Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
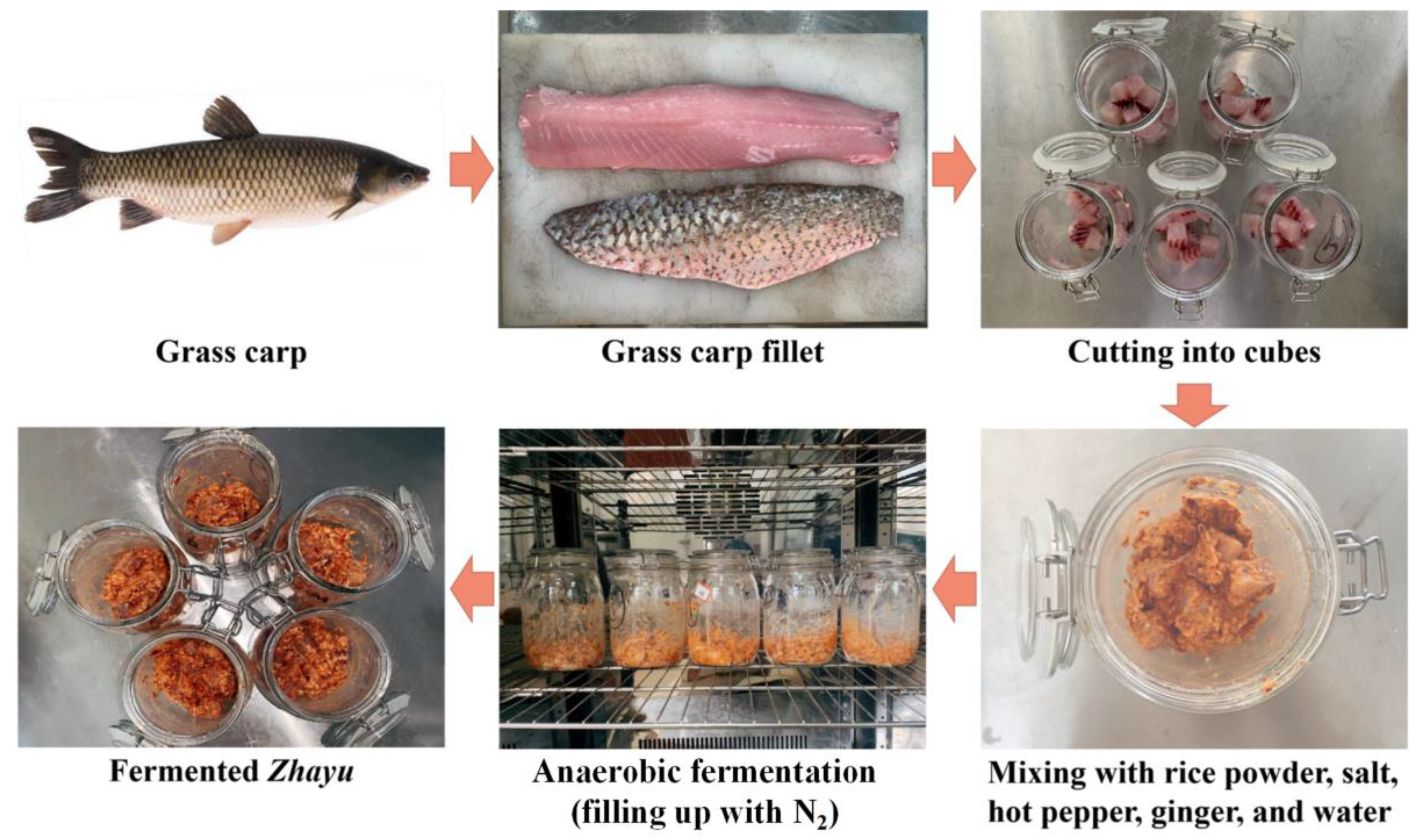
2.2. Preparation of Starter Culture
2.3. Sampling
2.4. Determination of pH, Titratable Acidity (TA), and Total Volatile Basic Nitrogen (TVB-N)
2.5. Determination of Crude Fat, Water-Soluble Protein, and Soluble Solids
2.6. Texture Analysis
2.7. Analysis of Flavor Characteristics of Zhayu
2.7.1. Sensory Analysis
2.7.2. Electronic Tongue (E-Tongue) Analysis
2.7.3. Electronic Nose (E-nose) Analysis
2.7.4. Determination of Free Amino Acids
2.7.5. GC-MS Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Safety Qualities
3.2. Nutritional Properties
3.3. Texture Properties
3.4. Taste Characteristics
3.5. Odor Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahanta, P.; Muzaddadi, A.U. Extension of shelflife of the fermented fish product, shidal by packaging in glass bottle and low temperature storage. Indian J. Fish 2013, 60, 135–143. [Google Scholar] [CrossRef]
- Paludan-Müller, C.; Madsen, M.; Sophanodora, P.; Gram, L.; Møller, P.L. Fermentation and microflora of plaa-som, a Thai fermented fish product prepared with different salt concentrations. Int. J. Food Microbiol. 2022, 73, 61–70. [Google Scholar] [CrossRef]
- Ijong, F.G.; Ohta, Y. Physicochemical and microbiological changes associated with Bakasang processing—A traditional Indonesian fermented fish sauce. J. Sci. Food Agric. 2015, 71, 69–74. [Google Scholar] [CrossRef]
- Devi, K.R.; Deka, M.; Jeyaram, K. Bacterial dynamics during yearlong spontaneous fermentation for production of ngari, a dry fermented fish product of Northeast India. Int. J. Food Microbiol. 2015, 199, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xia, W.; Yang, F.; Jiang, Q. Changes of biogenic amines in Chinese low-salt fermented fish pieces (Suan yu) inoculated with mixed starter cultures. Int. J. Food Sci. Technol. 2013, 48, 685–692. [Google Scholar] [CrossRef]
- Gao, P.; Wang, W.; Jiang, Q.; Xu, Y.; Xia, W. Effect of autochthonous starter cultures on the volatile flavour compounds of Chinese traditional fermented fish (Suan yu). Int. J. Food Sci. Technol. 2016, 51, 1630–1637. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Gao, P.; Xia, W.; Hua, Q.; Jiang, Q. Improvement of the quality stability of vacuum-packaged fermented fish (suanyu) stored at room temperature by irradiation and thermal treatments. Int. J. Food Sci. Technol. 2021, 56, 224–232. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Wu, Y.; Li, C.; Huang, H. Comparison of the microbial community and flavor compounds in fermented mandarin fish (Siniperca chuatsi): Three typical types of chinese fermented mandarin fish products. Food Res. Int. 2021, 144, 110365. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Wang, Y.; Li, L.; Yang, S. Contribution of autochthonous microbiota succession to flavor formation during chinese fermented mandarin fish (Siniperca chuatsi). Food Chem. 2021, 348, 129107. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, C.; Bao, R.; Liu, M.; Lin, X. Effects of flavourzyme addition on physicochemical properties, volatile compound components and microbial community succession of suanzhayu. Int. J. Food Microbiol. 2020, 334, 108839. [Google Scholar] [CrossRef]
- Gao, P.; Jiang, Q.; Xu, Y.; Xia, W. Esterase activities of autochthonous starter cultures to increase volatile flavour compounds in Chinese traditional fermented fish (Suan yu). Int. J. Food Prop. 2017, 20, S663–S672. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Wang, J.; Jiang, Q.; Xu, Y.; Qiu, Y.; Wang, H. Technological properties of Lactobacillus plantarum strains isolated from Chinese traditional low salt fermented whole fish. Food Control 2014, 40, 351–358. [Google Scholar] [CrossRef]
- Hua, Q.; Gao, P.; Xu, Y.; Xia, W.; Jiang, Q. Effect of commercial starter cultures on the quality characteristics of fermented fish-chili paste. LWT 2020, 122, 109016. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y. Influence of Lactobacillus plantarum on managing lipolysis and flavor generation of staphylococcus xylosus and saccharomyces cerevisiae in fish paste. LWT 2021, 140, 110709. [Google Scholar] [CrossRef]
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Physical properties and microstructure of commercial Som-fug, a fermented fish sausage. Eur. Food Res. Technol. 2005, 220, 520–525. [Google Scholar] [CrossRef]
- Feng, L.; Tang, N.; Liu, R.; Gong, M.; Wang, Z.; Guo, Y.; Wang, Y.; Zhang, Y.; Chang, M. The relationship between flavor formation, lipid metabolism, and microorganisms in fermented fish products. Food Funct. 2021, 12, 5685–5702. [Google Scholar] [CrossRef]
- Tan, R.C.; Ouyang, J.M.; Lu, X.L.; Xiong, S.B. Fermentation conditions of Yuzha by inoculated Lactobacillus plantarum and Pediococcus pentosaceus. Food Sci. 2007, 28, 268–272, (In Chinese with English abstract). [Google Scholar]
- Casquete, R.; Martin, A.; Benito, J.M.; Ruiz-Moyano, S.; Nevado, F.P. Impact of pre-selected autochthonous starter cultures on the flavor quality of iberian dry-fermented "salchichon" sausage with different ripening processes. J. Food Sci. 2011, 76, S535–S544. [Google Scholar] [CrossRef]
- Fei, Y.T.; Liu, D.M.; Luo, T.H.; Chen, G.; Wu, H.; Li, L.; Yu, Y.G.; Li, W. Molecular characterization of Lactobacillus plantarum DMDL 9010, a strain with efficient nitrite degradation capacity. PLoS ONE 2014, 9, 113792. [Google Scholar] [CrossRef]
- Ba, H.V.; Seo, H.W.; Seong, P.N.; Kang, S.M.; Kim, J.H. Lactobacillus plantarum (KACC 92189) as a potential probiotic starter culture for quality improvement of fermented sausages. Korean J. Food Sci. An. 2018, 38, 189–202. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists International Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1997. [Google Scholar]
- Cobb, B.F.; Alaniz, I.; Thompson, C.A. Biochemical and microbial studies on shrimp: Volatile nitrogen and amino nitrogen analysis. J. Food Sci. 1973, 38, 431–436. [Google Scholar] [CrossRef]
- Shin, J.M.; Hwang, Y.O.; Tu, O.J.; Jo, H.B.; Kim, J.H.; Chae, Y.Z.; Park, S.K. Comparison of different methods to quantify fat classes in bakery products. Food Chem. 2013, 136, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Liao, E.; Xu, Y.; Jiang, Q.; Xia, W. Characterisation of dominant autochthonous strains for nitrite degradation of Chinese traditional fermented fish. Int. J. Food Sci. Technol. 2018, 53, 2633–2641. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- ISO 4120:2004(E); Sensory Analysis-Methodology-Triangle Test. International Organization for Standardization (ISO): Geneva, Switzerland, 2004.
- ISO 4121:2003; Sensory Analysis-Guidelines for the Use of Quantitative Response Scales. International Organization for Standardization (ISO): Geneva, Switzerland, 2003.
- Luo, X.; Xiao, S.; Ruan, Q.; Gao, Q.; An, Y.; Hu, Y.; Xiong, S. Differences in flavor characteristics of frozen surimi products reheated by microwave, water boiling, steaming, and frying. Food Chem. 2022, 372, 131260. [Google Scholar] [CrossRef] [PubMed]
- Kováts, E. Gas-chromatographische Charakterisierung organischer Verbindungen. Teil 1: Retentionsindices aliphatischer Halogenide, Alkohole, Aldehyde und Ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Ortiz-Rivera, Y.; Sanchez-Vega, R.; Gutierrez-Mendez, N.; Leon-Felix, J.; Acosta-Muniz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef]
- Paukatong, K.V.; Kunawasen, S. Hazard analysis and critical control points (HACCP) generic model for the production of Thai fermented pork sausage (Nham). Berl. Munch. Tierarztl. Wochenschr. 2011, 114, 327–330. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, W.; Ge, C. Characterization of fermented silver carp sausages inoculated with mixed starter culture. LWT 2008, 41, 730–738. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, A.; Feng, L.; Gao, H. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. Int. J. Food Microbiol. 2016, 217, 146–155. [Google Scholar] [CrossRef]
- Xu, Y.; Xia, W.; Yang, F.; Kim, J.M.; Nie, X. Effect of fermentation temperature on the microbial and physicochemical properties of silver carp sausages inoculated with Pediococcus pentosaceus. Food Chem. 2010, 118, 512–518. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, P.; Xu, Y.; Xia, W.; Hua, Q.; Jiang, Q. Effect of storage conditions on microbiological characteristics, biogenic amines and physicochemical quality of low-salt fermented fish. J. Food Prot. 2020, 83, 1057–1065. [Google Scholar] [CrossRef]
- Sanz, B.; Selgas, D.; Parejo, I.; Ordóñez, J.A. Characteristics of lactobacilli isolated from dry fermented sausages. Int. J. Food Microbiol. 1988, 6, 199–205. [Google Scholar] [CrossRef]
- Montel, M.C.; Masson, F.; Talon, R. Bacterial role in flavour development. Meat Sci. 1998, 49, S111–S123. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Sun, L.; Dong, L.; Du, M. Dynamics of microbial communities, texture and flavor in suan zuo yu during fermentation. Food Chem. 2020, 332, 127364. [Google Scholar] [CrossRef]
- Maruji, Y.; Shimizu, M.; Murata, M.; Ando, M.; Sakaguchi, M.; Hirata, T. Multiple taste functions of the umami substances in muscle extracts of yellowtail and bastard halibut. Fisheries Sci. 2010, 76, 521–528. [Google Scholar] [CrossRef]
- Nasri, N.; Beno, N.; Septier, C.; Salles, C.; Thomas-Danguin, T. Cross-modal interactions between taste and smell: Odour-induced saltiness enhancement depends on salt level. Food Qual. Prefer. 2011, 22, 678–682. [Google Scholar] [CrossRef]
- Zhu, W.; Luan, H.; Bu, Y.; Li, J.; Zhang, Y. Changes in taste substances during fermentation of fish sauce and the correlation with protease activity. Food Res. Int. 2021, 144, 110349. [Google Scholar] [CrossRef]
- Gao, P.; Li, L.; Xia, W.; Xu, Y.; Liu, S. Valorization of nile tilapia (Oreochromis niloticus) fish head for a novel fish sauce by fermentation with selected lactic acid bacteria. LWT 2020, 129, 109539. [Google Scholar] [CrossRef]
- An, Y.; Qian, Y.L.; Magana, A.A.; Xiong, S.; Qian, M.C. Comparative characterization of aroma compounds in silver carp (Hypophthalmichthys molitrix), Pacific whiting (Merluccius productus), and Alaska pollock (Theragra chalcogramma) surimi by aroma extract dilution analysis, odor activity value, and aroma recombination studies. J. Agric. Food Chem. 2020, 68, 10403–10413. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Li, J.; Tu, Z.; Zhang, L.; Lin, D.; Sha, X.; Zeng, K.; Wang, H.; Pang, J.; Tang, P. Characterization of volatile compounds in grass carp (Ctenopharyngodon idellus) soup cooked using a traditional Chinese method by GC-MS. J. Food Process Pres. 2017, 41, 12995. [Google Scholar] [CrossRef]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of curing salts and probiotic cultures on the evolution of flavor compounds in dry-fermented sausages during ripening. Food Chem. 2016, 201, 334–338. [Google Scholar] [CrossRef]



| Inoculation Amounts (cfu/100 g) | Hardness (g) | Springiness | Chewiness (g) |
|---|---|---|---|
| Control | 250.16 ± 31.54 a | 1.00 ± 0.01 a | 163.4 ± 20.3 a |
| L. plantarum | |||
| 106 | 203.02 ± 26.84 bcd | 0.71 ± 0.06 ef | 45.53 ± 18.11 ef |
| 107 | 171.64 ± 16.33 de | 0.80 ± 0.19 ef | 42.86 ± 9.95 ef |
| 108 | 176.85 ± 21.91 cde | 0.88 ± 0.09 de | 54.19 ± 9.04 de |
| 109 | 186.78±20.6 cd | 0.75 ± 0.10 f | 31.66 ± 9.43 f |
| P. acidilactici | |||
| 106 | 178.21 ± 3.90 cd | 0.99 ± 0.01 ab | 111.4 ± 2.8 bc |
| 107 | 171.81 ± 32.46 de | 0.97 ± 0.02 b | 61.39 ± 2.65 de |
| 108 | 174.32 ± 26.46 cde | 0.79 ± 0.09 d | 67.31 ± 5.12 d |
| 109 | 162.28 ± 16.33 de | 0.84 ± 0.09 ef | 44.31 ± 6.26 ef |
| P. pentosaceus | |||
| 106 | 246.96 ± 26.27 ab | 0.87 ± 0.06 cd | 125.1 ± 3.6 b |
| 107 | 247.29 ± 35.79 ab | 0.84 ± 0.03 d | 94.07 ± 4.92 c |
| 108 | 220.27 ± 11.41 abc | 0.91 ± 0.03 c | 98.97 ± 19.33 c |
| 109 | 130.35 ± 31.86 e | 0.94 ± 0.06 bc | 109.6 ± 18.2 bc |
| FAA | Threshold (mg/100 g) | Taste | Concentration (mg/100 g) | TAV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SF | L.P. | P.A. | P.P. | SF | L.P. | P.A. | P.P. | |||
| Asp | 3 | umami | 5.51 ± 0.32 d | 45.6 ± 1.7 b | 42.1 ± 1.1 c | 52.1 ± 1.9 a | 1.84 | 15.19 | 14.05 | 17.36 |
| Glu | 5 | umami | 49.0 ± 3.3 d | 56.8 ± 2.7 b | 51.8 ± 1.6 c | 67.8 ± 2.47 a | 9.80 | 14.05 | 17.26 | 22.61 |
| Ser | 150 | sweet | 4.61 ± 0.26 a | 2.97 ± 0.31 c | 2.24 ± 0.06 d | 3.66 ± 0.13 b | 0.03 | 0.02 | 0.01 | 0.02 |
| Gly | 150 | sweet | 23.2 ± 1.3 a | 19.4 ± 0.8 c | 21.1 ± 0.5 b | 21.6 ± 0.7 bc | 0.15 | 0.13 | 0.14 | 0.14 |
| Thr | 260 | sweet | 9.26 ± 0.53 c | 14.2 ± 0.4 b | 17.8 ± 0.4 a | 18.4 ± 0.7 a | 0.04 | 0.05 | 0.07 | 0.07 |
| Ala | 60 | sweet | 58.5 ± 3.3 a | 44.3 ± 1.4 c | 45.2 ± 1.2 c | 47.5 ± 1.7 b | 0.97 | 0.74 | 0.75 | 0.79 |
| Pro | 300 | sweet/bitter | 51.9 ± 2.9 b | 51.8 ± 3.5 b | 46.5 ± 1.2 c | 56.6 ± 2.1 a | 0.17 | 0.17 | 0.15 | 0.19 |
| Lys | 50 | sweet/bitter | 12.6 ± 0.7 c | 45.1 ± 1.2 ab | 44.2 ± 1.1 b | 46.4 ± 1.6 a | 0.25 | 0.90 | 0.88 | 0.93 |
| Arg | 50 | bitter | 1.97 ± 0.11 b | 2.53 ± 0.36 a | 1.11 ± 0.03 d | 1.48 ± 0.05 c | 0.04 | 0.05 | 0.02 | 0.03 |
| Val | 40 | bitter | 30.3 ± 1.7 a | 25.5 ± 0.7 c | 26.2 ± 0.7 bc | 27.0 ± 0.9 b | 0.76 | 0.64 | 0.65 | 0.68 |
| Tyr | N.A. | bitter | 20.8 ± 1.2 c | 27.7 ± 0.7 a | 24.6 ± 0.6 b | 25.9 ± 0.9 b | ||||
| His | 20 | bitter | 30.4 ± 1.7 c | 76.4 ± 3.6 a | 79.8 ± 2.1 a | 70.8 ± 2.5 b | 1.52 | 3.82 | 3.99 | 3.54 |
| Leu | 190 | bitter | 50.5 ± 2.9 b | 53.4 ± 1.6 a | 50.9 ± 1.3 b | 53.5 ± 1.9 a | 0.27 | 0.28 | 0.27 | 0.28 |
| Ile | 90 | bitter | 23.2 ± 1.3 a | 18.1 ± 0.7 b | 17.5 ± 0.5 b | 18.4 ± 0.7 b | 0.26 | 0.20 | 0.19 | 0.20 |
| Phe | 90 | bitter | 38.2 ± 2.1 ab | 41.1 ± 1.0 a | 37.7 ± 0.9 b | 39.0 ± 1.4 ab | 0.42 | 0.46 | 0.42 | 0.43 |
| Met | 30 | bitter | 17.8 ± 1.0 a | 15.2 ± 0.6 b | 14.3 ± 0.3 c | 14.9 ± 0.5 bc | 0.59 | 0.51 | 0.48 | 0.50 |
| Cys | N.A. | salt | N.D. | N.D. | N.D. | N.D. | ||||
| Compounds | RI | Identification | Odor | Odor Threshold (μg/kg) | Relative Concentration (μg/kg) | |||
|---|---|---|---|---|---|---|---|---|
| SF | L.P. | P.A. | P.P. | |||||
| Aldehydes | ||||||||
| 2/3-methylbutanal | 923 | MS, RIL | malty | 1.5/0.5 b | 0.74 ± 0.13 a | 0.51 ± 0.05 c | 0.61 ± 0.04 b | 0.67 ± 0.07 b |
| hexanal | 1087 | MS, RI | grassy | 2.4 a | 2.52 ± 0.78 a | 0.48 ± 0.02 b | 0.49 ± 0.09 b | 0.46 ± 0.17 b |
| octanal | 1294 | MS, RI | citrus | 3.4 a | 0.28 ± 0.13 a | 0.11 ± 0.03 c | 0.15 ± 0.04 b | N.D. |
| (E)-2-heptenal | 1326 | MS, RI | green | 13 c | 0.48 ± 0.07 a | 0.41 ± 0.05 b | 0.45 ± 0.06 ab | 0.40 ± 0.04 b |
| nonanal | 1399 | MS, RI | citrus | 2.8 a | 0.09 ± 0.02 a | 0.11 ± 0.02 a | 0.12 ± 0.03 a | N.D. |
| (E)-2-octenal | 1435 | MS, RI | nutty | 3 a | 1.17 ± 0.03 a | 0.59 ± 0.05 b | 0.61 ± 0.09 b | 0.66 ± 0.07 b |
| benzaldehyde | 1508 | MS, RI | rosy | 350–3500 c | 1.35 ± 0.36 a | 1.07 ± 0.00 a | 1.17 ± 0.03 a | 1.02 ± 0.19 a |
| (E,E)-2,4-decadienal | 1820 | MS, RI | fishy, fatty | 0.027 a | 1.64 ± 0.11 a | 0.85 ± 0.05 b | 0.86 ± 0.06 b | 0.67 ± 0.12 c |
| Alcohols | ||||||||
| 1-propanol | 1049 | MS, RIL | alcoholic | 7000 c | 0.29 ± 0.02 c | 0.38 ± 0.16 b | 0.54 ± 0.00 b | 0.89 ± 0.05 a |
| 1-penten-3-ol | 1164 | MS, RI | grassy | 400 c | 0.76 ± 0.16 a | 0.56 ± 0.05 a | 0.42 ± 0.08 b | 0.65 ± 0.12 a |
| 1-pentanol | 1260 | MS, RIL | fusel-like | 4000 c | 3.35 ± 0.88 a | 1.93 ± 0.14 b | 1.75 ± 0.25 b | 1.85 ± 0.15 b |
| 2-heptanol | 1322 | MS, RIL | herbaceous | 41–81 c | 2.41 ± 0.35 b | 5.06 ± 0.02 a | 4.34 ± 0.21 a | 4.25 ± 0.83 a |
| 1-hexanol | 1359 | MS, RI | herbaceous | 2500 c | 7.19 ± 0.84 ab | 8.14 ± 0.31 a | 6.00 ± 0.42 b | 8.29 ± 0.88 a |
| (Z)-3-hexen-1-ol | 1385 | MS, RI | fishy | 3.9 b | 1.86 ± 0.38 a | 1.57 ± 0.09 a | 1.27 ± 0.07 b | 1.74 ± 0.13 a |
| 3-octanol | 1390 | MS, RIL | oily, nutty | 18–250 c | 0.46 ± 0.07 b | 0.67 ± 0.08 a | 0.67 ± 0.04 a | 0.62 ± 0.18 ab |
| 1-octen-3-ol | 1457 | MS, RI | mushroom | 1.2 a | 1.64 ± 0.34 a | 1.49 ± 0.29 a | 0.51 ± 0.06 b | 1.27 ± 0.48 a |
| 1-heptanol | 1468 | MS, RIL | woody | 3 c | 0.21 ± 0.03 c | 0.90 ± 0.07 a | 0.65 ± 0.06 b | 0.58 ± 0.07 b |
| 2-ethylhexanol | 1488 | MS, RIL | green, oily | 270,000 c | 0.41 ± 0.07 b | 1.01 ± 0.13 a | 0.67 ± 0.12 ab | 0.98 ± 0.27 a |
| 1-octanol | 1575 | MS, RIL | soapy | 110–130 c | 0.19 ± 0.03 c | 0.44 ± 0.01 a | 0.30 ± 0.01 b | 0.31 ± 0.09 b |
| 1-nonanol | 1663 | MS, RIL | soapy | 50 c | 0.15 ± 0.00 c | 0.73 ± 0.09 a | 0.36 ± 0.03 b | 0.43 ± 0.13 b |
| benzyl alcohol | 1880 | MS, RI | rosy | 1.2–1000 c | 0.73 ± 0.19 b | 0.87 ± 0.11 ab | 0.92 ± 0.02 a | 1.05 ± 0.18 a |
| phenylethyl alcohol | 1922 | MS, RI | rosy | 140 b | 0.43 ± 0.01 b | 0.54 ± 0.05 a | 0.56 ± 0.03 a | 0.67 ± 0.14 a |
| Terpenoids | ||||||||
| α-pinene | 1036 | MS, RIL | pine | 2.2 b | 3.41 ± 1.33 a | 1.41 ± 0.16 b | 1.93 ± 0.23 a | 2.43 ± 0.34 a |
| camphene | 1059 | MS, RIL | camphoraceous | N.A. | 4.06 ± 0.99 b | 3.03 ± 0.21 b | 4.53 ± 1.79 b | 7.72 ± 1.12 a |
| β-myrcene | 1170 | MS, RIL | balsamic | 1.2 b | 0.56 ± 0.15 a | 0.15 ± 0.01 b | 0.63 ± 0.09 a | 0.47 ± 0.12 a |
| D-limonene | 1204 | MS, RIL | lemon-like | 10 c | 0.70 ± 0.27 b | 0.56 ± 0.07 c | 1.14 ± 0.01 a | 1.02 ± 0.23 ab |
| β-phellandrene | 1219 | MS, RIL | citrus | 40–200 c | 0.77 ± 0.07 b | 0.87 ± 0.04 b | 1.23 ± 0.70 a | 1.03 ± 0.28 a |
| eucalyptol | 1230 | MS, RIL | camphoraceous | 12 c | 12.46 ± 2.01 b | 14.26 ± 0.53 a | 15.15 ± 1.68 a | 15.35 ± 4.12 ab |
| caryophyllene | 1570 | MS, RIL | woody | 64 c | N.D. | 2.35 ± 0.58 a | 1.22 ± 0.34 b | 2.33 ± 0.14 a |
| camphor | 1524 | MS, RIL | minty | 1000–1290 c | 0.36 ± 0.07 c | 0.69 ± 0.05 b | 0.80 ± 0.01 a | 0.83 ± 0.18 ab |
| linalool | 1554 | MS, RIL | lemon-like | 0.87 b | 5.23 ± 0.70 b | 6.83 ± 0.26 a | 6.79 ± 0.22 a | 7.28 ± 1.70 a |
| terpinen-4-ol | 1635 | MS, RIL | lilac-like | N.A. | 0.26 ± 0.06 b | 0.58 ± 0.05 a | 0.56 ± 0.04 a | 0.57 ± 0.12 a |
| citral | 1663 | MS, RIL | lemon-like | 32 c | 0.09 ± 0.00 b | 0.58 ± 0.08 a | 0.68 ± 0.02 a | 0.53 ± 0.21 a |
| α-terpineol | 1692 | MS, RIL | lilac-like | 330 c | 0.55 ± 0.05 b | 0.88 ± 0.10 a | 0.90 ± 0.04 a | 1.05 ± 0.31 a |
| borneol | 1696 | MS, RIL | piney | 140 c | 1.62 ± 0.21 b | 3.24 ± 0.45 a | 3.28 ± 0.02 a | 4.11 ± 1.24 a |
| β-bisabolene | 1721 | MS, RIL | balsamic | N.A. | 0.25 ± 0.00 b | 0.73 ± 0.21 a | 0.66 ± 0.08 a | 1.23 ± 0.41 a |
| neral | 1733 | MS, RIL | lemon-like | 30 c | 0.38 ± 0.01 b | 0.59 ± 0.06 a | 0.73 ± 0.01 a | 0.64 ± 0.22 a |
| nerol | 1808 | MS, RIL | rosy | 300 c | 0.15 ± 0.03 c | 0.32 ± 0.00 a | 0.24 ± 0.01 b | 0.24 ± 0.07 b |
| isogeraniol | 1812 | MS, RIL | rosy | N.A. | N.D. | 1.23 ± 0.19 ab | 1.40 ± 0.08 a | 1.03 ± 0.13 b |
| geraniol | 1857 | MS, RIL | rosy | 1.1 b | 0.33 ± 0.04 c | 1.91 ± 0.15 a | 0.92 ± 0.10 b | 1.09 ± 0.41 b |
| Ketones | ||||||||
| 2,3-butanedione | 968 | MS, RI | buttery | 1 b | 2.20 ± 0.93 a | 0.53 ± 0.02 b | 0.52 ± 0.03 b | 0.72 ± 0.17 b |
| 3-octanone | 1240 | MS, RIL | fruity | 21–50 c | 0.27 ± 0.12 ab | 0.15 ± 0.00 b | 0.09 ± 0.01 c | 0.31 ± 0.04 a |
| 3-hydroxy-2-butanone | 1286 | MS, RI | yogurt | 800 c | 15.11 ± 3.08 a | 1.87 ± 0.09 b | 1.56 ± 0.29 b | 1.55 ± 0.26 b |
| 6-methyl-5-hepten-2-one | 1341 | MS, RIL | green, fatty | 50 c | 0.87 ± 0.14 a | 0.41 ± 0.04 b | 0.56 ± 0.10 b | 0.67 ± 0.22 ab |
| Lactones | ||||||||
| butyrolactone | 1634 | MS, RI | sweet, buttery | 20,000–50,000 c | 0.26 ± 0.04 a | N.D. | N.D. | N.D. |
| lavender lactone | 1684 | MS, RIL | lavender | N.A. | 0.48 ± 0.09 a | 0.43 ± 0.03 a | 0.44 ± 0.03 a | 0.50 ± 0.03 a |
| γ-nonalactone | 2042 | MS, RI | coconut | 9.7 b | 0.10 ± 0.01 c | 0.19 ± 0.01 b | 0.19 ± 0.02 b | 0.23 ± 0.01 a |
| Acids | ||||||||
| acetic acid | 1445 | MS, RIL | acid | 99,000 b | 6.93 ± 0.33 c | 17.73 ± 1.57 a | 13.53 ± 0.94 b | 13.28 ± 2.07 b |
| butanoic acid | 1576 | MS, RI | rancid | 2400 b | 0.12 ± 0.01 c | 0.41 ± 0.07 a | 0.36 ± 0.05 a | 0.26 ± 0.02 b |
| 2/3-methylbutanoic acid | 1627 | MS, RI | rancid | 2200/490 b | 1.22 ± 0.21 a | 1.13 ± 0.07 a | 1.23 ± 0.24 a | 1.09 ± 0.10 a |
| pentanoic acid | 1720 | MS, RIL | rancid | 11,000 b | 0.10 ± 0.01 a | 0.08 ± 0.01 a | 0.09 ± 0.02 a | 0.07 ± 0.02 a |
| 4-methylpentanoic acid | 1817 | MS, RIL | rancid | 810 c | 0.20 ± 0.02 b | 0.42 ± 0.08 a | 0.18 ± 0.01 b | 0.22 ± 0.04 b |
| hexanoic acid | 1839 | MS, RI | rancid | 890 a | 0.53 ± 0.14 a | 0.35 ± 0.04 b | 0.42 ± 0.06 a | 0.28 ± 0.09 b |
| octanoic acid | 2089 | MS, RI | rancid | 3000 a | 0.05 ± 0.01 c | 0.12 ± 0.01 b | 0.19 ± 0.04 a | 0.10 ± 0.01 b |
| Esters | ||||||||
| ethyl acetate | 880 | MS, RI | fruity | 5–5000 d | 0.86 ± 0.11 c | 1.12 ± 0.01 a | 1.07 ± 0.11 b | 0.92 ± 0.08 bc |
| butyl acetate | 1078 | MS, RI | pineapple | 66 c | N.D. | 0.04 ± 0.02 a | 0.03 ± 0.03 a | N.D. |
| isoamyl acetate | 1126 | MS, RI | banana | 2 d | N.D. | 0.07 ± 0.01 b | 0.13 ± 0.01 a | N.D. |
| methyl salicylate | 1759 | MS, RIL | minty | 40 d | 0.34 ± 0.07 b | 1.28 ± 0.05 a | 1.22 ± 0.04 a | 1.45 ± 0.32 a |
| 2-phenylethyl butanoate | 1978 | MS, RIL | rosy | N.A. | 0.27 ± 0.04 ab | 0.29 ± 0.01 a | 0.22 ± 0.01 c | 0.25 ± 0.02 b |
| Phenols | ||||||||
| 2-methoxyphenol | 1862 | MS, RI | sweet | 0.84 b | 4.49 ± 0.81 a | 0.35 ± 0.03 c | 0.36 ± 0.01 c | 0.53 ± 0.05 b |
| phenol | 2008 | MS, RI | phenolic | 5900 c | 1.63 ± 0.34 a | 0.28 ± 0.01 c | 0.29 ± 0.01 c | 0.64 ± 0.05 b |
| 2-methoxy-4-vinylphenol | 2155 | MS, RI | phenolic | 5.1 b | 1.33 ± 0.19 a | 0.15 ± 0.01 c | 0.13 ± 0.02 c | 0.44 ± 0.09 b |
| eugenol | 2176 | MS, RIL | clove | 6–30 c | 2.22 ± 0.11 d | 8.29 ± 1.22 b | 6.32 ± 1.25 c | 11.23 ± 1.96 a |
| S-containing compounds | ||||||||
| dimethyl sulfide | 777 | MS, RIL | garlic | 0.84 a | 5.71 ± 1.73 c | 70.47 ± 6.99 a | 45.39 ± 3.73 b | 66.03 ± 11.74 a |
| dimethyl disulfide | 1107 | MS, RIL | fishy | 1.1 a | N.D. | 0.20 ± 0.07 a | N.D. | 0.09 ± 0.04 b |
| N-containing compounds | ||||||||
| 2-methylpyrazine | 1258 | MS, RIL | nutty, cocoa-like | 60–100,000 c | 0.89 ± 0.17 a | 0.52 ± 0.04 d | 0.68 ± 0.03 b | 0.64 ± 0.01 c |
| 2,3-dimethylpyrazine | 1339 | MS, RIL | nutty, cocoa-like | 2500–35,000 c | 0.37 ± 0.04 a | 0.13 ± 0.01 c | 0.24 ± 0.02 b | 0.11 ± 0.02 c |
| 2-ethyl-6-methylpyrazine | 1377 | MS, RIL | roasted | N.A. | 0.46 ± 0.08 a | 0.28 ± 0.01 b | 0.48 ± 0.07 a | 0.29 ± 0.01 b |
| trimethylpyrazine | 1399 | MS, RIL | baked potato | 400–1800 c | 0.13 ± 0.01 a | 0.11 ± 0.01 b | 0.14 ± 0.02 a | 0.11 ± 0.01 b |
| tetramethylpyrazine | 1472 | MS, RIL | musty, fermented, coffee | 1000–10,000 c | 0.66 ± 0.05 a | 0.49 ± 0.01 b | 0.64 ± 0.04 a | 0.48 ± 0.02 b |
| 4-methyl-5-thiazoleethanol | 2275 | MS, RI | nutty | 10,800 c | 0.20 ± 0.02 a | 0.10 ± 0.02 b | 0.14 ± 0.05 ab | 0.06 ± 0.00 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Cai, X.; Cong, L.; Hu, Y.; Liu, R.; Xiong, S.; Hu, X. Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria. Foods 2022, 11, 2756. https://doi.org/10.3390/foods11182756
An Y, Cai X, Cong L, Hu Y, Liu R, Xiong S, Hu X. Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria. Foods. 2022; 11(18):2756. https://doi.org/10.3390/foods11182756
Chicago/Turabian StyleAn, Yueqi, Xiaowen Cai, Lin Cong, Yang Hu, Ru Liu, Shanbai Xiong, and Xiaobo Hu. 2022. "Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria" Foods 11, no. 18: 2756. https://doi.org/10.3390/foods11182756
APA StyleAn, Y., Cai, X., Cong, L., Hu, Y., Liu, R., Xiong, S., & Hu, X. (2022). Quality Improvement of Zhayu, a Fermented Fish Product in China: Effects of Inoculated Fermentation with Three Kinds of Lactic Acid Bacteria. Foods, 11(18), 2756. https://doi.org/10.3390/foods11182756





