High-Throughput Flow Injection Analysis–Mass Spectrometry (FIA-MS) Fingerprinting for the Authentication of Tea Application to the Detection of Teas Adulterated with Chicory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Samples and Sample Treatment
2.3. Instrumentation
2.4. Data Analysis
2.4.1. Data Matrices
2.4.2. Chemometric Data Analysis
3. Results and Discussion
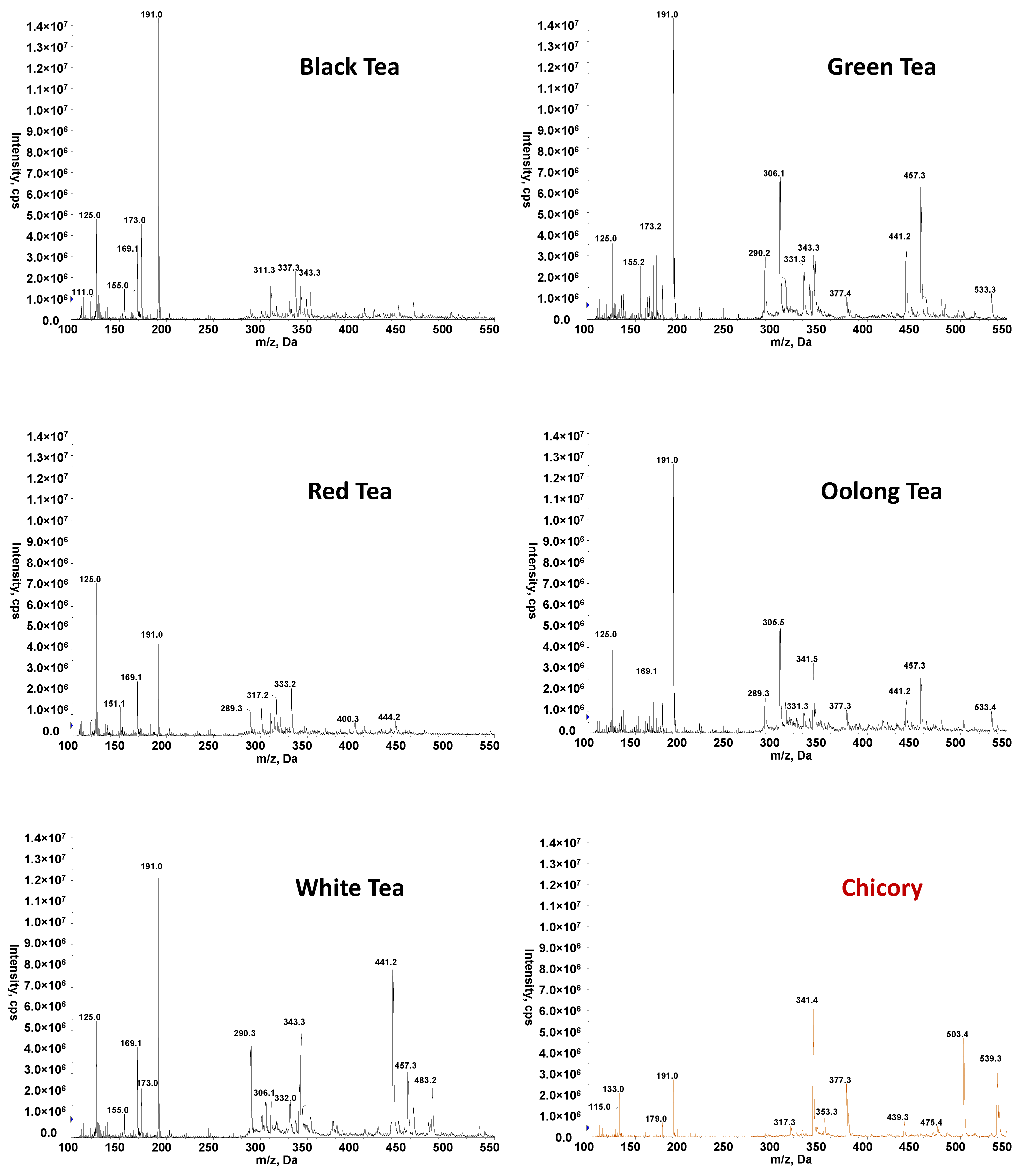
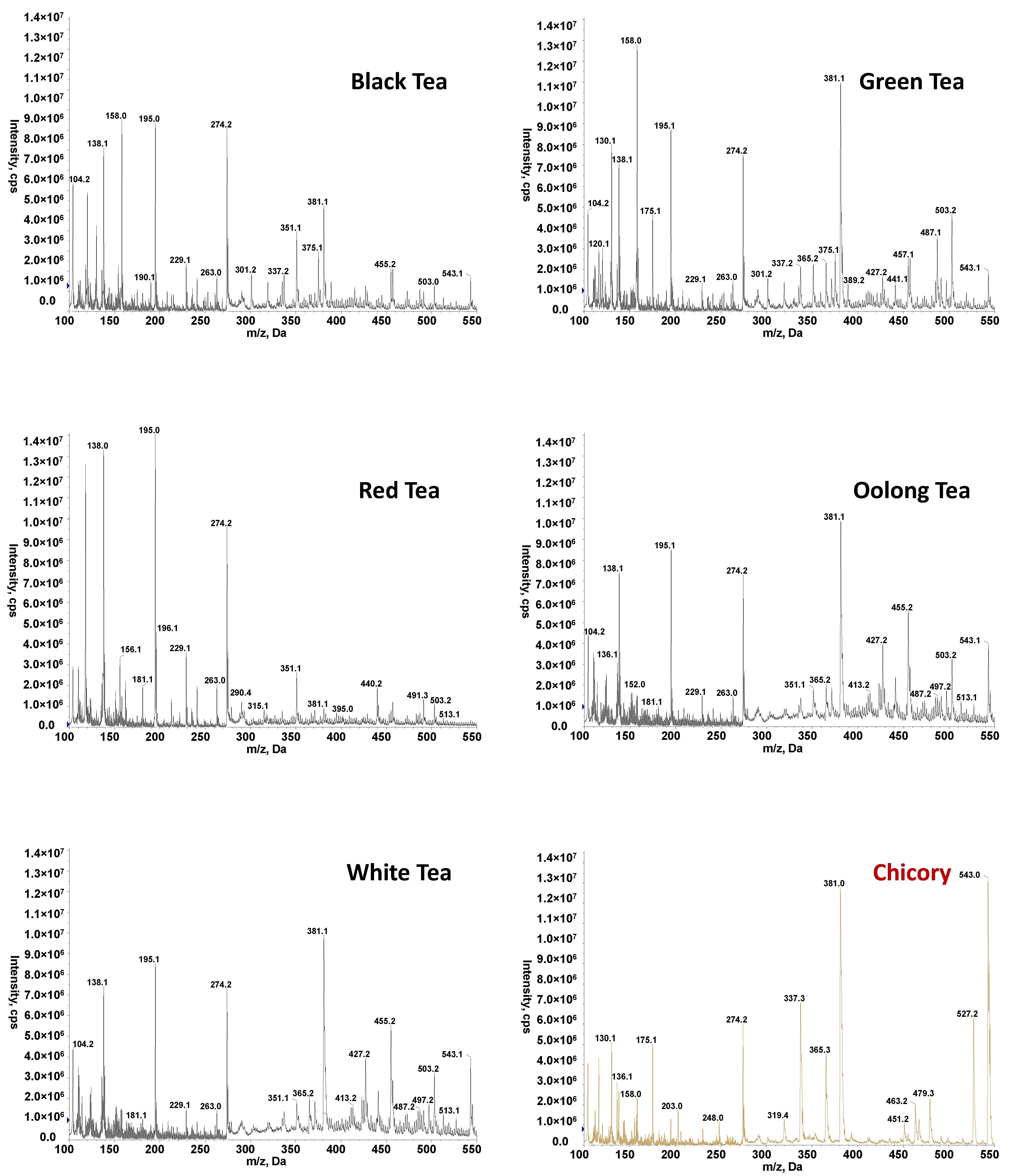
3.1. High-Throughput FIA-MS Fingerprints
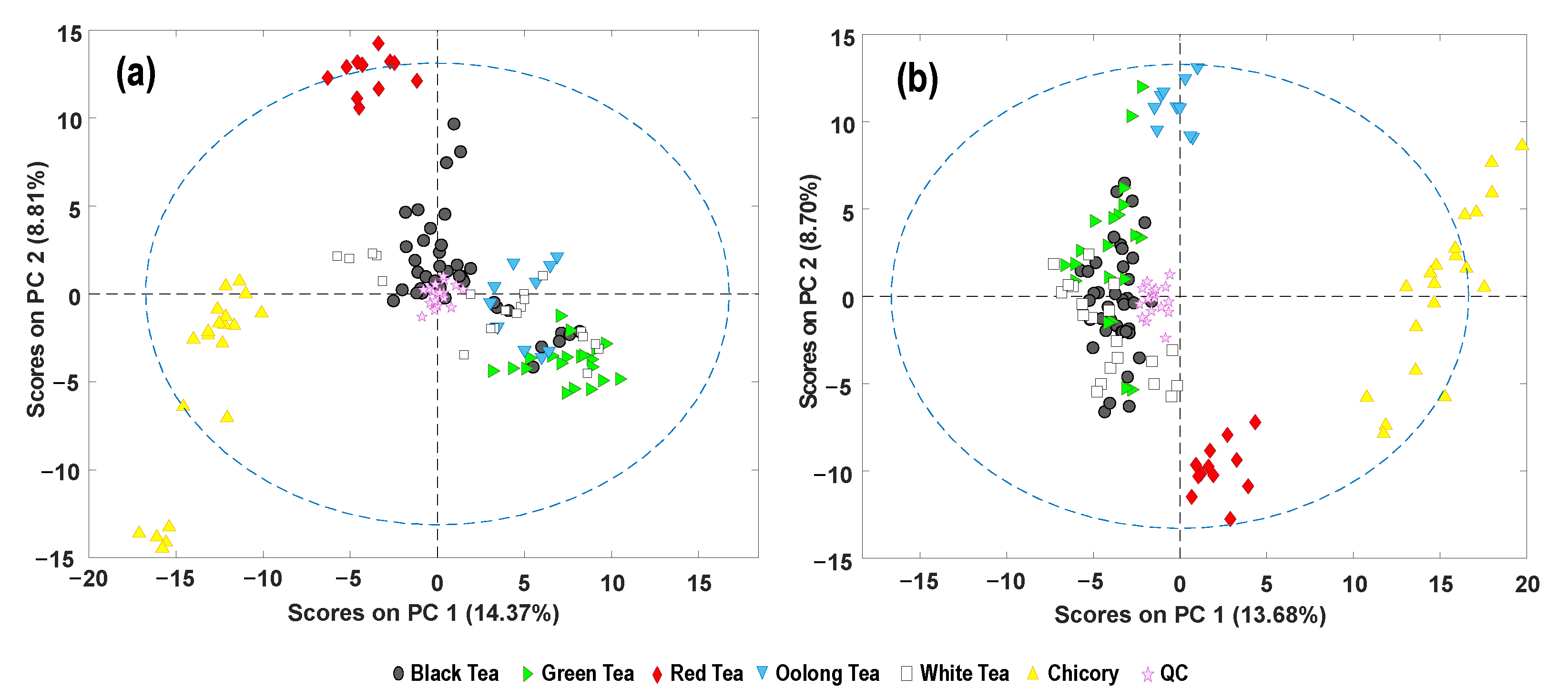
3.2. Exploratory Principal Component Analysis
3.3. Supervised Partial Least Squares–Discriminant Analysis
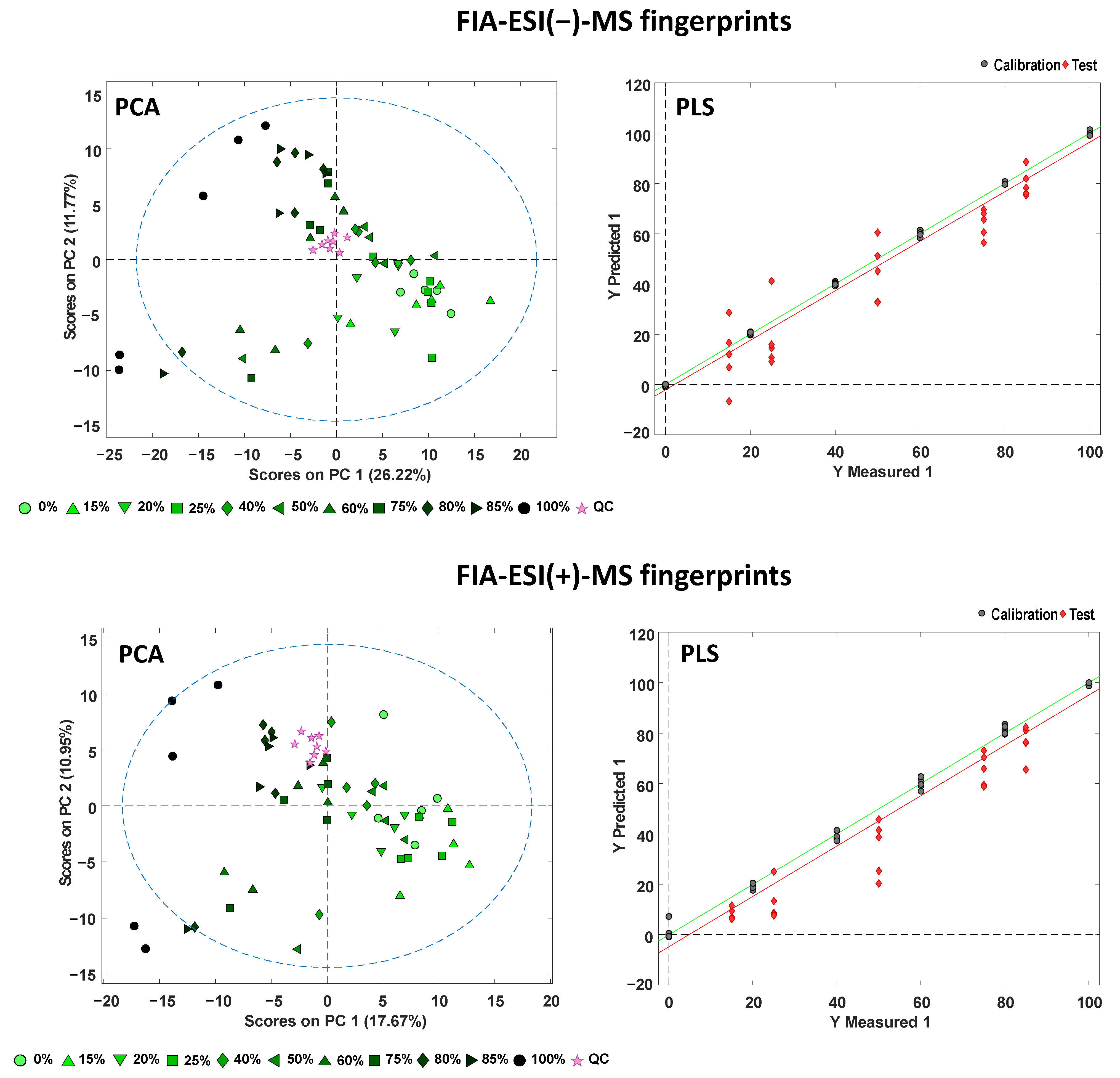
3.4. Detection and Quantitation of Chicory Adulterations in Tea by Partial Least Squares Regression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inarejos-García, A.M.; Helbig, I.; Klette, P.; Weber, S.; Maeder, J.; Morlock, G.E. Authentication of Commercial Powdered Tea Extracts (Camellia sinensis L.) by Gas Chromatography. ACS Food Sci. Technol. 2021, 1, 596–604. [Google Scholar] [CrossRef]
- Sanlier, N.; Gokcen, B.B.; Altuğ, M. Tea consumption and disease correlations. Trends Food Sci. Technol. 2018, 78, 95–106. [Google Scholar] [CrossRef]
- Jie, G.; Lin, Z.; Zhang, L.; Lv, H.; He, P.; Zhao, B. Free radical scavenging effect of Pu-erh tea extracts and their protective effect on oxidative damage in human fibroblast cells. J. Agric. Food Chem. 2006, 54, 8058–8064. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Piao, X.L.; Kim, J.M.; Kwon, S.W.; Park, J.H. Antilipogenic Effect of Green Tea Extract in C57BL/6J-Lepob/ob Mice. Phyther. Res. 2009, 23, 467–471. [Google Scholar] [CrossRef]
- Okello, E.J.; Savelev, S.U.; Perry, E.K. In vitro anti-β-secretase and dual anti-cholinesterase activities of Camellia sinensis L. (tea) relevant to treatment of dementia. Phyther. Res. 2004, 18, 624–627. [Google Scholar] [CrossRef]
- Spáčil, Z.; Nováková, L.; Solich, P. Comparison of positive and negative ion detection of tea catechins using tandem mass spectrometry and ultra high performance liquid chromatography. Food Chem. 2010, 123, 535–541. [Google Scholar] [CrossRef]
- Da Silva Pinto, M. Tea: A new perspective on health benefits. Food Res. Int. 2013, 53, 558–567. [Google Scholar] [CrossRef]
- Sang, S. Tea: Chemistry and Processing. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2016; pp. 268–272. ISBN 978-0-123-84953-3. [Google Scholar]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, L.; Lu, J. A study on chemical estimation of pu-erh tea quality. J. Sci. Food Agric. 2005, 85, 381–390. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Fang, C.H.; Wang, D. Feasibility study on identification of green, black and Oolong teas using near-infrared reflectance spectroscopy based on support vector machine (SVM). Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2007, 66, 568–574. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12–24. [Google Scholar] [CrossRef]
- Zambonin, C. Maldi-tof mass spectrometry applications for food fraud detection. Appl. Sci. 2021, 11, 3374. [Google Scholar] [CrossRef]
- European Comission. Food Fraud: What Does It Mean? Available online: https://ec.europa.eu/food/safety/food-fraud/whatdoes-it-mean_en (accessed on 5 May 2022).
- Van Ruth, S.M.; Huisman, W.; Luning, P.A. Food fraud vulnerability and its key factors. Trends Food Sci. Technol. 2017, 67, 70–75. [Google Scholar] [CrossRef]
- Lagiotis, G.; Stavridou, E.; Bosmali, I.; Osathanunkul, M.; Haider, N.; Madesis, P. Detection and quantification of cashew in commercial tea products using High Resolution Melting (HRM) analysis. J. Food Sci. 2020, 85, 1629–1634. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, S.; Tanwar, K. Recognition and Evaluation of Authenticity of Tea and Coffee. Int. J. Adv. Res. Sci. Commun. Technol. 2020, 11, 19–26. [Google Scholar]
- Pal, A.D.; Das, T. Analysis of adulteration in black tea. Int. J. Biol. Res. 2018, 3, 253–257. [Google Scholar]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food Ingredient–Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Cadot, P.; Kochuyt, A.M.; Van Ree, R.; Ceuppens, J.L. Oral allergy syndrome to chicory associated with birch pollen allergy. Int. Arch. Allergy Immunol. 2003, 131, 19–24. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Y.; Zhang, J.; Wang, G.; Chen, L.; Zhang, M.; Liu, T.; Liu, X.; Lu, C. Determining the geographical origin of Chinese green tea by linear discriminant analysis of trace metals and rare earth elements: Taking Dongting Biluochun as an example. Food Control. 2016, 59, 714–720. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Liao, R.; Zhao, J.; Yang, H.; Wang, F. Authentication of the geographical origin of Guizhou green tea using stable isotope and mineral element signatures combined with chemometric analysis. Food Control. 2021, 125, 107954. [Google Scholar] [CrossRef]
- Xia, W.; Li, Z.; Yu, C.C.; Liu, Z.; Nie, J.; Li, C.; Shao, S.; Zhang, Y.; Rogers, K.M.; Yuan, Y. Understanding processing, maturity and harvest period effects to authenticate early-spring Longjing tea using stable isotopes and chemometric analyses. Food Control. 2021, 124, 107907. [Google Scholar] [CrossRef]
- Fang, S.; Huang, W.J.; Wei, Y.; Tao, M.; Hu, X.; Li, T.; Kalkhajeh, Y.K.; Deng, W.W.; Ning, J. Geographical origin traceability of Keemun black tea based on its non-volatile composition combined with chemometrics. J. Sci. Food Agric. 2019, 99, 6937–6943. [Google Scholar] [CrossRef]
- Budínová, G.; Vláčil, D.; Mestek, O.; Volka, K. Application of infrared spectroscopy to the assessment of authenticity of tea. Talanta 1998, 47, 255–260. [Google Scholar] [CrossRef]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Sarker, S.D.; Nahar, L.; Al-Azizi, M.M.; Ashour, M.L. Authentication and discrimination of green tea samples using UV–vis, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2019, 164, 653–658. [Google Scholar] [CrossRef]
- Firmani, P.; De Luca, S.; Bucci, R.; Marini, F.; Biancolillo, A. Near infrared (NIR) spectroscopy-based classification for the authentication of Darjeeling black tea. Food Control. 2019, 100, 292–299. [Google Scholar] [CrossRef]
- Diniz, P.H.G.D.; Barbosa, M.F.; De Melo Milanez, K.D.T.; Pistonesi, M.F.; De Araújo, M.C.U. Using UV-Vis spectroscopy for simultaneous geographical and varietal classification of tea infusions simulating a home-made tea cup. Food Chem. 2016, 192, 374–379. [Google Scholar] [CrossRef]
- Peng, T.Q.; Yin, X.L.; Gu, H.W.; Sun, W.; Ding, B.; Hu, X.C.; Ma, L.A.; Wei, S.D.; Liu, Z.; Ye, S.Y. HPLC-DAD fingerprints combined with chemometric techniques for the authentication of plucking seasons of Laoshan green tea. Food Chem. 2021, 347, 128959. [Google Scholar] [CrossRef]
- Wang, L.; Wei, K.; Cheng, H.; He, W.; Li, X.; Gong, W. Geographical tracing of Xihu Longjing tea using high performance liquid chromatography. Food Chem. 2014, 146, 98–103. [Google Scholar] [CrossRef]
- Navratilova, K.; Hrbek, V.; Kratky, F.; Hurkova, K.; Tomaniova, M.; Pulkrabova, J.; Hajslova, J. Green tea: Authentication of geographic origin based on UHPLC-HRMS fingerprints. J. Food Compos. Anal. 2019, 78, 121–128. [Google Scholar] [CrossRef]
- Pons, J.; Bedmar, À.; Núñez, N.; Saurina, J.; Núñez, O. Tea and chicory extract characterization, classification and authentication by non-targeted HPLC-UV-FLD fingerprinting and chemometrics. Foods 2021, 10, 2935. [Google Scholar] [CrossRef]
- Ruíz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F. Flow Injection Analysis-Tandem Mass Spectrometry. In Fingerprinting Techniques in Food Authentication and Traceability; Siddiqi, K.S., Nollet, L.M.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 79–95. ISBN 978-1-138-19767-1. [Google Scholar]
- Sun, J.; Chen, P. A flow-injection mass spectrometry fingerprinting method for authentication and quality assessment of Scutellaria lateriflora-based dietary supplements. Anal. Bioanal. Chem. 2011, 401, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, J.; Mccoy, J.; Zhong, H.; Fletcher, E.J.; Harnly, J.; Chen, P. Spectrochimica Acta Part B Use of fl ow injection mass spectrometric fi ngerprinting and chemometrics for differentiation of three black cohosh species. Spectrochim. Acta Part B At. Spectrosc. 2015, 105, 121–129. [Google Scholar] [CrossRef]
- Campmajó, G.; Saurina, J.; Núñez, O. FIA–HRMS fingerprinting subjected to chemometrics as a valuable tool to address food classification and authentication: Application to red wine, paprika, and vegetable oil samples. Food Chem. 2022, 373, 131491. [Google Scholar] [CrossRef] [PubMed]
- Bedmar, À. Non-Targeted Fingerprinting Methodologies for the Authentication of Tea. Application to the Detection and Quantitation of Frauds in Adulterated Tea Samples with Chicory. Final Degree Research Project, Universitat de Barcelona. Available online: http://hdl.handle.net/2445/184649 (accessed on 5 May 2022).
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics. J. Chem. Inf. Comput. Sci. 1997, 38, 1254. [Google Scholar] [CrossRef]

| Sample Class | Sample Type (Codification) | Total Number of Samples |
|---|---|---|
| Tea | Black tea (B) | 39 |
| Green tea (G) | 20 | |
| Oolong tea (O) | 10 | |
| Red tea (R) | 12 | |
| White tea (W) | 20 | |
| Chicory | Chicory (C) | 20 |
| Tea (%) | Chicory (%) | Tea (%) | Chicory (%) | ||
|---|---|---|---|---|---|
| Calibration set | 100 | 0 | Validation set | 85 | 15 |
| 80 | 20 | 75 | 25 | ||
| 60 | 40 | 50 | 50 | ||
| 40 | 60 | 25 | 75 | ||
| 20 | 80 | 15 | 85 | ||
| 0 | 100 |
| FIA-MS: Positive-Ionization Mode | |||
|---|---|---|---|
| PLS-DA Model with 7 LVs | |||
| Class | Sensitivity (%) a | Specificity (%) b | False-positive remarks |
| Black tea | 94.8 | 100 | None |
| Green tea | 95.0 | 95.0 | Black tea (1); white tea (4) |
| Oolong tea | 100 | 100 | None |
| Red tea | 100 | 100 | None |
| White tea | 100 | 96.0 | Black tea (2); green tea (2) |
| Chicory | 100 | 100 | None |
| FIA-MS: Positive-Ionization Mode | |||
| PLS-DA Model with 6 LVs | |||
| Class | Sensitivity (%) a | Specificity (%) b | False-positive remarks |
| Black tea | 92.3 | 98.7 | White tea (1) |
| Green tea | 95.0 | 94.1 | Black tea (1); oolong tea (2); white tea (3) |
| Oolong tea | 90.0 | 96.4 | Green tea (4) |
| Red tea | 100 | 100 | None |
| White tea | 90.0 | 98.0 | Green tea (2) |
| Chicory | 100 | 100 | None |
| Green Tea Adulterated with Chicory | |||||||
|---|---|---|---|---|---|---|---|
| LVs | Calibration (R2) | Cross-Validation (R2) | Prediction (R2) | RMSEC (%) | RMSECV (%) | RMSEP (%) | |
| FIA-ESI(−)-MS fingerprints | 4 | 1.000 | 0.965 | 0.881 | 0.7 | 6.7 | 11.5 |
| FIA-ESI(+)-MS fingerprints | 4 | 0.997 | 0.960 | 0.935 | 2.0 | 7.2 | 12.8 |
| Black Tea Adulterated with Chicory | |||||||
| LVs | Calibration (R2) | Cross-Validation (R2) | Prediction (R2) | RMSEC (%) | RMSECV (%) | RMSEP (%) | |
| FIA-ESI(−)-MS fingerprints | 2 | 0.974 | 0.949 | 0.770 | 5.5 | 7.9 | 16.4 |
| FIA-ESI(+)-MS fingerprints | 2 | 0.971 | 0.944 | 0.946 | 5.8 | 8.5 | 7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilà, M.; Bedmar, À.; Saurina, J.; Núñez, O.; Sentellas, S. High-Throughput Flow Injection Analysis–Mass Spectrometry (FIA-MS) Fingerprinting for the Authentication of Tea Application to the Detection of Teas Adulterated with Chicory. Foods 2022, 11, 2153. https://doi.org/10.3390/foods11142153
Vilà M, Bedmar À, Saurina J, Núñez O, Sentellas S. High-Throughput Flow Injection Analysis–Mass Spectrometry (FIA-MS) Fingerprinting for the Authentication of Tea Application to the Detection of Teas Adulterated with Chicory. Foods. 2022; 11(14):2153. https://doi.org/10.3390/foods11142153
Chicago/Turabian StyleVilà, Mònica, Àlex Bedmar, Javier Saurina, Oscar Núñez, and Sònia Sentellas. 2022. "High-Throughput Flow Injection Analysis–Mass Spectrometry (FIA-MS) Fingerprinting for the Authentication of Tea Application to the Detection of Teas Adulterated with Chicory" Foods 11, no. 14: 2153. https://doi.org/10.3390/foods11142153
APA StyleVilà, M., Bedmar, À., Saurina, J., Núñez, O., & Sentellas, S. (2022). High-Throughput Flow Injection Analysis–Mass Spectrometry (FIA-MS) Fingerprinting for the Authentication of Tea Application to the Detection of Teas Adulterated with Chicory. Foods, 11(14), 2153. https://doi.org/10.3390/foods11142153









