Monitoring and Occurrence of Heavy PAHs in Pomace Oil Supply Chain Using a Double-Step Solid-Phase Purification and HPLC-FLD Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Samples
2.3. Oil Extraction
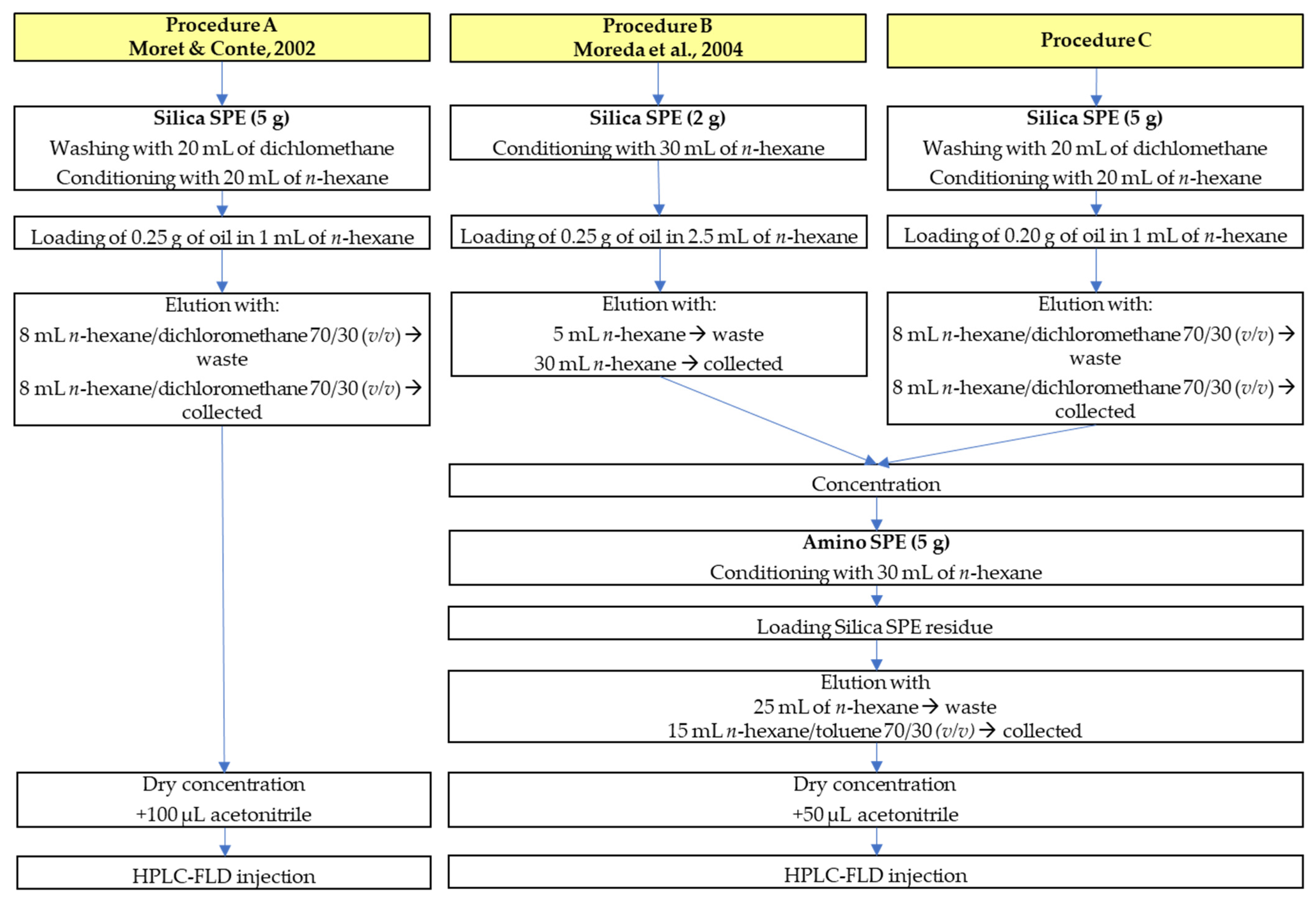
2.4. Sample Purification
2.5. HPLC-FLD Analysis
2.6. Method Performances
3. Results and Discussion
3.1. Method Optimization
3.2. Heavy PAHs along the Olive and Pomace Oil Supply Chain
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BaA | Benzo(a)anthracene |
| BaP | Benzo(a)pyrene |
| BbF | Benzo(b)fluoranthene |
| BghiP | Benzo(g,h,i)perylene |
| BkF | Benzo(k)fluoranthene |
| Ch | Chrysene |
| DAD | Diode-Array Detection |
| DBahA | Dibenzo(a,h)anthracene |
| EFSA | European Food Safety Authority |
| EU | European Union |
| FLD | Fluorometric Detector |
| GC | Gas Chromatography |
| GC×GC | Comprehensive Two-Dimensional Gas Chromatography |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| HPLC | High Performance Liquid Chromatography |
| IP | Indeno(1,2,3-cd)pyrene |
| LC | Liquid Chromatography |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LOD | Limit of Detection |
| LOQ | Limit of Quantification |
| MS | Mass Spectrometry |
| MS-MS | Tandem Mass Spectrometry |
| OM | Olive Mill |
| PAH4 | BaP, Ch, BaA, BbF |
| PAH8 | BaA, Chr, BbF, BaP, BkF, DBahA, BghiP, IP |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PF | Pomace Factory |
| RSD | Relative Standard Deviation |
| SD | Standard Deviation |
| SPE | Solid Phase Extraction |
| UV-Vis | Ultraviolet–Visible |
References
- Sánchez-Arévalo, C.M.; Olmo-García, L.; Fernández-Sánchez, J.F.; Carrasco-Pancorbo, A. Polycyclic aromatic hydrocarbons in edible oils: An overview on sample preparation, determination strategies, and relative abundance of prevalent compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3528–3573. [Google Scholar] [CrossRef]
- Mafra, I.; Amaral, J.S.; Oliveira, M.B.P.P. Polycyclic aromatic hydrocarbons (PAH) in olive oils and other vegetable oils; potential for carcinogenesis. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2010; Volume 54, pp. 489–498. ISBN 9780123744203. [Google Scholar]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, Analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Bertoz, V.; Purcaro, G.; Conchione, C.; Moret, S. A Review on the Occurrence and Analytical Determination of PAHs in Olive Oils. Foods 2021, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Guillen, M.D. Polycyclic aromatic compounds: Extraction and determination in food. Food Addit. Contam. 1994, 11, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Bolaños, P.; Frenich, A.G.; Vidal, J.L.M. Polycyclic aromatic hydrocarbons in food and beverages. Analytical methods and trends. J. Chromatogr. A 2010, 1217, 6303–6326. [Google Scholar] [CrossRef]
- Lee, M.L.; Novotny, M.V.; Bartle, K.D. Analytical Chemistry of Polycyclic Aromatic Compounds; Academic Press: New York, NY, USA, 1981. [Google Scholar] [CrossRef]
- Purcaro, G.; Moret, S.; Conte, L.S. Overview on polycyclic aromatic hydrocarbons: Occurrence, legislation and innovative determination in foods. Talanta 2013, 105, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Purcaro, G.; Barp, L.; Moret, S. Determination of hydrocarbon contamination in foods. A review. Anal. Methods 2016, 8, 5755–5772. [Google Scholar] [CrossRef]
- Dennis, M.J.; Massey, R.C.; Cripps, G.; Venn, I.; Howarth, N.; Lee, G. Factors affecting the polycyclic aromatic hydrocarbon content of cereals, fats and other food products. Food Addit. Contam. 1991, 8, 517–530. [Google Scholar] [CrossRef]
- Kiralan, S.S.; Erdogdu, F.; Tekin, A. Reducing polycyclic aromatic hydrocarbons (PAHs) formation in olive pomace oil using microwave pre-heating of olive pomace. Eur. J. Lipid Sci. Technol. 2016, 119, 1600241. [Google Scholar] [CrossRef]
- Moral, P.S.; Ruiz-Méndez, M.V. Production of pomace olive oil. Grasas Aceites 2006, 57, 47–55. [Google Scholar] [CrossRef]
- Gharby, S. Refining vegetable oils: Chemical and physical refining. Sci. World J. 2022, 6627013. [Google Scholar] [CrossRef] [PubMed]
- Kiralan, S.S.; Toptancı, I.; Tekin, A. Further Evidence on the Removal of Polycyclic Aromatic Hydrocarbons (PAHs) During Refining of Olive Pomace Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800381. [Google Scholar] [CrossRef]
- European Commission (EC). COMMISSION REGULATION (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, L215, 4–8. [Google Scholar]
- European Commission (EC). COMMISSION REGULATION (EU) No 836/2011 of 19 August 2011 amending Regulation (EC) No 333/2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs. Off. J. Eur. Union 2011, L215, 9–16. [Google Scholar]
- Šimko, P. Determination of polycyclic aromatic hydrocarbons in smoked meat products and smoke flavouring food additives. J. Chromatogr. B 2002, 770, 3–18. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, S.; Gong, G. Trends of research on polycyclic aromatic hydrocarbons in food: A 20-year perspective from 1997 to 2017. Trends Food Sci. Technol. 2019, 83, 86–98. [Google Scholar] [CrossRef]
- Bansal, V.; Kumar, P.; Kwon, E.E.; Kim, K.H. Review of the Quantification Techniques for Polycyclic Aromatic Hydrocarbons (PAHs) in Food Products. Food Sci. Nutr. 2017, 57, 3297–3312. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O. Polycyclic Aromatic Hydrocarbons in Foods: A Critical Review. Curr. Nutr. Food Sci. 2020, 16, 866–873. [Google Scholar] [CrossRef]
- Wu, S.; Gong, G.; Yan, K.; Sun, Y.; Zhang, L. Polycyclic Aromatic Hydrocarbons in Edible Oils and Fatty Foods: Occurrence, Formation, Analysis, Change and Control. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 93, pp. 59–112. ISBN 9780128206881. [Google Scholar]
- Moret, S.; Conte, L.S. Polycyclic aromatic hydrocarbons in edible fats and oils: Occurrence and analytical methods. J. Chromatogr. A 2000, 882, 245–253. [Google Scholar] [CrossRef]
- Arrebola, F.J.; Frenich, A.G.; Rodríguez, M.J.G.; Plaza-Bolaños, P.; Vidal, J.L.M. Determination of polycyclic aromatic hydrocarbons in olive oil by a completely automated headspace technique coupled to gas chromatography-mass spectrometry. Biol. Mass Spectrom. 2006, 41, 822–829. [Google Scholar] [CrossRef]
- Zachara, A.; Gałkowska, D.; Juszczak, L. Method Validation and Determination of Polycyclic Aromatic Hydrocarbons in Vegetable Oils by HPLC-FLD. Food Anal. Methods 2017, 10, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Yusty, M.L.; Daviña, J.C. Supercritical fluid extraction and high-performance liquid chromatography–fluorescence detection method for polycyclic aromatic hydrocarbons investigation in vegetable oil. Food Control 2005, 16, 59–64. [Google Scholar] [CrossRef]
- Costopoulou, D.; Vassiliadou, I.; Chrysafidis, D.; Bergele, K.; Tzavara, E.; Tzamtzis, V.; Leondiadis, L. Determination of PCDD/F, dioxin-like PCB and PAH levels in olive and olive oil samples from areas affected by the fires in summer 2007 in Greece. Chemosphere 2010, 79, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Dost, K.; Ideli, C. Determination of polycyclic aromatic hydrocarbons in edible oils and barbecued food by HPLC/UV–Vis detection. Food Chem. 2012, 133, 193–199. [Google Scholar] [CrossRef]
- Akdogan, A.; Buttinger, G.; Wenzl, T. Single laboratory validation of a saponification method for the determination of four polycyclic aromatic hydrocarbons in edible oils by HPLC-fluorescence detection. Food Addit. Contam. Part A 2016, 33, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Alomirah, H.; Al-Zenki, S.; Husain, A.; Sawaya, W.; Ahmed, N.; Gevao, B.; Kannan, K. Benzo[a]pyrene and total polycyclic aromatic hydrocarbons (PAHs) levels in vegetable oils and fats do not reflect the occurrence of the eight genotoxic PAHs. Food Addit. Contam. Part A 2010, 27, 869–878. [Google Scholar] [CrossRef]
- ISO 22959:2009; Animal and Vegetable Fats and Oils—Determination of Polycyclic Aromatic Hydrocarbons by on-Line Donor-Acceptor Complex Chromatography and HPLC with Fluorescence Detection. International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- Shi, L.-K.; Zhang, D.-D.; Liu, Y.-L. Survey of polycyclic aromatic hydrocarbons of vegetable oils and oilseeds by GC-MS in China. Food Addit. Contam. Part A 2016, 33, 603–611. [Google Scholar] [CrossRef]
- Belo, R.F.C.; Nunes, C.M.; dos Santos, E.V.; Augusti, D.V.; Pissinatti, R. Single laboratory validation of a SPE method for the determination of PAHs in edible oils by GC-MS. Anal. Methods 2012, 4, 4068–4076. [Google Scholar] [CrossRef]
- Gharbi, I.; Moret, S.; Chaari, O.; Issaoui, M.; Conte, L.S.; Lucci, P.; Hammami, M. Evaluation of hydrocarbon contaminants in olives and virgin olive oils from Tunisia. Food Control 2017, 75, 160–166. [Google Scholar] [CrossRef]
- Moret, S.; Conte, L. A rapid method for polycyclic aromatic hydrocarbon determination in vegetable oils. J. Sep. Sci. 2002, 25, 96–100. [Google Scholar] [CrossRef]
- Moreda, W.; Rodríguez-Acuña, R.; Pérez-Camino, M.D.C.; Cert, A. Determination of high molecular mass polycyclic aromatic hydrocarbons in refined olive pomace and other vegetable oils. J. Sci. Food Agric. 2004, 84, 1759–1764. [Google Scholar] [CrossRef]
- Guillén, M.D.; Sopelana, P.; Palencia, G. Polycyclic Aromatic Hydrocarbons and Olive Pomace Oil. J. Agric. Food Chem. 2004, 52, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; White, S.; MacArthur, R.; Petch, R.; Holland, J.; Damant, A. Single-laboratory validation of a GC/MS method for the determination of 27 polycyclic aromatic hydrocarbons (PAHs) in oils and fats. Food Addit. Contam. 2007, 24, 635–651. [Google Scholar] [CrossRef]
- Jira, W.; Ziegenhals, K.; Speer, K. Gas chromatography-mass spectrometry (GC-MS) method for the determination of 16 European priority polycyclic aromatic hydrocarbons in smoked meat products and edible oils. Food Addit. Contam. Part A 2008, 25, 704–713. [Google Scholar] [CrossRef]
- Purcaro, G.; Picardo, M.; Barp, L.; Moret, S.; Conte, L.S. Direct-immersion solid-phase microextraction coupled to fast gas chromatography mass spectrometry as a purification step for polycyclic aromatic hydrocarbons determination in olive oil. J. Chromatogr. A 2013, 1307, 166–171. [Google Scholar] [CrossRef]
- Gomez-Ruiz, J.A.; Cordeiro, F.; Lopez, P.; Wenzl, T. Optimisation and validation of programmed temperature vaporization (PTV) injection in solvent vent mode for the analysis of the 15+1 EU-priority PAHs by GC–MS. Talanta 2009, 80, 643–650. [Google Scholar] [CrossRef]
- Zhou, R.-Z.; Jiang, J.; Mao, T.; Zhao, Y.-S.; Lu, Y. Multiresidue analysis of environmental pollutants in edible vegetable oils by gas chromatography–tandem mass spectrometry. Food Chem. 2016, 207, 43–50. [Google Scholar] [CrossRef]
- Veyrand, B.; Brosseaud, A.; Sarcher, L.; Varlet, V.; Monteau, F.; Marchand, P.; Andre, F.; Le Bizec, B. Innovative method for determination of 19 polycyclic aromatic hydrocarbons in food and oil samples using gas chromatography coupled to tandem mass spectrometry based on an isotope dilution approach. J. Chromatogr. A 2007, 1149, 333–344. [Google Scholar] [CrossRef]
- Drabova, L.; Tomaniova, M.; Kalachova, K.; Kocourek, V.; Hajslova, J.; Pulkrabova, J. Application of solid phase extraction and two-dimensional gas chromatography coupled with time-of-flight mass spectrometry for fast analysis of polycyclic aromatic hydrocarbons in vegetable oils. Food Control 2013, 33, 489–497. [Google Scholar] [CrossRef]
- Purcaro, G.; Morrison, P.; Moret, S.; Conte, L.S.; Marriott, P.J. Determination of polycyclic aromatic hydrocarbons in vegetable oils using solid-phase microextraction–comprehensive two-dimensional gas chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2007, 1161, 284–291. [Google Scholar] [CrossRef]
- Shi, L.-K.; Liu, Y.-L.; Liu, H.-M.; Zhang, M.-M. One-step solvent extraction followed by liquid chromatography–atmospheric pressure photoionization tandem mass spectrometry for the determination of polycyclic aromatic hydrocarbons in edible oils. Anal. Bioanal. Chem. 2015, 407, 3605–3616. [Google Scholar] [CrossRef] [PubMed]
- Hollosi, L.; Wenzl, T. Development and optimisation of a dopant assisted liquid chromatographic-atmospheric pressure photo ionisation-tandem mass spectrometric method for the determination of 15+1 EU priority PAHs in edible oils. J. Chromatogr. A 2011, 1218, 23–31. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Polycyclic aromatic hydrocarbons in food - Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 724, 1–114. [Google Scholar] [CrossRef]
- Purcaro, G.; Moret, S.; Conte, L.S. Rapid SPE-HPLC determination of the 16 European priority polycyclic aromatic hydrocarbons in olive oils. J. Sep. Sci. 2008, 31, 3936–3944. [Google Scholar] [CrossRef]


| PAH | Time (min) | λex (nm) | λem (nm) |
|---|---|---|---|
| BaA, Ch | 23.0 | 270 | 390 |
| BbF | 28.0 | 260 | 430 |
| BkF, BaP | 30.7 | 256 | 410 |
| DbahA, BghiP | 34.0 | 290 | 410 |
| IP | 36.4 | 290 | 484 |
| Procedure B | Procedure C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAH | Spike (μg/kg) | Recovery (%) | SD | RSD% | Recovery (%) | SD | RSD% | LOD (μg/kg) | LOQ (μg/kg) | Linearity Range | HorRat |
| BaA | 1.0 | 71.1 | 22.4 | 31.5 | 56.5 | 14.1 | 24.9 | 0.03 | 0.1 | 0.1–59.9 | 1.13 |
| Ch | 1.0 | 100.1 | 33.0 | 32.9 | 78.1 | 18.3 | 23.4 | 0.21 | 0.7 | 0.7–59.5 | 1.06 |
| BbF | 2.0 | 114.7 | 4.6 | 4.0 | 109.4 | 5.5 | 5.0 | 0.06 | 0.2 | 0.2–89.8 | 0.23 |
| BkF | 1.0 | 108.6 | 5.5 | 5.1 | 101.6 | 5.3 | 5.2 | 0.03 | 0.1 | 0.1–59.6 | 0.24 |
| BaP | 1.0 | 104.0 | 4.9 | 4.7 | 98.8 | 4.6 | 4.7 | 0.03 | 0.1 | 0.1–59.5 | 0.21 |
| DBahA | 2.0 | 101.5 | 1.2 | 1.2 | 96.3 | 1.6 | 1.6 | 0.03 | 0.1 | 0.1–89.1 | 0.07 |
| BghiP | 2.0 | 119.6 | 9.7 | 8.1 | 102.7 | 7.0 | 6.8 | 0.09 | 0.3 | 0.3–89.2 | 0.31 |
| IP | 1.0 | 115.6 | 5.7 | 4.9 | 94.2 | 10.7 | 11.4 | 0.03 | 0.1 | 0.1–59.5 | 0.52 |
| Olive Mill | Sample Type | Extracted Oil (%) | BaA | Ch | BbF | BkF | BaP | DBahA | BghiP | IP | PAH4 | PAH8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OM1 | Picholine olives | 26.6 | 0.2 | 2.7 | 0.1 | <0.1 | 0.1 | <0.1 | 0.2 | 0.1 | 3.1 | 3.4 |
| Olive paste | 33.4 | 0.3 | 2.4 | 0.1 | 0.2 | 0.1 | 0.1 | 0.3 | 0.3 | 2.9 | 3.8 | |
| Virgin olive oil | <0.1 | 1.3 | 0.1 | <0.1 | 0.1 | <0.1 | 0.2 | 0.2 | 1.5 | 1.9 | ||
| Residual pomace oil | 8.1 | 1.8 | 7.9 | 1.9 | 0.9 | 0.5 | <0.1 | <0.3 | 0.5 | 12.1 | 13.5 | |
| OM2 | Ghiacciola olives | 19.7 | <0.1 | <0.7 | 0.1 | <0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.6 |
| Virgin olive oil | <0.1 | <0.7 | <0.2 | <0.1 | <0.1 | <0.1 | <0.3 | 0.1 | - | 0.1 | ||
| Residual pomace oil | 4.3 | 0.6 | 3.3 | 2.1 | 0.7 | 0.5 | 0.3 | 0.4 | 0.7 | 6.5 | 8.6 | |
| OM3 | Bianchera olives | 31.5 (solvent) | 0.4 | 3.1 | 0.4 | <0.1 | 0.1 | <0.1 | 0.1 | 0.2 | 4 | 4.3 |
| Bianchera olives | (centrifugation) | <0.1 | 0.9 | 0.1 | <0.1 | 0.1 | 0.1 | <0.3 | 0.2 | 1.1 | 1.4 | |
| Virgin olive oil-01 * | 0.1 | 2.9 | 0.1 | 0.1 | 0.1 | <0.1 | 0.2 | 0.2 | 3.2 | 3.7 | ||
| Virgin olive oil-02 ** | <0.1 | 1 | <0.2 | <0.1 | 0.1 | <0.1 | 0.4 | 0.1 | 1.1 | 0.7 | ||
| Residual pomace oil | 7.2 | 1.5 | 6.2 | 2.2 | 0.7 | 0.1 | 0.1 | 0.3 | 0.6 | 10 | 11.7 | |
| OM4 | Virgin olive oil | <0.1 | 0.2 | <0.2 | <0.1 | 0.1 | <0.1 | <0.3 | 0.1 | 0.3 | 0.4 | |
| Residual pomace oil | 3.5 | 0.9 | 5 | 0.8 | 0.4 | 0.5 | <0.1 | 0.3 | 0.5 | 7.2 | 8.4 | |
| OM5 | Virgin olive oil | <0.1 | 0.4 | <0.2 | <0.1 | 0.1 | <0.1 | 0.1 | 0.2 | 0.5 | 0.8 | |
| Residual pomace oil *** | 16 | 0.2 | 1.4 | 0.1 | 0.2 | 0.3 | <0.1 | 0.2 | 0.3 | 2 | 2.7 |
| OM/PF | Sample Type | Extracted Oil % | BaA | Ch | BbF | BkF | BaP | DBahA | BghiP | IP | PAH4 | PAH8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OM1 | fresh pomace | 8.1 | 1.8 | 7.9 | 1.9 | 0.9 | 0.5 | <0.1 | <0.3 | 0.5 | 12.1 | 13.5 |
| OM2 | fresh pomace | 4.3 | 0.6 | 3.3 | 2.1 | 0.7 | 0.5 | 0.3 | 0.4 | 0.7 | 6.5 | 8.6 |
| 1–2 days pomace | 4.6 | 0.9 | 4.1 | 0.7 | 0.4 | 0.4 | 0.1 | 0.5 | 0.3 | 6.1 | 7.4 | |
| 10–20 days pomace | 5.7 | 71.4 | 26.4 | 11.3 | 4.9 | 5.5 | 0.5 | 3.1 | 2.7 | 114.6 | 125.8 | |
| OM3 | fresh pomace | 14.9 | 1.5 | 6.2 | 2.2 | 0.7 | 0.5 | 0.1 | 0.3 | 0.6 | 10.4 | 12.1 |
| 10–20 days pomace—surface | 5 | 2.8 | <0.7 | 4.5 | 1.6 | 1.8 | 0.2 | 1.5 | 1.3 | 9.1 | 13.7 | |
| 10–20 days pomace—depth | 4.4 | 0.7 | 1.6 | 0.4 | 0.3 | 0.5 | <0.1 | 0.4 | 0.4 | 3.2 | 4.3 | |
| 30 days pomace—surface—clean area | 2.6 | 0.1 | 0.5 | 1.9 | 0.1 | 0.6 | 0.2 | 0.4 | 0.7 | 3.1 | 4.5 | |
| OM4 | 2–3 days pomace | 3.5 | 0.9 | 5,0 | 0.8 | 0.4 | 0.5 | <0.1 | 0.3 | 0.5 | 7.2 | 8.4 |
| 7–10 days pomace | 4.3 | 1.1 | 5.8 | 0.9 | 0.5 | 0.5 | 0.4 | 0.2 | 0.3 | 8.3 | 9.7 | |
| OM5 | fresh pomace—decanter | 0.5 | 1.7 | 0.2 | 0.3 | 0.3 | 0.4 | 0.2 | 0.5 | 2.7 | 4.1 | |
| fresh pomace—sinolea | 16 | 0.2 | 1.4 | 0.1 | 0.2 | 0.3 | <0.1 | 0.2 | 0.3 | 2,0 | 2.7 | |
| OM6 | fresh pomace | 3.8 | 3,0 | 11.6 | 0.2 | 4.8 | 4.2 | 0.7 | 4.5 | 2.1 | 18.8 | 30.9 |
| OM7 | fresh pomace | 5.9 | 5.2 | 13.7 | 1.7 | 4.3 | 6.1 | 0.3 | 2.8 | 4.1 | 26.7 | 38.2 |
| PF1 | fresh pomace freshly conferred | 3.9 | 1.1 | 5.9 | 1.4 | 0.7 | 1.5 | <0.1 | 1.1 | 1.1 | 9.9 | 12.8 |
| fresh pomace conferred by 15 days | 2.5 | 95.5 | 44.5 | 30.2 | 11.9 | 24.2 | 1.1 | 21.1 | 22.2 | 194.4 | 250.7 | |
| dry pomace | 5.6 | 319 | 901.5 | 197.7 | 69.1 | 58.2 | 15 | 97.2 | 152.5 | 1476.4 | 1810.2 | |
| oil/hexane mixture | 1052.7 | 1533 | 1481.9 | 462.3 | 1064.1 | 97.2 | 1002.8 | 1271.4 | 5131.7 | 7965.4 | ||
| crude oil | 2816.5 | 3613.2 | 2888.6 | 886 | 3081.2 | 190.5 | 1955.7 | 2127.2 | 12399.5 | 17558.9 | ||
| oil from fresh exhausted pomace | 1.3 | 357 | 477.1 | 753.3 | 238.3 | 819.6 | 96 | 1237.5 | 1345.4 | 2407 | 5324.2 | |
| PF2 | fresh pomace 7 days—surface | 6.9 | 0.3 | 5.9 | 1 | 0.4 | 0.8 | 0.1 | 0.9 | 0.8 | 8 | 10.2 |
| fresh pomace 7 days—depth | 5.4 | 0.1 | 3.8 | 0.8 | <0.1 | 0.5 | 0.1 | 0.3 | 0.6 | 5.2 | 6.2 | |
| fresh pomace 7 days—yard contact | 6.1 | 0.3 | 4.1 | 0.6 | <0.1 | 0.9 | 0.1 | 0.8 | 0.5 | 5.9 | 7.3 | |
| fresh pomace 2–3 days | 3.3 | 0.1 | 2.8 | 0.6 | <0.1 | 0.4 | <0.1 | 0.1 | 0.4 | 3.9 | 4.4 | |
| fresh pomace 15–20 days | 3.6 | 126.7 | 219.8 | 104.8 | 46.2 | 67.6 | 4 | 43.1 | 45.1 | 518.9 | 657.3 | |
| fresh pomace entering the drying plant | 4.5 | <0.1 | 2 | 0.5 | <0.1 | 0.8 | <0.1 | 0.5 | 0.3 | 1.5 | 4.1 | |
| dry pomace | 5.1 | 65.1 | 93.7 | 40.1 | 15.2 | 34.1 | 4.3 | 29 | 27.6 | 233 | 309.1 | |
| oil/hexane mixture | 151.1 | 357.3 | 128.1 | 54.7 | 23.1 | 5.7 | 149.2 | 138.4 | 659.6 | 1007.6 | ||
| crude oil | 307.6 | 576.6 | 306 | 117.2 | 349.8 | 37.3 | 465.5 | 485.3 | 1540 | 2645.3 | ||
| exhausted pomace | 0.2 | 355 | 798.3 | 222.8 | 81.9 | 200.5 | 26 | 164.9 | 207.8 | 1576.6 | 2057.2 | |
| hexane pre-extraction | <0.1 | <0.7 | <0.2 | <0.1 | <0.1 | <0.1 | <0.3 | <0.1 | - | - | ||
| hexane post-extraction | <0.1 | 1.2 | 0.6 | 0.4 | 0.2 | 0.1 | 0.6 | 0.3 | 2 | 3.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barp, L.; Moret, S.; Purcaro, G. Monitoring and Occurrence of Heavy PAHs in Pomace Oil Supply Chain Using a Double-Step Solid-Phase Purification and HPLC-FLD Determination. Foods 2022, 11, 2737. https://doi.org/10.3390/foods11182737
Barp L, Moret S, Purcaro G. Monitoring and Occurrence of Heavy PAHs in Pomace Oil Supply Chain Using a Double-Step Solid-Phase Purification and HPLC-FLD Determination. Foods. 2022; 11(18):2737. https://doi.org/10.3390/foods11182737
Chicago/Turabian StyleBarp, Laura, Sabrina Moret, and Giorgia Purcaro. 2022. "Monitoring and Occurrence of Heavy PAHs in Pomace Oil Supply Chain Using a Double-Step Solid-Phase Purification and HPLC-FLD Determination" Foods 11, no. 18: 2737. https://doi.org/10.3390/foods11182737
APA StyleBarp, L., Moret, S., & Purcaro, G. (2022). Monitoring and Occurrence of Heavy PAHs in Pomace Oil Supply Chain Using a Double-Step Solid-Phase Purification and HPLC-FLD Determination. Foods, 11(18), 2737. https://doi.org/10.3390/foods11182737






