Growth and Spoilage Potential of an Aeromonas salmonicida Strain in Refrigerated Atlantic Cod (Gadus morhua) Stored under Various Modified Atmospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Experimental Design
2.2. Inoculum Preparation
2.3. Packing and Storage Conditions
2.4. Sampling and Bacterial Quantification
2.5. Quantification of Metabolites by Nuclear Magnetic Resonance (NMR)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Headspace Gas Composition in Packages
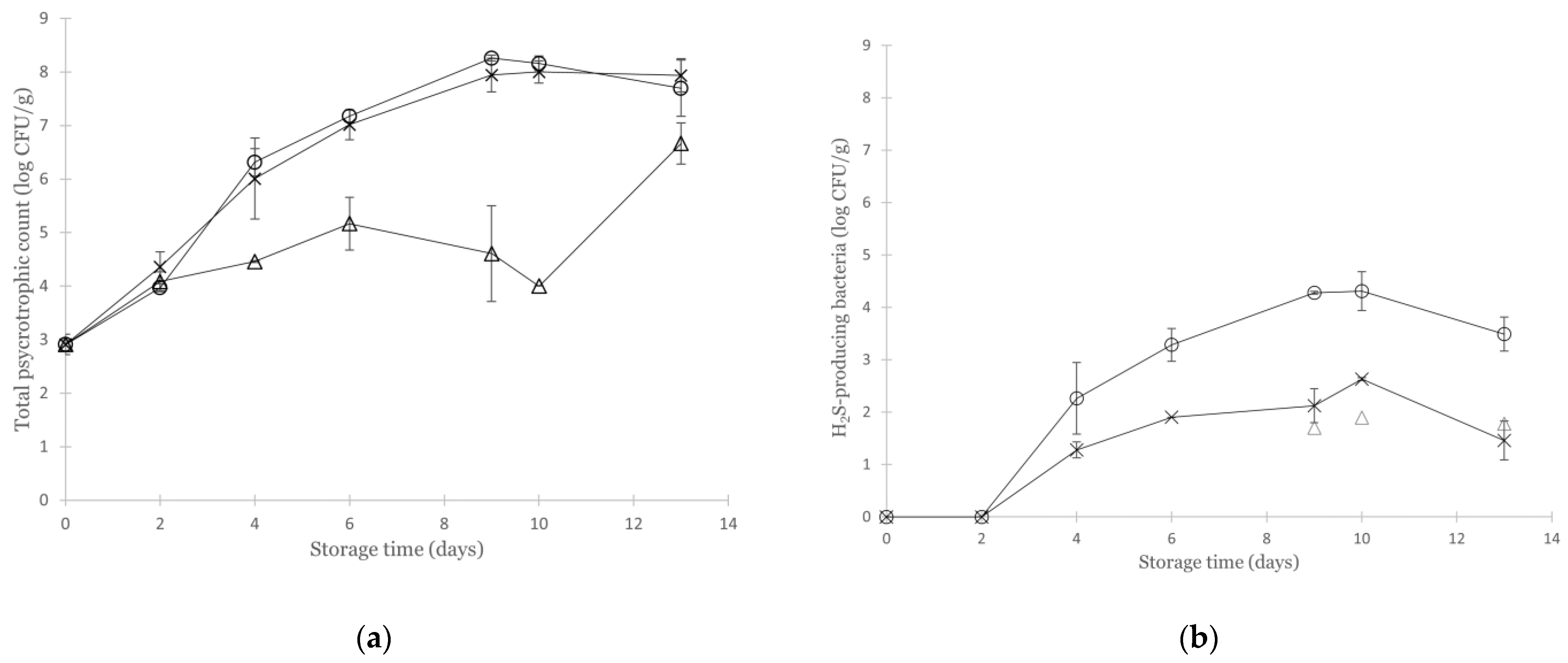
3.2. Effect of Various Modified Atmospheres on the Microbial Quality of Non-Inoculated Cod
3.3. Growth of Aeromonas Salmonicida in Cod under Various Modified Atmospheres
3.4. Spoilage Metabolite Production under Various Modified Atmospheres
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheng, L.; Wang, L. The microbial safety of fish and fish products: Recent advances in understanding its significance, contamination sources, and control strategies. Compr. Rev. Food. Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Hoel, S.; Vadstein, O.; Jakobsen, A.N. The significance of mesophilic Aeromonas spp. in minimally processed ready-to-eat seafood. Microorganisms 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, D.; Luo, Y. Characterization of the microbiota in lightly salted bighead carp (Aristichthys nobilis) fillets stored at 4 °C. Food Microbiol. 2017, 62, 106–111. [Google Scholar] [CrossRef]
- Hoel, S.; Vadstein, O.; Jakobsen, A.N. Growth of mesophilic Aeromonas salmonicida in an experimental model of nigiri sushi during cold storage. Int. J. Food Microbiol. 2018, 285, 1–6. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.-D. Effect of temperature on the growth kinetics and predictive growth model of Aeromonas hydrophila on squid (Sepioteuthis sepioidea). Food Sci. Biotechnol. 2014, 23, 307–312. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Meziti, A.; Kormas, K.A.; Boziaris, I.S. Indigenous and spoilage microbiota of farmed sea bream stored in ice identified by phenotypic and 16S rRNA gene analysis. Food Microbiol. 2013, 33, 85–89. [Google Scholar] [CrossRef]
- Beaz-Hidalgo, R.; Agüeria, D.; Latif-Eugenín, F.; Yeannes, M.I.; Figueras, M.J. Molecular characterization of Shewanella and Aeromonas isolates associated with spoilage of Common carp (Cyprinus carpio). FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef]
- Hozbor, M.C.; Saiz, A.I.; Yeannes, M.I.; Fritz, R. Microbiological changes and its correlation with quality indices during aerobic iced storage of sea salmon (Pseudopercis semifasciata). LWT 2006, 39, 99–104. [Google Scholar] [CrossRef]
- Don, S.; Xavier, K.A.M.; Devi, S.T.; Nayak, B.B.; Kannuchamy, N. Identification of potential spoilage bacteria in farmed shrimp (Litopenaeus vannamei): Application of relative rate of spoilage models in shelf life-prediction. LWT 2018, 97, 295–301. [Google Scholar] [CrossRef]
- Huang, Q.; Jiao, X.; Yan, B.; Zhang, N.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W.; Fan, D. Changes in physicochemical properties of silver carp (Hypophthalmichthys molitrix) surimi during chilled storage: The roles of spoilage bacteria. Food Chem. 2022, 387, 132847. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Hoel, S.; Lunestad, B.T.; Lerfall, J.; Jakobsen, A.N. Aeromonas spp. isolated from ready-to-eat seafood on the Norwegian market: Prevalence, putative virulence factors and antimicrobial resistance. J. Appl. Microbiol. 2021, 130, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.Å.; Langsrud, S.; Berget, I.; Gaarder, M.Ø.; Moen, B. High oxygen packaging of Atlantic cod fillets inhibits known spoilage organisms, but sensory quality is not improved due to the growth of Carnobacterium/Carnobacteriaceae. Foods 2021, 10, 1754. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Jeksrud, W.K.; Rosnes, J.T. A review of modified atmosphere packaging of fish and fishery products—Significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 2002, 37, 107–127. [Google Scholar] [CrossRef]
- Olafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.M.; Henehan, G.; Nielsen, J.; Nilsen, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Vogel Birte, F.; Venkateswaran, K.; Satomi, M.; Gram, L. Identification of Shewanella baltica as the most important H2S-producing species during iced storage of Danish marine fish. Appl. Env. Microbiol. 2005, 71, 6689–6697. [Google Scholar] [CrossRef]
- Sørensen, J.S.; Bøknæs, N.; Mejlholm, O.; Dalgaard, P. Superchilling in combination with modified atmosphere packaging resulted in long shelf-life and limited microbial growth in Atlantic cod (Gadus morhua L.) from capture-based-aquaculture in Greenland. Food Microbiol. 2020, 88, 103405. [Google Scholar] [CrossRef]
- Dalgaard, P.; Gram, L.; Huss, H.H. Spoilage and shelf-life of cod fillets packed in vacuum or modified atmospheres. Int. J. Food Microbiol. 1993, 19, 283–294. [Google Scholar] [CrossRef]
- Dalgaard, P.; Mejlholm, O.; Christiansen, T.J.; Huss, H.H. Importance of Photobacterium phosphoreum in relation to spoilage of modified atmosphere-packed fish products. Lett. Appl. Micorbiol. 1997, 24, 373–378. [Google Scholar] [CrossRef]
- Kuuliala, L.; Al Hage, Y.; Ioannidis, A.G.; Sader, M.; Kerckhof, F.M.; Vanderroost, M.; Boon, N.; De Baets, B.; De Meulenaer, B.; Ragaert, P.; et al. Microbiological, chemical and sensory spoilage analysis of raw Atlantic cod (Gadus morhua) stored under modified atmospheres. Food Microbiol. 2018, 70, 232–244. [Google Scholar] [CrossRef]
- Wang, T.; Sveinsdóttir, K.; Magnússon, H.; Martinsdóttir, E. Combined application of modified atmosphere packaging and superchilled storage to extend the shelf life of fresh cod (Gadus morhua) loins. J. Food Sci. 2008, 73, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Hovda, M.B.; Lunestad, B.T.; Sivertsvik, M.; Rosnes, J.T. Characterisation of the bacterial flora of modified atmosphere packaged farmed Atlantic cod (Gadus morhua) by PCR-DGGE of conserved 16S rRNA gene regions. Int. J. Food Microbiol. 2007, 117, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.Å.; Mørkøre, T.; Rudi, K.; Rødbotten, M.; Bjerke, F.; Eie, T. Quality changes of prerigor filleted Atlantic salmon (Salmo salar L.) packaged in modified atmosphere using CO2 emitter, traditional MAP, and vacuum. J. Food Sci. 2009, 74, M242–M249. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.Å.; Moen, B.; Rødbotten, M.; Berget, I.; Pettersen, M.K. Effect of vacuum or modified atmosphere packaging (MAP) in combination with a CO2 emitter on quality parameters of cod loins (Gadus morhua). Food Pack Shelf Life 2016, 9, 29–37. [Google Scholar] [CrossRef]
- Sivertsvik, M. The optimized modified atmosphere for packaging of pre-rigor filleted farmed cod (Gadus morhua) is 63mL/100mL oxygen and 37mL/100mL carbon dioxide. LWT 2007, 40, 430–438. [Google Scholar] [CrossRef]
- Debevere, J.; Boskou, G. Effect of modified atmosphere packaging on the TVB/TMA-producing microflora of cod fillets. Int. J. Food Microbiol. 1996, 31, 221–229. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Mørkøre, T.; Rudi, K.; Olsen, E.; Eie, T. Quality Changes during Refrigerated Storage of MA-Packaged Pre-rigor Fillets of Farmed Atlantic Cod (Gadus morhua L.) Using Traditional MAP, CO2 Emitter, and Vacuum. J. Food Sci. 2007, 72, 423–430. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Rødbotten, M.; Lea, P.; Rotabakk, B.T.; Birkeland, S.; Pettersen, M.K. Effect of Transport Packaging and Repackaging into Modified Atmosphere on Shelf Life and Quality of Thawed Atlantic Cod Loins. Pack Technol. Sci. 2015, 28, 925–938. [Google Scholar] [CrossRef]
- Jakobsen, A.N.; Shumilina, E.; Lied, H.; Hoel, S. Growth and spoilage metabolites production of a mesophilic Aeromonas salmonicida strain in Atlantic salmon (Salmo salar L.) during cold storage in modified atmosphere. J. Appl. Microbiol. 2020, 129, 935–946. [Google Scholar] [CrossRef]
- Stohr, V.; Joffraud, J.J.; Cardinal, M.; Leroi, F. Spoilage potential and sensory profile associated with bacteria isolated from cold-smoked salmon. Food Res. Int. 2001, 34, 797–806. [Google Scholar] [CrossRef]
- Macé, S.; Cardinal, M.; Jaffrès, E.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.-F.; Dousset, X.; Joffraud, J.-J. Evaluation of the spoilage potential of bacteria isolated from spoiled cooked whole tropical shrimp (Penaeus vannamei) stored under modified atmosphere packaging. Food Microbiol. 2014, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hoel, S.; Mehli, L.; Bruheim, T.; Vadstein, O.; Jakobsen, A.N. Assessment of microbiological quality of retail fresh sushi from selected sources in Norway. J. Food Protect 2015, 78, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Nordic Committee on Food Analysis. No. 150, Mesophilic Aeromonas Species. Quantification in Foods and Feeds. NMKL. 2004. Available online: https://www.nmkl.org/product/mesophilic-aeromonas-species-determination-in-foods-and-feeds/ (accessed on 1 July 2022).
- Nordic Committee on Food Analysis. No. 184, Aerobic Count and Specific Spoilage Organisms in Fish and Fish Products. NMKL. 2006. Available online: https://www.nmkl.org/product/aerobic-count-and-specific-spoilage-organisms-in-fish-and-fish-products/ (accessed on 1 July 2022).
- Shumilina, E.; Ciampa, A.; Capozzi, F.; Rustad, T.; Dikiy, A. NMR approach for monitoring post-mortem changes in Atlantic salmon fillets stored at 0 and 4 degrees C. Food Chem. 2015, 184, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Rosnes, J.T.; Jeksrud, W.K. Solubility and absorption rate of carbon dioxide into non-respiring foods. Part 2: Raw fish fillets. J. Food Eng. 2004, 63, 451–458. [Google Scholar] [CrossRef]
- Abel, N.; Rotabakk, B.T.; Lerfall, J. Effect of salt on CO2 solubility in salmon (Salmo salar L.) stored in modified atmosphere. J. Food Eng. 2020, 278, 109946. [Google Scholar] [CrossRef]
- Rotabakk, B.T.; Birkeland, S.; Lekang, O.I.; Sivertsvik, M. Enhancement of modified atmosphere packaged farmed Atlantic halibut (Hippoglossus hippoglossus) fillet quality by soluble gas stabilization. Food Sci. Technol. Int. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Lerfall, J.; Bjørge Thomassen, G.M.; Jakobsen, A.N. Quality of fresh saithe (Pollachius virens) in modified atmosphere packages as affected by the gas composition. Food Pack Shelf Life 2018, 18, 147–156. [Google Scholar] [CrossRef]
- Lerfall, J.; Shumilina, E.; Jakobsen, A.N. The significance of Shewanella sp. strain HSO12, Photobacterium phosphoreum strain HS254 and packaging gas composition in quality deterioration of fresh saithe fillets. LWT 2022, 154, 112636. [Google Scholar] [CrossRef]
- Broekaert, K.; Heyndrickx, M.; Herman, L.; Devlieghere, F.; Vlaemynck, G. Seafood quality analysis: Molecular identification of dominant microbiota after ice storage on several general growth media. Food Microbiol. 2011, 28, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Huff-Lonergan, E.; Sebranek, J.G.; Lonergan, S.M. High-oxygen modified atmosphere packaging system induces lipid and myoglobin oxidation and protein polymerization. Meat Sci. 2010, 85, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological spoilage and investigation of volatile profile during storage of sea bream fillets under various conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Verdos, G.I.; Haroutounian, S.A.; Boziaris, I.S. The dynamics of Pseudomonas and volatilome during the spoilage of gutted sea bream stored at 2 °C. Food Contr. 2015, 55, 257–265. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Boziaris, I.S. The evolution of knowledge on seafood spoilage microbiota from the 20th to the 21st century: Have we finished or just begun? Trends Food Sci. Technol. 2022, 120, 236–247. [Google Scholar] [CrossRef]
- Tryfinopoulou, P.; Drosinos, E.H.; Nychas, G.J.E. Performance of Pseudomonas CFC-selective medium in the fish storage ecosystems. J. Microbiol. Methods 2001, 47, 243–247. [Google Scholar] [CrossRef]
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef]
- Howgate, P. A review of the kinetics of degradation of inosine monophosphate in some species of fish during chilled storage. Int. J. Food Sci. Technol. 2006, 41, 341–353. [Google Scholar] [CrossRef]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of melanosis and microbial growth in Pacific white shrimp (Litopenaeus vannamei) using high CO2 modified atmosphere packaging. Food Chem. 2020, 312, 126114. [Google Scholar] [CrossRef]
- Boskou, G.; Debevere, J. Reduction of trimethylamine oxide by Shewanella spp. under modified atmospheres in vitro. Food Microbiol. 1997, 14, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Hagen, I.V.; Helland, A.; Bratlie, M.; Midttun, Ø.; McCann, A.; Sveier, H.; Rosenlund, G.; Mellgren, G.; Ueland, P.M.; Gudbrandsen, O.A. TMAO, creatine and 1-methylhistidine in serum and urine are potential biomarkers of cod and salmon intake: A randomised clinical trial in adults with overweight or obesity. Eur. J. Nutr. 2020, 59, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Ryder, J.; Karunasagar, I.; Ababouch, L. (Eds.) Assessment and Management of Seafood Safety and QUALITY: Current Practices and Emerging Issues; FAO: Rome, Italy, 2014; p. 432. [Google Scholar]
- Van Ruth, S.M.; Brouwer, E.; Koot, A.; Wijtten, M. Seafood and water management. Foods 2014, 3, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Packaging Atmosphere | |
|---|---|
| Inoculated | CO2/O2 (67/33%) |
| CO2/N2 (67/33%) | |
| Vacuum | |
| Non-inoculated | CO2/O2 (67/33%) |
| CO2/N2 (67/33%) | |
| Vacuum |
| Non-Inoculated | Inoculated | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | CO2/O2 (67/33%) | CO2/N2 (67/33%) | Vacuum | CO2/O2 (67/33%) | CO2/N2 (67/33%) | Vacuum | PD | PA | PI | PM | |
| IMP | |||||||||||
| 0 * | 0.17 ± 0.13 a, x | 0.17 ± 0.13 a, x | 0.17 ± 0.13 a, x | 0.17 ± 0.13 a, x | 0.17 ± 0.13 a, x | 0.17 ± 0.13 a, x | |||||
| 6 | 0.05 ± 0.0 ab, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.08 ± 0.05 b, x | 0.01 ± 0.01 a, x | 0.0 ± 0.0 a, x | |||||
| 13 | 0.01 ± 0.01 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | |||||
| GLM | <0.001 | 0.734 | 0.877 | <0.001 | |||||||
| HxR | 0 * | 0.37 ± 0.08 a, x | 0.37 ± 0.08 a, y | 0.37 ± 0.08 a, y | 0.37 ± 0.08 a, x | 0.37 ± 0.08 a, y | 0.37 ± 0.08 a, y | ||||
| 6 | 0.44 ± 0.02 b, x | 0.17 ± 0.16 ab, xy | 0.21 ± 0.16 ab, xy | 0.41 ± 0.01 b, x | 0.41 ± 0.05 b, y | 0.05 ± 0.02 a, y | |||||
| 13 | 0.37 ± 0.06 b, x | 0.01 ± 0.00 a, x | 0.0 ± 0.0 a, x | 0.36 ± 0.11 b, x | 0.01 ± 0.01 a, x | 0.0 ± 0.0 a, x | |||||
| GLM | <0.001 | <0.001 | 0.837 | <0.001 | |||||||
| Hx | 0 * | 0.04 ± 0.01 a, x | 0.04 ± 0.01 a, x | 0.04 ± 0.01 a, x | 0.04 ± 0.01 a, x | 0.04 ± 0.01 a, x | 0.04 ± 0.01 a, x | ||||
| 6 | 0.08 ± 0.01 a, x | 0.33 ± 0.15 ab, y | 0.45 ± 0.24 ab, xy | 0.09 ± 0.01 ab, y | 0.16 ± 0.05 ab, y | 0.49 ± 0.05 b, y | |||||
| 13 | 0.17 ± 0.04 a, y | 0.50 ± 0.02 b, y | 0.52 ± 0.01 b, y | 0.14 ± 0.01 a, z | 0.50 ± 0.02 b, z | 0.60 ± 0.00 c, z | |||||
| GLM | <0.001 | <0.001 | 0.821 | <0.001 | |||||||
| Non-Inoculated | Inoculated | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | CO2/O2 (67/33%) | CO2/N2 (67/33%) | Vacuum | CO2/O2 (67/33%) | CO2/N2 (67/33%) | Vacuum | PD | PA | PI | PM | |
| TMA | 0 * | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | 0.0 ± 0.0 a, x | ||||
| 6 | 0.0 ± 0.0 a, x | 1.58 ± 1.10 ab, x | 1.26 ± 0.57 ab, x | 0.04 ± 0.04 a, x | 0.39 ± 0.19 ab, x | 1.84 ± 0.27 b, y | |||||
| 13 | 0.09 ± 0.10 a, x | 5.61 ± 0.36 c, y | 5.18 ± 0.50 c, y | 0.09 ± 0.12 a, x | 3.35 ± 0.66 b, y | 5.30 ± 0.17 c, z | |||||
| GLM | <0.001 | <0.001 | 0.352 | <0.001 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoel, S.; Lerfall, J.; Jakobsen, A.N. Growth and Spoilage Potential of an Aeromonas salmonicida Strain in Refrigerated Atlantic Cod (Gadus morhua) Stored under Various Modified Atmospheres. Foods 2022, 11, 2757. https://doi.org/10.3390/foods11182757
Hoel S, Lerfall J, Jakobsen AN. Growth and Spoilage Potential of an Aeromonas salmonicida Strain in Refrigerated Atlantic Cod (Gadus morhua) Stored under Various Modified Atmospheres. Foods. 2022; 11(18):2757. https://doi.org/10.3390/foods11182757
Chicago/Turabian StyleHoel, Sunniva, Jørgen Lerfall, and Anita Nordeng Jakobsen. 2022. "Growth and Spoilage Potential of an Aeromonas salmonicida Strain in Refrigerated Atlantic Cod (Gadus morhua) Stored under Various Modified Atmospheres" Foods 11, no. 18: 2757. https://doi.org/10.3390/foods11182757
APA StyleHoel, S., Lerfall, J., & Jakobsen, A. N. (2022). Growth and Spoilage Potential of an Aeromonas salmonicida Strain in Refrigerated Atlantic Cod (Gadus morhua) Stored under Various Modified Atmospheres. Foods, 11(18), 2757. https://doi.org/10.3390/foods11182757







