Resistant Protein: Forms and Functions
Abstract
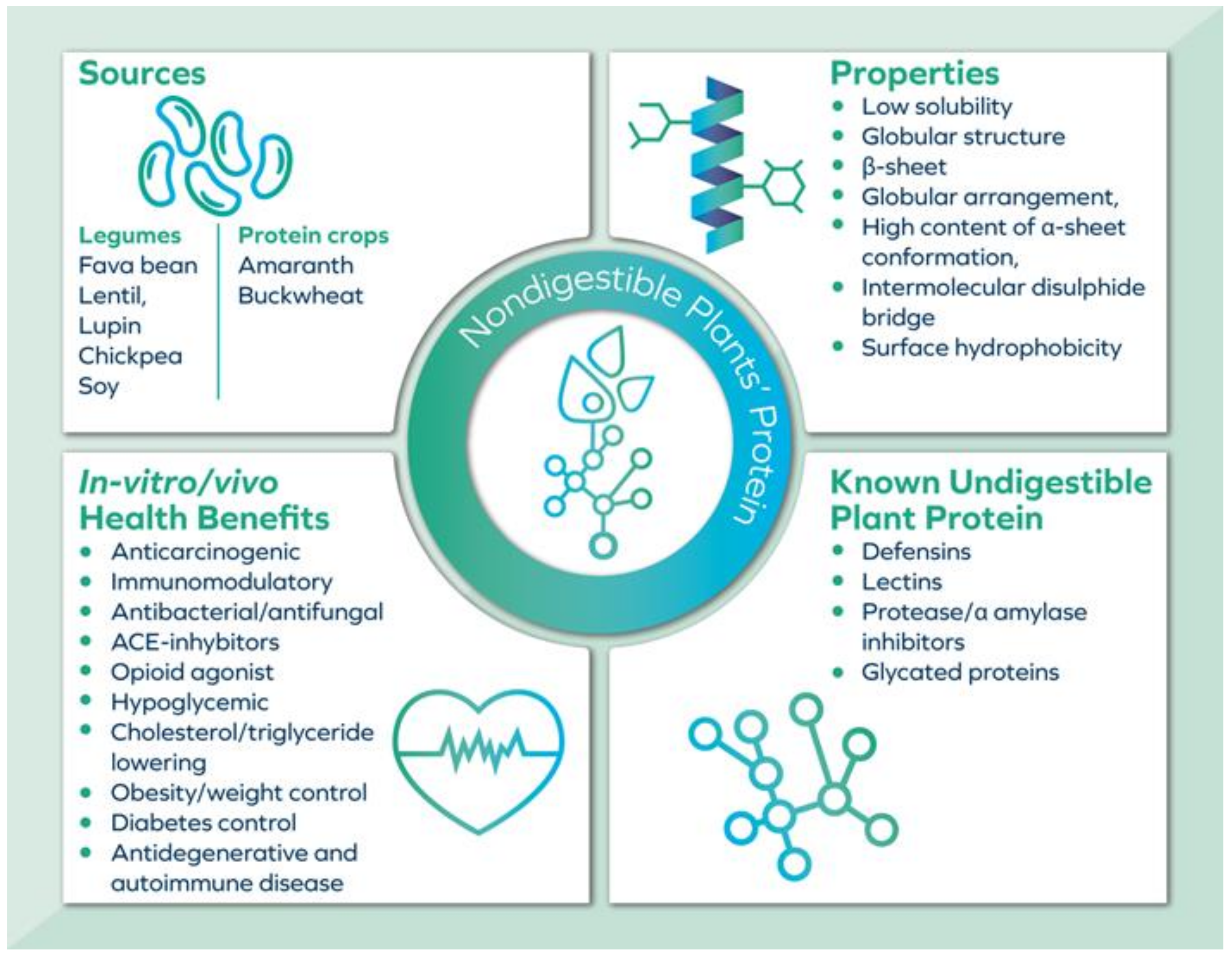
:1. Introduction
2. Resistant Plant Protein: Functions
3. Resistant Plant Protein: Forms
4. Limitations
5. Perspectives
- -
- Open a completely new research field on resistant protein, combining the interests of food scientists and food engineers to develop strategies for designing future foods.
- -
- Advance the discipline of nutritional science by delivering comprehensive investigations elucidating the role of resistant protein in the maintenance of health and the prevention of non-communicable disease. The scientific advancement in this research field would significantly impact the future of food nutrition, public health and food policy development.
- -
- Allow the food industry, in conjunction with national and international health authorities, to implement health promotion dietary strategies (personalised ingredients/foods) by establishing a pipeline in which the characterised resistant plant protein will be linked to NCD prevention for the potential as diet-based therapeutics.
- -
- Endorse and facilitate a dietary shift to include resistant plant protein by consumers seeking a healthier, nutritious and more sustainable diet.
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. United Nations Environment Programme; United Nations: New York, NY, USA, 2012. [Google Scholar]
- Parodi, A.; Leip, A.; De Boer, I.J.M.; Slegers, P.M.; Ziegler, F.; Temme, E.H.; Herrero, M.; Tuomisto, H.; Valin, H.; Van Middelaar, C.E.; et al. The Potential of Future Foods for Sustainable and Healthy Diets. Nat. Sustain. 2018, 1, 782–789. [Google Scholar] [CrossRef]
- Tzachor, A.; Richards, C.E.; Holt, L. Future Foods for Risk-Resilient Diets. Nat. Food 2021, 2, 326–329. [Google Scholar] [CrossRef]
- Horton, P.; Long, S.P.; Smith, P.; Banwart, S.A.; Beerling, D.J. Technologies to Deliver Food and Climate Security through Agriculture. Nat. Plants 2021, 7, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, A.P.; South, P.F.; Bernacchi, C.J.; Ort, D.R. Alternative Pathway to Photorespiration Protects Growth and Productivity at Elevated Temperatures in a Model Crop. Plant Biotechnol. J. 2022, 20, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Lawson, T. From Green to Gold: Agricultural Revolution for Food Security. J. Exp. Bot. 2020, 71, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy Balance and Obesity: What Are the Main Drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Action Plan for Implementation of the European Strategy for the Prevention and Control of Noncommunicable Diseases 2012−2016; World Health Organization: Copenhagen, Denmark, 2012. [Google Scholar]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, Inefficiencies and Waste in the Global Food System. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef]
- Aiking, H. Future Protein Supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- de Boer, J.; Aiking, H. Prospects for Pro-Environmental Protein Consumption in Europe: Cultural, Culinary, Economic and Psychological Factors. Appetite 2018, 121, 29–40. [Google Scholar] [CrossRef]
- Hayashi, K.; Oita, A.; Lassaletta, L.; Shindo, J.; Shibata, H.; Sakurai, G.; Eguchi, S. Reducing Nitrogen Footprints of Consumer-Level Food Loss and Protein Overconsumption in Japan, Considering Gender and Age Differences. Environ. Res. Lett. 2018, 13, 124027. [Google Scholar] [CrossRef] [Green Version]
- de Boer, J.; Aiking, H. Strategies towards Healthy and Sustainable Protein Consumption: A Transition Framework at the Levels of Diets, Dishes, and Dish Ingredients. Food Qual. Prefer. 2019, 73, 171–181. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for Protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Keshavarzian, A.; Mutlu, G.M. Hyperalbuminemia and Elevated Transaminases Associated with High-Protein Diet. Scand. J. Gastroenterol. 2006, 41, 759–760. [Google Scholar] [CrossRef]
- Delimaris, I. Adverse Effects Associated with Protein Intake above the Recommended Dietary Allowance for Adults. ISRN Nutr. 2013, 2013, 126929. [Google Scholar] [CrossRef]
- Southgate, D.A.T.; Johnson, I.T.; Fenwick, G.R. (Eds.) Nutrient Availability: Chemical and Biological Aspects; Royal Society of Chemistry: Cambridge, UK, 1989; pp. 10–12. [Google Scholar]
- Fleming, R.M. The Effect of High-Protein Diets on Coronary Blood Flow. Angiology 2000, 51, 817–826. [Google Scholar] [CrossRef]
- Gautam, B.P.S.; Gondwal, M.; Kishore, N. Adverse Effect in Human Beings Associated with Excess Dietary Protein Intake. In Biomedical Applications of Natural Proteins; Kumar, D., Kundapur, R., Eds.; Springer: New Delhi, India, 2015. [Google Scholar]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Sugano, M.; Goto, S.; Yamada, Y.; Yoshida, K.; Hashimoto, Y.; Matsuo, T.; Kimoto, M. Cholesterol-Lowering Activity of Various Undigested Fractions of Soybean Protein in Rats. J. Nutr. 1990, 120, 977–985. [Google Scholar] [CrossRef]
- Ogawa, T.; Gatchalian-Yee, M.; Sugano, M.; Kimoto, M.; Matsuo, T.; Hashimoto, Y. Hypocholesterolemic Effect of Undigested Fraction of Soybean Protein in Rats Fed No Cholesterol. Biosci. Biotechnol. Biochem. 1992, 56, 1845–1848. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G.; Cappelloni, M.; Pusztai, A. Perspectives into Factors Limiting in Vivo Digestion of Legume Proteins: Antinutritional Compounds or Storage Proteins? J. Agric. Food. Chem. 2000, 48, 742–749. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-Aligned Dietary Fiber Definitions Help to Bridge the ‘Fiber Gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Davidson, M.H.; McDonald, A. Fiber: Forms and Functions. In Proceedings of the Nutrition Research; Elsevier: Amsterdam, The Netherlands, 1998; Volume 18, pp. 617–624. [Google Scholar]
- Calixto, F.D.S. La Fibra Dietética En Nutrición y Salud. ANS. Aliment. Nutr. Salud 1997, 4, 17–21. [Google Scholar]
- Trowell, H. Dietary Fiber Definitions. Am. J. Clin. Nutr. 1988, 48, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Goñi, I.; Mañas, E.; Abia, R. Klason Lignin, Condensed Tannins and Resistant Protein as Dietary Fibre Constituents: Determination in Grape Pomaces. Food Chem. 1991, 39, 299–309. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary Fibre from Edible Seaweeds: Chemical Structure, Physicochemical Properties and Effects on Cholesterol Metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Morita, T.; Kasaoka, S.; Oh-hashi, A.; Ikai, M.; Numasaki, Y.; Kiriyama, S. Resistant Proteins Alter Cecal Short-Chain Fatty Acid Profiles in Rats Fed High Amylose Cornstarch. J. Nutr. 1998, 128, 1156–1164. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; FAO: Rome, Italy, 2013. [Google Scholar]
- Morita, T.; Kasaoka, S.; Kiriyama, S. Physiological Functions of Resistant Proteins: Proteins and Peptides Regulating Large Bowel Fermentation of Indigestible Polysaccharide. J. AOAC Int. 2004, 87, 792–796. [Google Scholar] [CrossRef]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kato, N. Feeding of Buckwheat Protein Extract Reduces Hepatic Triglyceride Concentration, Adipose Tissue Weight, and Hepatic Lipogenesis in Rats. J. Nutr. Biochem. 1996, 7, 555–559. [Google Scholar] [CrossRef]
- He, T.; Giuseppin, M.L.F. Slow and Fast Dietary Proteins Differentially Modulate Postprandial Metabolism. Int. J. Food. Sci. Nutr. 2014, 65, 386–390. [Google Scholar] [CrossRef]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural Aspects of Legume Proteins and Nutraceutical Properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, S.; Das, M.; Dwivedi, P.D. A Comprehensive Review of Legume Allergy. Clin. Rev. Allergy Immunol. 2013, 45, 30–46. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.C.; Kris-Etherton, P.M. Soy Protein Reduces Serum Cholesterol by Both Intrinsic and Food Displacement Mechanisms. J. Nutr. 2010, 140, 2302S–2311S. [Google Scholar] [CrossRef] [Green Version]
- Vital, D.A.L.; de Mejía, E.G.; Dia, V.P.; Loarca-Piña, G. Peptides in Common Bean Fractions Inhibit Human Colorectal Cancer Cells. Food Chem. 2014, 157, 347–355. [Google Scholar] [CrossRef]
- Scarafoni, A.; Magni, C.; Duranti, M. Molecular Nutraceutics as a Mean to Investigate the Positive Effects of Legume Seed Proteins on Human Health. Trends Food Sci. Technol. 2007, 18, 454–463. [Google Scholar] [CrossRef]
- Pryme, I.F.; Bardocz, S.; Pusztai, A.; Ewen, S.W.B. Suppression of Growth of Tumour Cell Lines in Vitro and Tumours in Vivo by Mistletoe Lectins. Histol. Histopathol. 2006, 21, 285–299. [Google Scholar]
- Światecka, D.; Małgorzata, I.; Aleksander, Ś.; Henryk, K.; Elzbieta, K. The Impact of Glycated Pea Proteins on Bacterial Adhesion. Food Res. Int. 2010, 43, 1566–1576. [Google Scholar] [CrossRef]
- Barrett, M.L.; Udani, J.K. A Proprietary Alpha-Amylase Inhibitor from White Bean (Phaseolus Vulgaris): A Review of Clinical Studies on Weight Loss and Glycemic Control. Nutr. J. 2011, 10, 24. [Google Scholar] [CrossRef]
- Birk, Y. Protease Inhibitors of Plant Origin and Role of Protease Inhibitors in Human Nutrition Overview. In Protease Inhibitors as Cancer Chemopreventive Agents; Troll, W., Kennedy, A.R., Eds.; Springer US: Boston, MA, USA, 1993; pp. 97–106. ISBN 978-1-4615-2882-1. [Google Scholar]
- Hellinger, R.; Gruber, C.W. Peptide-Based Protease Inhibitors from Plants. Drug Discov. Today 2019, 24, 1877–1889. [Google Scholar] [CrossRef]
- Barbole, R.S.; Saikhedkar, N.; Giri, A. Plant Peptides as Protease Inhibitors for Therapeutic and Agricultural Applications. In Natural Products as Enzyme Inhibitors: An Industrial Perspective; Maheshwari, V.L., Patil, R.H., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 25–57. ISBN 978-981-19-0932-0. [Google Scholar]
- Wan, X.S.; Serota, D.G.; Ware, J.H.; Crowell, J.A.; Kennedy, A.R. Detection of Bowman-Birk Inhibitor and Anti-Bowman-Birk Inhibitor Antibodies in Sera of Humans and Animals Treated With Bowman-Birk Inhibitor Concentrate. Nutr. Cancer 2002, 43, 167–173. [Google Scholar] [CrossRef]
- Atzler, J.J.; Ispiryan, L.; Gallagher, E.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Enzymatic Degradation of FODMAPS via Application of β-Fructofuranosidases and α-Galactosidases—A Fundamental Study. J. Cereal Sci. 2020, 95, 102993. [Google Scholar] [CrossRef]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. Characterization of the FODMAP-Profile in Cereal-Product Ingredients. J. Cereal Sci. 2020, 92, 102916. [Google Scholar] [CrossRef]
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Gibson, P.R. Controversies and Reality of the FODMAP Diet for Patients with Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2019, 34, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, J.J.; Dayre Mcnally, J.; Navidzadeh, A.; Beauregard, M. Development of an Optimized Feeding Technology for Dairy Cows. Appl. Biochem. Biotechnol. 2000, 87, 247–264. [Google Scholar] [CrossRef]
- Finot, P.-A. The Absorption and Metabolism of Modified Amino Acids in Processed Foods. J. AOAC Int. 2005, 88, 894–903. [Google Scholar] [CrossRef]
- Murray, M.; Coughlan, M.T.; Gibbon, A.; Kumar, V.; Marques, F.Z.; Selby-Pham, S.; Snelson, M.; Tsyganov, K.; Williamson, G.; Woodruff, T.M.; et al. Reduced Growth, Altered Gut Microbiome and Metabolite Profile, and Increased Chronic Kidney Disease Risk in Young Pigs Consuming a Diet Containing Highly Resistant Protein. Front. Nutr. 2022, 9, 816749. [Google Scholar] [CrossRef]
- Amonsou, E.O.; Taylor, J.R.; Emmambux, M.N.; Duodu, K.G.; Minnaar, A. Highly Viscous Dough-Forming Properties of Marama Protein. Food Chem. 2012, 134, 1519–1526. [Google Scholar] [CrossRef]
- Kårlund, A.; Gómez-Gallego, C.; Turpeinen, A.M.; Palo-Oja, O.M.; El-Nezami, H.; Kolehmainen, M. Protein Supplements and Their Relation with Nutrition, Microbiota Composition and Health: Is More Protein Always Better for Sportspeople? Nutrients 2019, 11, 829. [Google Scholar] [CrossRef]
- Dallas, D.C.; Sanctuary, M.R.; Qu, Y.; Khajavi, S.H.; van Zandt, A.E.; Dyandra, M.; Frese, S.A.; Barile, D.; German, J.B. Personalizing Protein Nourishment. Crit. Rev. Food Sci. Nutr. 2017, 57, 3313–3331. [Google Scholar] [CrossRef]
- Fuller, M. Determination of Protein and Amino Acid Digestibility in Foods Including Implications of Gut Microbial Amino Acid Synthesis. Br. J. Nutr. 2012, 108, S238–S246. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tomé, D. Intestinal Luminal Nitrogen Metabolism: Role of the Gut Microbiota and Consequences for the Host. Pharmacol. Res. 2013, 68, 114–126. [Google Scholar] [CrossRef]
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; van Mil, S.W.C. Protein Fermentation in the Gut; Implications for Intestinal Dysfunction in Humans, Pigs, and Poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G159–G170. [Google Scholar] [CrossRef]
- Portune, K.J.; Beaumont, M.; Davila, A.M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut Microbiota Role in Dietary Protein Metabolism and Health-Related Outcomes: The Two Sides of the Coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef] [Green Version]
- Windey, K.; de Preter, V.; Verbeke, K. Relevance of Protein Fermentation to Gut Health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus Vulgaris Lectins: A Systematic Review of Characteristics and Health Implications. Crit. Rev. Food Sci. Nutr. 2018, 58, 70–83. [Google Scholar] [CrossRef] [Green Version]
- Sudheendra, C.V.K.; Hazra, T.; Solanki, A.; Ramani, V. Anti-Nutritive and Therapeutic Properties of Lectins. In Biological and Chemical Hazards in Food and Food Products; Apple Academic Press: Waretown, NJ, USA, 2021; ISBN 9781774637135. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannini, E.; Sahin, A.W.; Arendt, E.K. Resistant Protein: Forms and Functions. Foods 2022, 11, 2759. https://doi.org/10.3390/foods11182759
Zannini E, Sahin AW, Arendt EK. Resistant Protein: Forms and Functions. Foods. 2022; 11(18):2759. https://doi.org/10.3390/foods11182759
Chicago/Turabian StyleZannini, Emanuele, Aylin W. Sahin, and Elke K. Arendt. 2022. "Resistant Protein: Forms and Functions" Foods 11, no. 18: 2759. https://doi.org/10.3390/foods11182759





