 0.01 ± 0.01 c | 0.03 ± 0.02 c | 0.45 ± 0.02 a | 0.24 ± 0.13 b | n.f. | — | — | — | — |
3.6. Effect of BZ25 Addition on NK Activity
3.7. Effect of BZ25 Addition on Bas
3.7.1. Changes in Bas during Fermentation
3.7.2. Changes in Total Biogenic Amines (TBA) Content
3.7.3. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Su, H.; Song, H.-L. Comparison of four extraction methods, SPME, DHS, SAFE, versus SDE, for the analysis of flavor compounds in natto. Food Anal. Methods 2018, 11, 343–354. [Google Scholar] [CrossRef]
- Li, D.; Hou, L.; Hu, M.; Gao, Y.; Tian, Z.; Fan, B.; Li, S.; Wang, F. Recent advances in nattokinase-enriched fermented soybean Foods: A review. Foods 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Horii, Y.; Watanabe, S.; Kubo, Y.; Koguchi, K.; Hoshi, Y.; Matsumoto, K.-I.; Soda, K. Comparison of soybean cultivars for enhancement of the polyamine contents in the fermented soybean natto using Bacillus subtilis (natto). Biosci. Biotechnol. Biochem. 2017, 81, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ge, C.; Yuan, W.; Zhu, R.; Zhang, W.; Du, L.; Xue, J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J. Sci. Food Agric. 2010, 90, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, C.-Y.; Wang, J.; Man, X.; Shi, Q.; Nie, F.; Nan, J.; Min, J.Z. Simultaneous determination of free DL-amino acids in natto with novel fluorescent derivatization by UPLC-FL. Food Anal. Methods 2021, 14, 1099–1109. [Google Scholar] [CrossRef]
- Kojima, A.; Ikehara, S.; Kamiya, K.; Kajita, E.; Sato, Y.; Kouda, K.; Tamaki, J.; Kagamimori, S.; Iki, M. Natto intake is inversely associated with osteoporotic fracture risk in postmenopausal Japanese women. J. Nutr. 2020, 150, 599–605. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Li, G.; Zhang, M.; Niu, T.; Kang, X.; Zhao, H.; Chen, J.; Sun, E.; Li, Y. Effect of Bifidobacterium animalis subsp. lactis MN-Gup on constipation and the composition of gut microbiota. Benef. Microbes 2021, 12, 31–42. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zhang, Y.; He, L.; Zhang, C.; Liu, X. Different effects of soybean protein and its derived peptides on the growth and metabolism of Bifidobacterium animalis subsp. animalis JCM 1190. Food Funct. 2021, 12, 5731–5744. [Google Scholar] [CrossRef]
- Wang, H.K.; Ng, Y.K.; Koh, E.; Yao, L.; Chien, A.S.; Lin, H.X.; Lee, Y.K. RNA-Seq reveals transcriptomic interactions of Bacillus subtilis natto and Bifidobacterium animalis subsp. lactis in whole soybean solid-state co-fermentation. Food Microbiol. 2015, 51, 25–32. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.-J.; Mei, W.-C.; Li, T.; Tao, Y.-S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 239, 495–501. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. UPLC-Q-TOF-MS based metabolomics and chemometric analyses for green tea fermented with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. Curr. Res. Food Sci. 2022, 5, 471–478. [Google Scholar] [CrossRef]
- Tronchoni, J.; Curiel, J.A.; Morales, P.; Torres-Pérez, R.; Gonzalez, R. Early transcriptional response to biotic stress in mixed starter fermentations involving Saccharomyces cerevisiae and Torulaspora delbrueckii. Int. J. Food Microbiol. 2017, 241, 60–68. [Google Scholar] [CrossRef]
- Lan, G.; Li, C.; He, L.; Zeng, X.; Zhu, Q. Effects of different strains and fermentation method on nattokinase activity, biogenic amines, and sensory characteristics of natto. J. Food Sci. Technol. 2020, 57, 4414–4423. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, X.; He, Z.; Su, H.; Li, W.; Ren, X. Amino acid and microbial community dynamics during the fermentation of hong qu glutinous rice wine. Food Microbiol. 2020, 90, 103467. [Google Scholar] [CrossRef]
- Kabelova, I.; Dvorakova, M.; Cizkova, H.; Dostalek, P.; Melzoch, K. Determination of free amino acids in cheeses from the Czech market. Czech J. Food Sci. 2009, 27, 143–150. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef]
- Aredes, R.S.; Peixoto, F.C.; Sphaier, L.A.; Marques, F.F.D. Evaluation of craft beers through the direct determination of amino acids by capillary electrophoresis and principal component analysis. Food Chem. 2021, 344, 7. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows in vitro cytotoxicity at concentrations that have been found in foods. Food Chem. 2020, 331, 127303. [Google Scholar] [CrossRef]
- Swider, O.; Roszko, M.L.; Wojcicki, M.; Szymczyk, K. Biogenic amines and free amino acids in traditional fermented vegetables-dietary risk evaluation. J. Agric. Food Chem. 2020, 68, 856–868. [Google Scholar] [CrossRef]
- Larqué, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef]
- Kalač, P. Health effects and occurrence of dietary polyamines: A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Y.; Qian, J.; Wu, S.; Ji, Y.; Liu, Y.; Zeng, J.; Gong, A. Nattokinase crude extract inhibits hepatocellular carcinoma growth in mice. J. Microbiol. Biotechnol. 2019, 29, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Unrean, P.; Nguyen, N.H.A. Metabolic pathway analysis and kinetic studies for production of nattokinase in Bacillus subtilis. Bioprocess Biosyst. Eng. 2013, 36, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, M.; You, X.; Sela, D.A.; Xiao, H.; McClements, D.J. Encapsulation of bifidobacterium in alginate microgels improves viability and targeted gut release. Food Hydrocoll. 2021, 116, 106634. [Google Scholar] [CrossRef]
- Tian, P.; Zhan, P.; Tian, H.; Wang, P.; Lu, C.; Zhao, Y.; Ni, R.; Zhang, Y. Analysis of volatile compound changes in fried shallot (Allium cepa L. var. aggregatum) oil at different frying temperatures by GC-MS, OAV, and multivariate analysis. Food Chem. 2021, 345, 128748. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, G.; Tian, X.; He, L.; Li, C.; Zeng, X.; Wang, X. Effect of fermentation parameters on natto andits thrombolytic property. Foods 2021, 10, 2547. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.V.V.; Mahanama, K.R.R.; Somasiri, H.P.P.S.; Punyasiri, P.A.N. Validation of a reversed-phase high-performance liquid chromatographic method for the determination of free amino acids in rice using L-theanine as the internal standard. Food Chem. 2018, 240, 196–203. [Google Scholar] [CrossRef]
- Lu, M.; Gao, Z.; Xing, S.; Long, J.; Li, C.; He, L.; Wang, X. Purification, characterization, and chemical modification of Bacillus velezensis SN-14 fibrinolytic enzyme. Int. J. Biol. Macromol. 2021, 177, 601–609. [Google Scholar] [CrossRef]
- Kim, B.; Byun, B.Y.; Mah, J.-H. Biogenic amine formation and bacterial contribution in natto products. Food Chem. 2012, 135, 2005–2011. [Google Scholar] [CrossRef]
- Li, L.; Wen, X.; Wen, Z.; Chen, S.; Wang, L.; Wei, X. Evaluation of the biogenic amines formation and degradation abilities of Lactobacillus curvatus from Chinese bacon. Front. Microbiol. 2018, 9, 1015. [Google Scholar] [CrossRef]
- Yoon, W.-K.; Garcia, C.V.; Kim, C.-S.; Lee, S.-P. Fortification of Mucilage and GABA in Hovenia dulcis Extract by Co fermentation with Bacillus subtilis HA and Lactobacillus plantarum EJ2014. Food Sci. Technol. Res. 2018, 24, 265–271. [Google Scholar] [CrossRef]
- Bujna, E.; Farkas, N.A.; Tran, A.M.; Dam, M.S.; Nguyen, Q.D. Lactic acid fermentation of apricot juice by mono- and mixed cultures of probiotic Lactobacillus and Bifidobacterium strains. Food Sci. Biotechnol. 2018, 27, 547–554. [Google Scholar] [CrossRef]
- Li, S.; Tang, S.; He, Q.; Hu, J.; Zheng, J. Changes in proteolysis in fermented milk produced by streptococcus thermophilus in co-culture with lactobacillus plantarum or bifidobacterium animalis subsp. lactis during refrigerated storage. Molecules 2019, 24, 3699. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarska, K.; Taylor, M.; Piyasiri, U.; Frank, D. Flavor and metabolite profiles of meat, meat substitutes, and traditional plant-based high-protein food products available in australia. Foods 2021, 10, 801. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Pielech-Przybylska, K. Pyrazines biosynthesis by Bacillus strains isolated from natto fermented soybean. Biomolecules 2021, 11, 1736. [Google Scholar] [CrossRef]
- Kimura, K.; Kubo, Y. Flavor development during natto fermentation. J. Jpn. Soc. Food Sci. Technol. Nippon Shokuhin Kagaku Kogaku Kaishi 2017, 64, 379–384. [Google Scholar] [CrossRef]
- Chen, L.-L.; Wang, J.-L.; Hu, Y.; Qian, B.-J.; Yao, X.-M.; Wang, J.-F.; Zhang, J.-H. Computational design of glutamate dehydrogenase in Bacillus subtilis natto. J. Mol. Model. 2013, 19, 1919–1927. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.-B.; Kim, Y.-S. Determination of key volatile compounds related to long-term fermentation of soy sauce. J. Food Sci. 2019, 84, 2758–2776. [Google Scholar] [CrossRef]
- He, G.; Huang, J.; Liang, R.; Wu, C.; Zhou, R. Comparing the differences of characteristic flavour between natural maturation and starter culture for Mucor-type Douchi. Food Sci. Technol. 2016, 51, 1252–1259. [Google Scholar] [CrossRef]
- Sadineni, V.; Kondapalli, N.; Obulam, V.S.R. Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann. Microbiol. 2012, 62, 1353–1360. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, B.; Zhao, M.; Zheng, F.; Huang, M.; Sun, J.; Sun, X.; Li, H. Characterization of the key odorants in chinese Zhima aroma-type Baijiu by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2016, 64, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qin, D.; Wu, Z.; Sun, B.; Sun, X.; Huang, M.; Sun, J.; Zheng, F. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chem. 2019, 284, 100–107. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, Z.L.J. Complilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2003. [Google Scholar]
- Sun, J.; Li, Q.; Luo, S.; Zhang, J.; Huang, M.; Chen, F.; Zheng, F.; Sun, X.; Li, H. Characterization of key aroma compounds in Meilanchun sesame flavor style baijiu by application of aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission/addition experiments. RSC Adv. 2018, 8, 23757–23767. [Google Scholar] [CrossRef]
- Shi, J.; Tong, G.; Yang, Q.; Huang, M.; Ye, H.; Liu, Y.; Wu, J.; Zhang, J.; Sun, X.; Zhao, D. Characterization of key aroma compounds in tartary buckwheat (fagopyrum tataricum gaertn.) by means of sensory-directed flavor analysis. J. Agric. Food Chem. 2021, 69, 11361–11371. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.-T.; Schwab, W.; Wan, X. Effect of the roasting degree on flavor quality of large-leaf yellow tea. Food Chem. 2021, 347, 129016. [Google Scholar] [CrossRef]
- Gu, S.; Wang, X.; Tao, N.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC x GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
- Kum, S.-J.; Yang, S.-O.; Lee, S.M.; Chang, P.-S.; Choi, Y.H.; Lee, J.J.; Hurh, B.S.; Kim, Y.-S. Effects of aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang). J. Agic. Food Chem. 2015, 63, 1401–1418. [Google Scholar] [CrossRef]
- Murray, N.M.; O’Riordan, D.; Jacquier, J.-C.; O’Sullivan, M.; Holton, T.A.; Wynne, K.; Robinson, R.C.; Barile, D.; Nielsen, S.D.; Dallas, D.C. Peptidomic screening of bitter and nonbitter casein hydrolysate fractions for insulinogenic peptides. J. Dairy Sci. 2018, 101, 2826–2837. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Koizumi, R.; Itoyama, R.; Ichisawa, M.; Negishi, J.; Sakuma, R.; Furusho, T.; Sagane, Y.; Takano, K. Free amino acids in potato (solanum tuberosum) may cause egumi-taste in food products. Potato Res. 2019, 62, 305–314. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Hornack, K.; Reilly, D. Increased taste thresholds of amino acids with age. Am. J. Clin. Nutr. 1979, 32, 1622–1627. [Google Scholar] [CrossRef]
- Troise, A.D.; Wiltafsky, M.; Fogliano, V.; Vitaglione, P. The quantification of free Amadori compounds and amino acids allows to model the bound Maillard reaction products formation in soybean products. Food Chem. 2018, 247, 29–38. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Sennewald, K.; Gagnon, J. Comparison of taste qualities and thresholds of D- and L-amino acids. Physiol. Behav. 1981, 27, 51–59. [Google Scholar] [CrossRef]
- Chen, D.-W.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Chen, K.; Gao, C.; Han, X.; Li, D.; Wang, H.; Lu, F. Co-fermentation of lentils using lactic acid bacteria and Bacillus subtilis natto increases functional and antioxidant components. J. Food Sci. 2021, 86, 475–483. [Google Scholar] [CrossRef]
- Mayer, H.K.; Fiechter, G. UHPLC analysis of biogenic amines in different cheese varieties. Food Control 2018, 93, 9–16. [Google Scholar] [CrossRef]
- Santos, M.M. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Spermine and spermidine are cytotoxic towards intestinal cell cultures, but are they a health hazard at concentrations found in foods. Food Chem. 2018, 269, 321–326. [Google Scholar] [CrossRef]
- Shukla, S.; Lee, J.S.; Park, H.-K.; Yoo, J.-A.; Hong, S.-Y.; Kim, J.-K.; Kim, M. Effect of novel starter culture on reduction of biogenic amines, quality improvement, and sensory properties of doenjang, a traditional Korean soybean fermented sauce variety. J. Food Sci. 2015, 80, M1794–M1803. [Google Scholar] [CrossRef]
- Zhao, D.; Shi, D.; Sun, J.; Li, A.; Sun, B.; Zhao, M.; Chen, F.; Sun, X.; Li, H.; Huang, M.; et al. Characterization of key aroma compounds in Gujinggong Chinese Baijiu by gas chromatography-olfactometry, quantitative measurements, and sensory evaluation. Food Res. Int. 2018, 105, 616–627. [Google Scholar] [CrossRef]







| Number | Name | Aroma Description | RI | ID | Threshold Value (µg/L) a | OAV | |||
|---|---|---|---|---|---|---|---|---|---|
| SS | SFB | NMB | NMBB | ||||||
| Amines | |||||||||
| A1 | N,N-dimethyl-Methylamine | Fish | 2081.3 | MS, RI, Std | 8–23 d | — | — | 7–20 | — |
| Esters | |||||||||
| B1 | Hexanoic acid ethyl ester | Fruit, pineapple, banana aroma | 1233.3 | MS, RI | 55.3 b | <1 | <1 | 10 | 98 |
| B2 | Octanoic acid ethyl ester | Cream, milk | 1436 | MS, RI | 13 c | 1 | 28 | 4 | 20 |
| B3 | Benzoic acid ethyl ester | Camomile, flower, celery, fruit | 1676.6 | MS, RI | 0.6 d | 193 | 239 | — | 16 |
| B4 | 2-Methyl-propanoic acid ethyl ester | Fruity | 969 | MS, RI | 58 e | 49 | — | 3 | 3 |
| B11 | Benzeneacetic acid methyl ester | Jasmine flower, sweet, fruit | 1767.6 | MS, RI | 0.16 h | — | 1347 | 1171 | — |
| Pyrazines | |||||||||
| C1 | Trimethyl-pyrazine | Nutty, cocoa-like, roasted | 1407.7 | MS, RI | 50–96 g | 40–77 | 36–90 | 62–119 | 171–328 |
| C2 | 2,5-Dimethyl-pyrazine | Cocoa, roasted nut, roast beef | 1325.7 | MS, RI | 1700 i | 1 | 1 | 5 | 14 |
| C3 | 2-Ethyl-3,5-dimethyl-pyrazine | Roasted potato | 1465 | MS, RI | 7.5 b | 2 | 74 | 182 | 10 |
| C4 | 2-Ethyl-5-methyl-pyrazine | Nutty | 1394.4 | MS, RI | 16 j | — | 8 | 10 | 7 |
| Ketones | |||||||||
| D2 | 5-Methyl-2-hexanone | — | 1141.8 | MS, RI | 62–89 d | 1 | 2–3 | 17–24 | 11–16 |
| D3 | 2,3-Hexadecanone | Butter, caramel, fruit | 2079.1 | MS, RI | 7.3 d | 12 | 4 | 33 | 72 |
| D4 | 2,3-Butanedione | Sweet, cream | 984.7 | MS, RI | 5 e | — | 33 | — | 644 |
| D5 | 2-Nonanone | Sweet, coconut | 1342.7 | MS, RI | 32 d | 4 | 42 | 46 | 46 |
| D7 | 2-Heptanone | Mild medicinal fragrance | 1181.3 | MS, RI | 1 i | 1287 | 2376 | 982 | — |
| D8 | 3-Octanone | Herb, butter | 1251.9 | MS, RI | 1.3 g | 602 | 24 | 172 | — |
| D9 | 2,3-Pentanedione | Caramel, nuts | 1061.6 | MS, RI | 30 d | 6 | 11 | 7 | — |
| D10 | Acetone | irritating flavors | 819.8 | MS, RI | 1100 d | 25 | 16 | 7 | — |
| D19 | 6-Methyl-5-hepten-2-one | Pepper, mushroom | 1338.9 | MS, RI | 68 d | 16 | — | — | — |
| Acids | |||||||||
| E1 | Acetic acid | Stimulative sour | 1451.1 | MS, RI | 13–150 g | 13–485 | — | 62–715 | 26–301 |
| E2 | 2-Ethyl-butanoic acid | Fruit milchigs | 1380.5 | MS, RI | 93–460 d | — | — | 6–29 | 1–7 |
| Aldehydes | |||||||||
| F1 | Decanal | Sweet flowery, wax | 1502 | MS, RI | 0.1 i | 5510 | 3739 | — | 6675 |
| F2 | Nonanal | Flowery, citrus, fat, wax fragrant | 1396.1 | MS, RI | 3.1 g | 151 | 124 | 40 | 278 |
| F3 | 3-Methyl-butanal | Malt, unpleasant smell | 919.7 | MS, RI | 31.6 d | 505 | — | 16 | — |
| F4 | 2-Methyl-butanal | Cocoa, almond | 916.3 | MS, RI | 1 d | — | — | 540 | — |
| F5 | Octanal | Crude oil smell | 1284.6 | MS, RI | 0.9 j | 316 | — | 120 | — |
| F6 | Phenylacetaldehyde | Fengxinzi taste | 1647 | MS, RI | 4 j | 108 | 41 | 30 | — |
| F8 | Benzaldehyde | Cherry, nuts, bitter almond aroma | 1528.8 | MS, RI, Std | 85 g | 80 | 25 | 26 | — |
| F9 | Hexanal | Grass, fat | 1082.9 | MS, RI | 230 g | 22 | <1 | <1 | — |
| Alcohols | |||||||||
| G1 | 1-Octen-3-ol | Mushroom, green | 1447.3 | MS, RI | 10 g | 168 | 13 | 6 | 931 |
| G2 | Trans-geraniol | Flowery, lemon aroma | 1845.3 | MS, RI | 1 f | — | 208 | — | 189 |
| G3 | 3-Octanol | Herbaceous, melon, citrus-like odor | 1389.8 | MS, RI | 18 d | — | 7 | — | 39 |
| G4 | 1-Hexanol | Green, fowery | 1350.3 | MS, RI | 34 g | — | 52 | — | 12 |
| G9 | 2-Methyl-3-hexanol | — | 1370.8 | MS, RI | 46–81 d | — | — | 11–29 | — |
| G20 | Eucalyptol | — | 1206.7 | MS, RI, Std | 4.6 d | 73 | — | — | — |
| Aromatics | |||||||||
| H1 | 2-Methoxy-phenol | Spicy, medicine fragrance | 1869.1 | MS, RI | 1.5 g | 465 | 2200 | 569 | 495 |
| H2 | Biphenyl | — | 2005 | MS, RI | 0.5 d | 9 | 11 | 5 | 65 |
| H3 | Phenol | Phenol | 2010.6 | MS, RI | 21 g | 25 | 19 | 30 | 37 |
| Furans | |||||||||
| I1 | 2-Pentyl-furan | Bean, green | 1230.1 | MS, RI | 5.8 i | 502 | 126 | 35 | 78 |
| I2 | 2,3-Dihydro-benzofuran | — | 2399 | MS, RI | 48 d | 2 | 3 | 3 | 13 |
| I3 | 2-Ethyl-furan | Malt fragrance | 957.3 | MS, RI | 2.3 i | 7694 | 2029 | 334 | — |
| Others | |||||||||
| J1 | Hexadecane | Alkane | 1405 | MS, RI, Std | 25 i | — | — | 84 | 56 |
| J2 | Undecane | Alkane | 1090.5 | MS, RI, Std | 1578 i | — | — | <1 | <1 |
| J3 | Styrene | Balsamic, gasoline | 1259.6 | MS, RI | 65 d | 2 | 2 | — | 16 |
| J6 | Naphthalene | — | 1757.7 | MS, RI | 6 d | — | — | — | 43 |
| J7 | Anethole | Fennel, spicy, licorice smell | 1839 | MS, RI | 100 f | — | — | 4 | 20 |
| J13 | Dimethyl trisulfide | Sulfuric, strong onion odor | 1380.1 | MS, RI | 14 g | 19 | 19 | 2 | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Lan, G.; Tian, X.; He, L.; Li, C.; Tao, H.; Zeng, X.; Wang, X. Effect of Adding Bifidobacterium animalis BZ25 on the Flavor, Functional Components and Biogenic Amines of Natto by Bacillus subtilis GUTU09. Foods 2022, 11, 2674. https://doi.org/10.3390/foods11172674
Zhang Q, Lan G, Tian X, He L, Li C, Tao H, Zeng X, Wang X. Effect of Adding Bifidobacterium animalis BZ25 on the Flavor, Functional Components and Biogenic Amines of Natto by Bacillus subtilis GUTU09. Foods. 2022; 11(17):2674. https://doi.org/10.3390/foods11172674
Chicago/Turabian StyleZhang, Qifeng, Guangqun Lan, Xueyi Tian, Laping He, Cuiqin Li, Han Tao, Xuefeng Zeng, and Xiao Wang. 2022. "Effect of Adding Bifidobacterium animalis BZ25 on the Flavor, Functional Components and Biogenic Amines of Natto by Bacillus subtilis GUTU09" Foods 11, no. 17: 2674. https://doi.org/10.3390/foods11172674