Synergistic Hypolipidemic Effects and Mechanisms of Phytochemicals: A Review
Abstract
:1. Introduction
2. Synergistic Hypolipidemic Effects between Phytochemicals of the Same Category
2.1. Synergistic Hypolipidemic Effects of Flavonoids
2.2. Synergistic Hypolipidemic Effects of Polysaccharides
2.3. Synergistic Hypolipidemic Effects of Polyphenols
2.4. Synergistic Hypolipidemic Effects of Other Phytochemicals
3. Synergistic Hypolipidemic Effects between Different Categories of Phytochemicals
3.1. Synergistic Hypolipidemic Effects of Flavonoids with Other Categories of Phytochemicals
3.2. Synergistic Hypolipidemic Effects of Polysaccharides with Other Categories of Phytochemicals
3.3. Synergistic Hypolipidemic Effects of Polyphenols with Other Categories of Phytochemicals
3.4. Synergistic Hypolipidemic Effects of other Different Categories of Phytochemicals
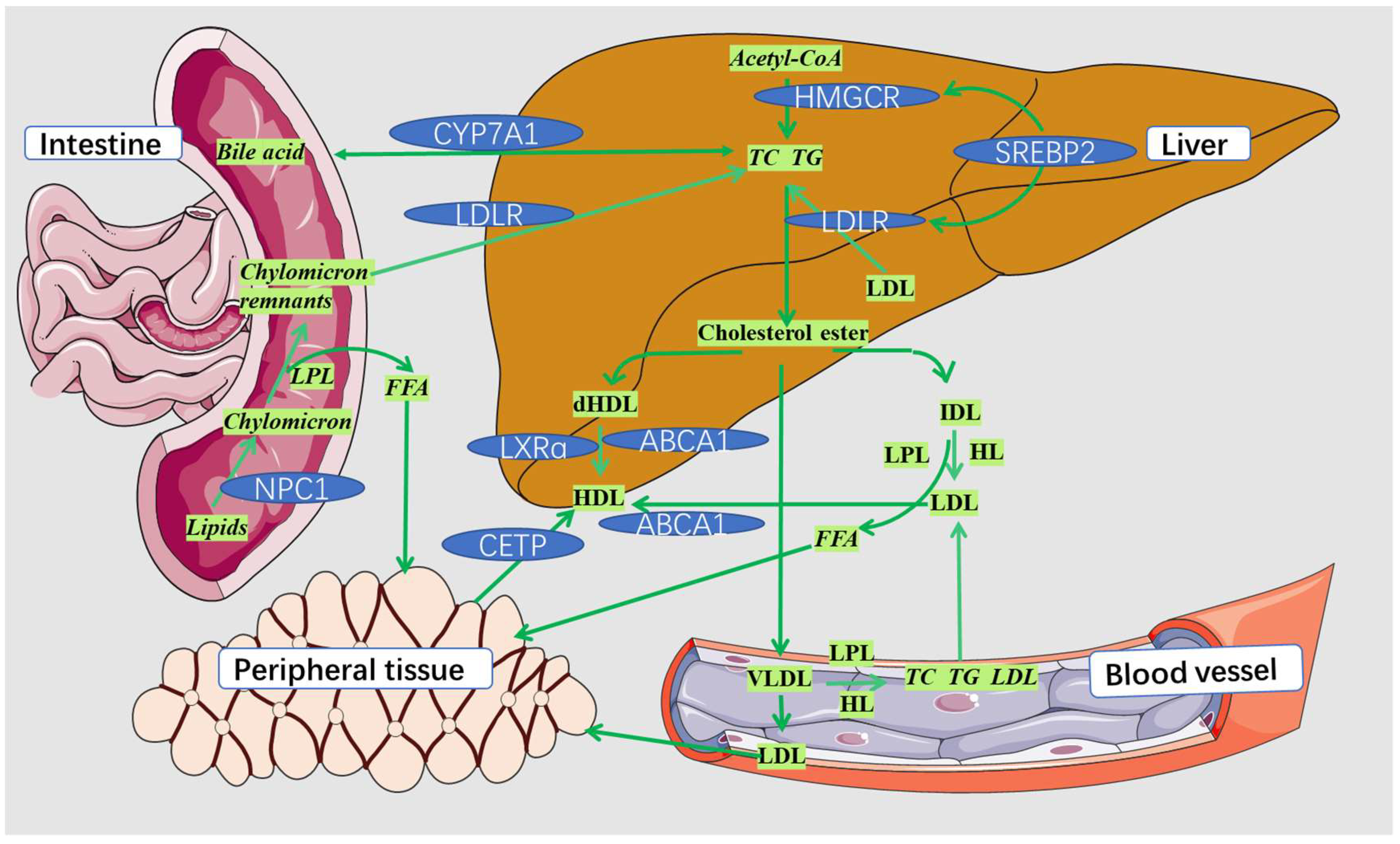
4. The Synergistic Hypolipidemic Mechanisms
4.1. The Synergistic Hypolipidemic Mechanisms Based on the Pathway Analysis
4.1.1. Synergistic Hypolipidemic Mechanisms Based on the Same Pathway
4.1.2. Synergistic Hypolipidemic Mechanisms Based on Different Pathways
4.2. Target-Based Analysis of Synergistic Mechanisms
4.2.1. Synergistic Hypolipidemic Effects Based on the Same Target
4.2.2. Synergistic Hypolipidemic Effects Based on Multi-Target
4.3. Other Mechanisms
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boden, W.E.; Bhatt, D.L.; Toth, P.P.; Ray, K.K.; Chapman, M.J.; Luscher, T.F. Profound reductions in first and total cardiovascular events with icosapent ethyl in the REDUCE-IT trial: Why these results usher in a new era in dyslipidaemia therapeutics. Eur. Heart J. 2020, 41, 2304. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care 2013, 40, 195. [Google Scholar] [CrossRef]
- Nesto, R.W. Beyond low-density lipoprotein: Addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am. J. Cardiovasc. Drug 2005, 5, 379–387. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskina, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Fernandez-Friera, L.; Penalvo, J.L.; Fernandez-Ortiz, A.; Ibanez, B.; Lopez-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; de Vega, V.M.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort the PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef]
- Gupta, M.; Blumenthal, C.; Chatterjee, S.; Bandyopadhyay, D.; Jain, V.; Lavie, C.J.; Virani, S.S.; Ray, K.K.; Aronow, W.S.; Ghosh, R.K. Novel emerging therapies in atherosclerosis targeting lipid metabolism. Expert Opin. Investig. Drug 2020, 29, 611–622. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Boutari, C.; Karagiannis, A.; Athyros, V.G. Rosuvastatin and ezetimibe for the treatment of dyslipidemia and hypercholesterolemia. Expert Rev. Cardiovasc. Ther. 2021, 19, 575–580. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Dong, J.L.; Yu, Z. Pleiotropic use of Statins as non-lipid-lowering drugs. Int. J. Biol. Sci. 2020, 16, 2704–2711. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Xia, Y.; Xu, J.F.; Li, Q.Y.; Zhang, C.H.; Li, M.H. Effects of phytochemicals from plant-based functional foods on hyperlipidemia and their underpinning mechanisms. Trends Food Sci. Technol. 2020, 103, 304–320. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Gong, X.; Ji, M.Y.; Xu, J.P.; Zhang, C.H.; Li, M.H. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. 2020, 60, 2303–2326. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G.Y. Salvia miltiorrhiza in Treating Cardiovascular Diseases: A Review on Its Pharmacological and Clinical Applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef]
- Ul Islam, S.; Ahmed, M.B.; Ahsan, H.; Lee, Y.S. Recent Molecular Mechanisms and Beneficial Effects of Phytochemicals and Plant-Based Whole Foods in Reducing LDL-C and Preventing Cardiovascular Disease. Antioxidants 2021, 10, 784. [Google Scholar] [CrossRef]
- Feldman, F.; Koudoufio, M.; Desjardins, Y.; Spahis, S.; Delvin, E.; Levy, E. Efficacy of Polyphenols in the Management of Dyslipidemia: A Focus on Clinical Studies. Nutrients 2021, 13, 672. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, J.N.; Luo, L.Y.; Zhang, D.Y. Effects of Pu-erh tea aqueous extract (PTAE) on blood lipid metabolism enzymes. Food Funct. 2015, 6, 2008–2016. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotla, E.; Oniszczuk, A. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.Q.; Li, S.P.; Ye, X.L.; Li, X.G.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef]
- Gao, J.L.; Chen, G.; He, H.Q.; Liu, C.; Xiong, X.J.; Li, J.; Wang, J. Therapeutic Effects of Breviscapine in Cardiovascular Diseases: A Review. Front. Pharmacol. 2017, 8, 289. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [Green Version]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Pinto, M.D. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Brit. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.Y.; Zhang, B.; Deng, Z.Y. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2021, 62, 5658–5677. [Google Scholar] [CrossRef]
- Zhang, L.J.; Virgous, C.; Si, H.W. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Leng, E.N.; Xiao, Y.; Mo, Z.T.; Li, Y.Q.; Zhang, Y.Y.; Deng, X.S.; Zhou, M.; Zhou, C.C.; He, Z.X.; He, J.Y.; et al. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complement. Altern. Med. 2018, 18, 122. [Google Scholar] [CrossRef]
- Aslam, M.; Ladilov, Y. Emerging Role of cAMP/AMPK Signaling. Cells 2022, 11, 308. [Google Scholar] [CrossRef]
- Casanova, L.M.; Costa, S.S. Synergistic Interactions in Natural Products: Therapeutic Potential and Challenges. Rev. Virtual Quim. 2017, 9, 575–595. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, D.H.; Choi, H.I.; Ahn, J.; Jang, Y.J.; Ha, T.Y.; Jung, C.H. Synergistic lipid-lowering effects of Zingiber mioga and Hippophae rhamnoides extracts. Exp. Ther. Med. 2020, 20, 2270–2278. [Google Scholar] [CrossRef]
- Yari, Z.; Cheraghpour, M.; Alavian, S.M.; Hedayati, M.; Eini-Zinab, H.; Hekmatdoost, A. The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: An open-labeled randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 99–111. [Google Scholar] [CrossRef]
- Yusof, H.M.; Sarah, N.M.L.; Lam, T.W.; Kassim, M.N.I. Hypolipidemic effects of quercetin and kaempferol in human hepatocellular carcinoma (HepG2) cells. Int. Food Res. J. 2018, 25, 241–245. [Google Scholar]
- Ma, Q.Q.; Cui, Y.L.; Xu, S.Y.; Zhao, Y.Y.; Yuan, H.D.; Piao, G.C. Synergistic Inhibitory Effects of Acacetin and 11 OTher. Flavonoids Isolated from Artemisia sacrorum on Lipid Accumulation in 3T3-L1 Cells. J. Agric. Food Chem. 2018, 66, 12931–12940. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of Catechin, Epicatechin, and Rutin: Optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 2021, 88, 108520. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.T.; Liu, S.Y.; Kuang, Y.J.; Jiang, Z.M.; Lin, Y.; Xie, Z.S.; Liu, E.H. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J. EthnoPharmacol. 2021, 264, 113380. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.I.; Su, C.H.; Lu, M.K.; Lai, M.N.; Ng, L.T. Synergistic anti-inflammatory effects of different polysaccharide components from Xylaria nigripes. J. Food Biochem. 2021, 45, e13694. [Google Scholar] [CrossRef]
- Li, Q.; Fang, X.J.; Chen, H.J.; Han, Y.C.; Liu, R.L.; Wu, W.J.; Gao, H.Y. Retarding effect of dietary fibers from bamboo shoot (Phyllostachys edulis) in hyperlipidemic rats induced by a high-fat diet. Food Funct. 2021, 12, 4696–4706. [Google Scholar] [CrossRef]
- Hannan, P.A.; Khan, J.A.; Ullah, I.; Ullah, S. Synergistic combinatorial antihyperlipidemic study of selected natural antioxidants; modulatory effects on lipid profile and endogenous antioxidants. Lipids Health Dis. 2016, 15, 151. [Google Scholar] [CrossRef]
- Cirico, T.L.; Ornaye, S.T. Additive or synergetic effects of phenolic compounds on human low density lipoprotein oxidation. Food Chem. Toxicol. 2006, 44, 510–516. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Lu, H.; Guo, T.; Liu, X. Hypolipidemic Effects of Numb-Tasting Components of Zanthoxylum bungeanum Combined with Capsaicin at Various Ratios on Rats. Food Sci. 2014, 35, 231–235. [Google Scholar]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant. Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef]
- Alseekh, S.; de Souza, L.P.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef]
- Juca, M.M.; Cysne, F.M.S.; de Almeida, J.C.; Mesquita, D.D.; Barriga, J.R.D.; Ferreira, K.C.D.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.; Honorio, J.E.R.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Huff, M.W. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can. J. Cardiol. 2010, 26, 17A–21A. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Cappello, A.R.; Dolce, V.; Iacopetta, D.; Martello, M.; Fiorillo, M.; Curcio, R.; Muto, L.; Dhanyalayam, D. Bergamot (Citrus bergamia Risso) Flavonoids and Their Potential Benefits in Human Hyperlipidemia and Atherosclerosis: An Overview. Mini Rev. Med. Chem. 2016, 16, 619–629. [Google Scholar] [CrossRef]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Drangova, M.; Huff, M.W. Intervention with citrus flavonoids reverses obesity and improves metabolic syndrome and atherosclerosis in obese Ldlr−/− mice. J. Lipid Res. 2018, 59, 1714–1728. [Google Scholar] [CrossRef]
- Zhang, T.T.; Jiang, J.G. Active ingredients of traditional Chinese medicine in the treatment of diabetes and diabetic complications. Expert Opin. Inv. Drug 2012, 21, 1625–1642. [Google Scholar] [CrossRef]
- Qin, X.X.; Lu, Y.F.; Peng, Z.; Yao, Y.C.; Fan, S.X. Systematic Chemical Analysis Approach Reveals Superior Antioxidant Capacity via the Synergistic Effect of Flavonoid Compounds in Red Vegetative Tissues. Front Chem. 2018, 6, 9. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Wang, J.S.; Zhang, W.; Zhu, D.; Zhu, X.L.; Pang, X.F.; Qu, W.J. Hypolipidaemic and hypoglycaemic effects of total flavonoids from seed residues of Hippophae rhamnoides L. in mice fed a high-fat diet. J. Sci. Food Agric. 2011, 91, 1446–1451. [Google Scholar] [CrossRef]
- Zhao, E.; Yang, J.; Zhao, S.; Fan, J. Research Progress on Extraction Process and Functional Activities of Flavonoids from Leaves of Hioppophae rhamnoides. MPB 2019, 17, 4096–4101. [Google Scholar]
- Wu, Y.-Q.; Xu, R.-A. Research advances on hypolipidemic effect of polysaccharides. Chin. J. Chin. Mater. Med. 2018, 43, 3451–3459. [Google Scholar]
- Luan, F.; Zou, J.B.; Rao, Z.L.; Ji, Y.F.; Lei, Z.Q.; Peng, L.X.; Yang, Y.; He, X.R.; Zeng, N. Polysaccharides from Laminaria japonica: An insight into the current research on structural features and biological properties. Food Funct. 2021, 12, 4254–4283. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B.J. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef]
- Xie, J.H.; Jin, M.L.; Morris, G.A.; Zha, X.Q.; Chen, H.Q.; Yi, Y.; Li, J.E.; Wang, Z.J.; Gao, J.; Nie, S.P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. 2016, 56, S60–S84. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, X.; Wang, Y.; Ling, Y.; Li, P.; Zhang, F. Research Progress on Pretreatment and Analysis Methods for Polysaccharides in Traditional Chinese Medicine. J. Instr. Anal. 2020, 39, 1168–1175. [Google Scholar]
- Yang, X.; Feng, Y.-S.; Tong, S.-S.; Yu, J.-N.; Xu, X.-M. Study on mechanism and structure-activity relationship of hypolipidemic polysaccharides: A review. Chin. J. Chin. Mater. Med. 2018, 43, 4011–4018. [Google Scholar]
- Zhang, T.; Miller, M.C.; Zheng, Y.; Zhang, Z.Y.; Xue, H.T.; Zhao, D.Y.; Su, J.Y.; Mayo, K.H.; Zhou, Y.F.; Tai, G.H. Macromolecular assemblies of complex polysaccharides with galectin-3 and their synergistic effects on function. Biochem. J. 2017, 474, 3849–3868. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, J.; Luo, Z.; Li, S.P.; Zhao, J. Synergistic immunomodulatory effect of complex polysaccharides from seven herbs and their major active fractions. Int. J. Biol. Macromol. 2020, 165, 530–541. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metab. Clin. Exp. 2012, 61, 1058–1066. [Google Scholar] [CrossRef]
- Xiao, R.Q.; Grinstaff, M.W. Chemical synthesis of polysaccharides and polysaccharide mimetics. Prog. Polym. Sci. 2017, 74, 78–116. [Google Scholar] [CrossRef]
- Zhang, L.; McClements, D.J.; Wei, Z.L.; Wang, G.Q.; Liu, X.B.; Liu, F.G. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Vittorio, O.; Iemma, F.; Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Picci, N. Polyphenol Conjugates and Human Health: A Perspective Review. Crit. Rev. Food Sci. 2016, 56, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.O. Antioxidant capacities of individual and combined phenolics in a model system. Food Chem. 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Blade, C.; Arola, L.; Salvado, M.J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Zhang, Z.S.; Yu, H.J.; Huang, Y.; Chen, Z.Y. Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and up-regulation of CYP7A1. J. Nutr. Biochem. 2010, 21, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Ballington, Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Vittorio, O.; Cirillo, G.; Boyer, C. Enhancing the therapeutic effects of polyphenols with macromolecules. Polym. Chem. 2016, 7, 1529–1544. [Google Scholar] [CrossRef]
- Ariyarathna, I.R.; Karunaratne, D.N. Microencapsulation stabilizes curcumin for efficient delivery in food applications. Food Packaging Shelf 2016, 10, 79–86. [Google Scholar] [CrossRef]
- Zhuang, T.X.; Liu, X.H.; Wang, W.; Song, J.; Zhao, L.; Ding, L.L.; Yang, L.; Zhou, M.M. Dose-Related Urinary Metabolic Alterations of a Combination of Quercetin and Resveratrol-Treated High-Fat Diet Fed Rats. Front. Pharmacol. 2021, 12, 655563. [Google Scholar] [CrossRef]
- Park, H.J.; Yang, J.Y.; Ambati, S.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Baile, C.A. Combined Effects of Genistein, Quercetin, and Resveratrol in Human and 3T3-L1 Adipocytes. J. Med. Food 2008, 11, 773–783. [Google Scholar] [CrossRef]
- Huang, W.; Ye, X.; Li, X.; Zhao, Z.; Lan, P.; Wang, L.; Liu, M.; Gao, Y.; Zhu, J.; Li, P.; et al. The inhibition activity of chemical constituents in hawthorn frult and their synergistic action to HMG-CoA reductase. Chin. J. Chin. Mater. Med. 2010, 35, 2428–2431. [Google Scholar]
- Gao, J.; Zhang, M.Q.; Niu, R.X.; Gu, X.; Hao, E.W.; Hou, X.T.; Deng, J.G.; Bai, G. The combination of cinnamaldehyde and kaempferol ameliorates glucose and lipid metabolism disorders by enhancing lipid metabolism via AMPK activation. J. Funct. Foods 2021, 83, 104556. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.D.; Xu, J.L.; Zhou, Z.K. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, L.; Wang, B.J.; Zhang, Z.J.; Liu, H.; Zhang, Y.N.; Liu, J.F. Synergistic Hypoglycemic Effects of Pumpkin Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice. Molecules 2019, 24, 955. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. High molecular weight oat beta-glucan enhances lipid-lowering effects of phytosterols. A randomised controlled trial. Clin. Nutr. 2020, 39, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Remesy, C.; Demigne, C. Apple pectin and a polyphenol-rich apple concentrate are more effective togeTher. than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Zhou, F.; Ouyang, J.; Wang, Q.Y.; Li, Y.L.; Wu, J.L.; Huang, J.A.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Abdullah; Tian, W.; Song, M.; Cao, Y.; Xiao, J. Co-delivery of EGCG and lycopene via a pickering double emulsion induced synergistic hypolipidemic effect. Food Funct. 2022, 13, 3419–3430. [Google Scholar] [CrossRef]
- Hasimun, P.; Sukandar, E.Y.; Adnyana, I.K.; Tjahjono, D.H. Synergistic Effect of Curcuminoid and S-methyl Cysteine in Regulation of Cholesterol Homeostasis. Int. J. Pharmacol. 2011, 7, 268–272. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Z.J.; Shen, H.L.; Yin, M.; Tang, K.X. Effects of artesunate and ursolic acid on hyperlipidemia and its complications in rabbit. Eur. J. Pharm. Sci. 2013, 50, 366–371. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Amin, R.S.; Atteia, H.H.; El-Zeiky, R.R.; Al-Gabri, N.A. New Insight on a Combination of Policosanol and 10-Dehydrogingerdione Phytochemicals as Inhibitors for Platelet Activation Biomarkers and Atherogenicity Risk in Dyslipidemic Rabbits: Role of CETP and PCSK9 Inhibition. Appl. Biochem. Biotech. 2018, 186, 805–815. [Google Scholar] [CrossRef]
- Ahmad, M.; Sultana, M.; Raina, R.; Pankaj, N.K.; Verma, P.K.; Prawez, S. Hypoglycemic, Hypolipidemic, and Wound Healing Potential of Quercetin in Streptozotocin-Induced Diabetic Rats. Pharmacogn. Mag. 2017, 13, S633–S639. [Google Scholar] [PubMed]
- Berrougui, H.; Grenier, G.; Loued, S.; Drouin, G.; Khalil, A. A new insight into resveratrol as an atheroprotective compound: Inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis 2009, 207, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Milton, I.; Portillo, M.P. The combination of resveratrol and quercetin enhances the individual effects of these molecules on triacylglycerol metabolism in white adipose tissue. Eur. J. Nutr. 2016, 55, 341–348. [Google Scholar] [CrossRef]
- Yang, J.Y.; Della-Fera, M.A.; Rayalam, S.; Ambati, S.; Hartzell, D.L.; Park, H.J.; Baile, C.A. Baile, Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008, 82, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Q.; Ma, W.N.; Tian, F.; Shen, H.Y.; Zhou, M.M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. PhytoTher. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Li, J.E.; Futawaka, K.; Yamamoto, H.; Kasahara, M.; Tagami, T.; Liu, T.H.; Moriyama, K. Cinnamaldehyde Contributes to Insulin Sensitivity by Activating PPAR delta, PPAR gamma, and RXR. Am. J. Chin. Med. 2015, 43, 879–892. [Google Scholar] [CrossRef]
- Song, H.M.; Sun, Z.X. Hypolipidaemic and hypoglycaemic properties of pumpkin polysaccharides. 3 Biotech. 2017, 7, 159. [Google Scholar] [CrossRef]
- Yuan, G.Z.; Shi, S.; Jia, Q.L.; Shi, J.; Shi, S.J.; Zhang, X.S.; Shou, X.T.; Zhu, X.P.; Hu, Y.H. Use of Network Pharmacology to Explore the Mechanism of Gegen (Puerariae lobatae Radix) in the Treatment of Type 2 Diabetes Mellitus Associated with Hyperlipidemia. Evid. Based Compl. Alt. 2021, 2021, 6633402. [Google Scholar] [CrossRef]
- Tang, T.; Song, J.J.; Li, J.; Wang, H.W.; Zhang, Y.; Suo, H.Y. A synbiotic consisting of Lactobacillus plantarum S58 and hull-less barley beta-glucan ameliorates lipid accumulation in mice fed with a high-fat diet by activating AMPK signaling and modulating the gut microbiota. Carbohyd. Polym. 2020, 243, 116398. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaragoud, G.; Rajath, S.; Mahendra, V.P.; Kumar, G.S.; Krishna, A.G.G.; Kumar, G.S. Hypolipidemic mechanism of oryzanol components- ferulic acid and phytosterols. Biochem. Bioph. Res. Commun. 2016, 476, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ker, Y.B.; Peng, C.H.; Chyau, C.C.; Peng, R.Y. Soluble Polysaccharide Composition and myo-Inositol Content Help Differentiate the Antioxidative and Hypolipidemic Capacity of Peeled Apples. J. Agric. Food Chem. 2010, 58, 4660–4665. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Tamuly, S.; Das Purkayastha, M.; Dutta, B.; Barman, C.; Kalita, D.J.; Boro, R.; Agarwal, S. Green tea leaves extract with low concentration of EGCG can provide health benefits without causing renal damage. Acta Aliment. Hung. 2021, 50, 369–382. [Google Scholar] [CrossRef]
- Ortsater, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjoholm, A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. 2012, 9, 11. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, M.Z.; Zhang, Y.B.; Wen, B.B.; An, H.M.; Ou, X.C.; Xiong, Y.F.; Lin, H.Y.; Liu, Z.H.; Huang, J.A. Coadministration of epigallocatechin-3-gallate (EGCG) and caffeine in low dose ameliorates obesity and nonalcoholic fatty liver disease in obese rats. PhytoTher. Res. 2019, 33, 1019–1026. [Google Scholar] [CrossRef]
- Sugiura, C.; Nishimatsu, S.; Moriyama, T.; Ozasa, S.; Kawada, T.; Sayama, K. Sayama, Catechins and Caffeine Inhibit Fat Accumulation in Mice through the Improvement of Hepatic Lipid Metabolism. J. Obes. 2012, 2012, 520510. [Google Scholar] [CrossRef]
- Saeedi, F.; Farkhondeh, T.; Roshanravan, B.; Amirabadizadeh, A.; Ashrafizadeh, M.; Samarghandian, S. Curcumin and blood lipid levels: An updated systematic review and meta-analysis of randomised clinical trials. Arch. Physiol. Biochem. 2020. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.Y.; Dong, B.H.; Jiang, Y.X.; Yu, L.Y.; Chen, Z.M.; Hu, C.J.; Xu, R.C. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Ke, L.I.N.; Taiping, Z.; Heyun, Z. Separation and Purification of Ursolic Acid (UA) from Hawthorn Fruits and Hypolipidemic Effect of UA on Mice. Nat. Prod. Res. Dev. 2007, 19, 1052–1054. [Google Scholar]
- Wang, Y.L.; Wang, Z.J.; Shen, H.L.; Yin, M.; Tang, K.X. The hypolipidemic effect of artesunate and ursolic acid in rats. Pak. J. Pharm. Sci. 2015, 28, 871–874. [Google Scholar]
- Guo, Y.L.; Xu, R.X.; Zhu, C.G.; Wu, N.Q.; Cui, Z.P.; Li, J.J. Policosanol Attenuates Statin-Induced Increases in Serum Proprotein Convertase Subtilisin/Kexin Type 9 When Combined with Atorvastatin. Evid. Based Compl. Alt. 2014, 2014, 926087. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Park, G.S.; Lee, S.Y.; Kim, J.Y.; Kim, Y.K. The Conformation and CETP Inhibitory Activity of 10 -Dehydrogingerdione Isolated from Zingiber officinale. Arch. Pharm. Res. 2011, 34, 727–731. [Google Scholar] [CrossRef]
- Zou, L.; Shen, Y.; Huang, X.; Ding, X. Progress in Research on Hypolipidemic Mechanisms of Functional Food Components. Food Sci. 2016, 37, 239–244. [Google Scholar]
- Colombo, A.; Dinkel, L.; Muller, S.A.; Monasor, L.S.; Schifferer, M.; Cantuti-Castelvetri, L.; Konig, J.; Vidatic, L.; Bremova-Ertl, T.; Lieberman, A.P.; et al. Loss of NPC1 enhances phagocytic uptake and impairs lipid trafficking in microglia. Nat. Commun. 2021, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Tan, M.Y.; Zhang, R.J.; Qiu, M.Y.; Fu, Y.S.; Xie, X.J.; Gu, H.F. Acid-Sensing Ion Channel 1/Calpain1 Activation Impedes Macrophage ATP-Binding Cassette Protein A1-Mediated Cholesterol Efflux Induced by Extracellular Acidification. Front. Physiol. 2022, 12, 2442. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Kastelein, J.J.P.; Ray, K.K.; Ginsberg, H.N.; Chapman, J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; et al. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef]
- Ference, B.A.; Majeed, F.; Penumetcha, R.; Flack, J.M.; Brook, R.D. Effect of Naturally Random Allocation to Lower Low-Density Lipoprotein Cholesterol on the Risk of Coronary Heart Disease Mediated by Polymorphisms in NPC1L1, HMGCR, or Both. J. Am. Coll. Cardiol. 2015, 65, 1552–1561. [Google Scholar] [CrossRef]
- Tao, R.Y.; Xiong, X.W.; DePinho, R.A.; Deng, C.X.; Dong, X.C. Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6. J. Lipid Res. 2013, 54, 2745–2753. [Google Scholar] [CrossRef]
- Feng, X.J.; Zhang, L.; Xu, S.W.; Shen, A.Z. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog. Lipid Res. 2020, 77, 101006. [Google Scholar] [CrossRef]
- Wang, Y.P.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [Green Version]
- Ference, B.A.; Kastelein, J.J.P.; Ginsberg, H.N.; Chapman, J.; Nicholls, S.J.; Ray, K.K.; Packard, C.J.; Laufs, U.; Brook, R.D.; Oliver-Williams, C.; et al. Association of Genetic Variants Related to CETP Inhibitors and Statins with Lipoprotein Levels and Cardiovascular Risk. JAMA 2017, 318, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.F.; Tang, X.; Shi, Y.H.; Le, G.W. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, T.H. Recent development in acetyl-CoA carboxylase inhibitors and their potential as novel drugs. Future Med. Chem. 2020, 12, 533–561. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.Y.; Li, D.J.; Huang, W.Y. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schon, M.R.; Abumrad, N.A.; Bluher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef]
- Wu, B.N.; Kuo, K.K.; Chen, Y.H.; Chang, C.T.; Huang, H.T.; Chai, C.Y.; Dai, Z.K.; Chen, I.J. Theophylline-Based KMUP-1 Improves Steatohepatitis via MMP-9/IL-10 and Lipolysis via HSL/p-HSL in Obese Mice. Int. J. Mol. Sci. 2016, 17, 1345. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Baron, M.; Bouhlel, M.A.; Vanhoutte, J.; Copin, C.; Sebti, Y.; Derudas, B.; Mayi, T.; Bories, G.; Tailleux, A.; et al. Human Atherosclerotic Plaque Alternative Macrophages Display Low Cholesterol Handling but High Phagocytosis Because of Distinct Activities of the PPAR gamma and LXR alpha Pathways. Circ. Res. 2011, 108, 985–995. [Google Scholar] [CrossRef]
- Srivastava, R.A.K.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Pan, D.A. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 2002, 30, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Bio. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Kwon, D.Y.; Yoon, S.H. AMP-activated protein kinase: A potential target for the diseases prevention by natural occurring polyphenols. New Biotechnol. 2009, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Ma, J.Q.; Sun, J.M.; Cheng, C.; Feng, Z.J.; Jiang, H.; Yang, W. Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway. Nutrients 2017, 9, 959. [Google Scholar] [CrossRef]
- Zhao, L.; Cen, F.; Tian, F.; Li, M.J.; Zhang, Q.; Shen, H.Y.; Shen, X.C.; Zhou, M.M.; Du, J. Combination treatment with quercetin and resveratrol attenuates high fat diet-induced obesity and associated inflammation in rats via the AMPK alpha 1/SIRT1 signaling pathway. Exp. Ther. Med. 2017, 14, 5942–5948. [Google Scholar]
- Bitterman, J.L.; Chung, J.H. Metabolic effects of resveratrol: Addressing the controversies. Cell Mol. Life Sci. 2015, 72, 1473–1488. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Pan, Y.; Wang, R.; Kang, L.L.; Xue, Q.C.; Wang, X.N.; Kong, L.D. Quercetin inhibits AMPK/TXNIP activation and reduces inflammatory lesions to improve insulin signaling defect in the hypothalamus of high fructose-fed rats. J. Nutr. Biochem. 2014, 25, 420–428. [Google Scholar] [CrossRef]
- Deng, N.; He, Z.Q.; Guo, R.X.; Zheng, B.S.; Li, T.; Liu, R.H. Highland Barley Whole Grain (Hordeum vulgare L.) Ameliorates Hyperlipidemia by Modulating Cecal Microbiota, miRNAs, and AMPK Pathways in Leptin Receptor-Deficient db/db Mice. J. Agric. Food Chem. 2020, 68, 11735–11746. [Google Scholar] [CrossRef]
- Bae, I.Y.; Kim, S.M.; Lee, S.; Lee, H.G. Effect of enzymatic hydrolysis on cholesterol-lowering activity of oat beta-glucan. New Biotechnol. 2010, 27, 85–88. [Google Scholar] [CrossRef]
- De Smet, E.; Mensink, R.P.; Plat, J. Effects of plant sterols and stanols on intestinal cholesterol metabolism: Suggested mechanisms from past to present. Mol. Nutr. Food Res. 2012, 56, 1058–1072. [Google Scholar] [CrossRef]
- Mulati, A.; Ma, S.B.; Zhang, H.B.; Ren, B.; Zhao, B.T.; Wang, L.F.; Liu, X.N.; Zhao, T.; Kamanova, S.; Sair, A.T.; et al. Sea-Buckthorn Flavonoids Alleviate High-Fat and High-Fructose Diet-Induced Cognitive Impairment by Inhibiting Insulin Resistance and Neuroinflammation. J. Agric. Food Chem. 2020, 68, 5835–5846. [Google Scholar] [CrossRef] [PubMed]
- Martin-Navarro, C.M.; Lorenzo-Morales, J.; Machin, R.P.; Lopez-Arencibia, A.; Garcia-Castellano, J.M.; de Fuentes, I.; Loftus, B.; Maciver, S.K.; Valladares, B.; Pineroa, J.E. Inhibition of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase and Application of Statins as a Novel Effective Therapeutic Approach against Acanthamoeba Infections. Antimicrob. Agents Chemother. 2013, 57, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.; Sousa, S.F. An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef]
- Egom, E.E.A.; Hafeez, H. Biochemistry of Statins. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 73, pp. 127–168. [Google Scholar]
- Susilowati, R.; Jannah, J.; Maghfuroh, Z.; Kusuma, M.T. Kusuma, Antihyperlipidemic effects of apple peel extract in high-fat diet-induced hyperlipidemic rats. J. Adv. Pharm. Technol. 2020, 11, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Kostakou, P.M.; Mikhailidis, D.P. Cholesterol Ester Transfer Protein (CETP), Postprandial Lipemia and Hypolipidemic Drugs. Curr Med. Chem. 2009, 16, 4345–4360. [Google Scholar] [CrossRef] [PubMed]
- El-Seweidy, M.M.; Asker, M.E.; Eldahmy, S.I.; Atteia, H.H.; Abdallah, M.A. Haemostatic risk factors in dyslipidemic rabbits: Role of 10-dehydrogingerdione as a new hypolipemic agent. J. Thromb. Thrombolys 2015, 39, 196–202. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.M.; Kim, S.J.; Lee, E.Y.; Kim, J.R.; Cho, K.H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef]
- Xue, Z.H.; Zhang, Q.; Yu, W.C.; Wen, H.C.; Hou, X.N.; Li, D.; Kou, X.H. Potential Lipid-Lowering Mechanisms of Biochanin A. J. Agric. Food Chem. 2017, 65, 3842–3850. [Google Scholar] [CrossRef]
- Xue, Z.H.; Hou, X.N.; Yu, W.C.; Wen, H.C.; Zhang, Q.; Li, D.; Kou, X.H. Lipid metabolism potential and mechanism of CPe-III from chickpea (Cicer arietinum L). Food Res. Int. 2018, 104, 126–133. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic db/db Mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef]
- Stoeckman, A.K.; Towle, H.C. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J. Biol. Chem. 2002, 277, 27029–27035. [Google Scholar] [CrossRef]
- Yang, R.M.; Chu, X.X.; Sun, L.; Kang, Z.Y.; Ji, M.; Yu, Y.; Liu, Y.; He, Z.D.; Gao, N.N. Hypolipidemic activity and mechanisms of the total phenylpropanoid glycosides from Ligustrum robustum (Roxb.) Blume by AMPK-SREBP-1c pathway in hamsters fed a high-fat diet. PhytoTher. Res. 2018, 32, 715–722. [Google Scholar] [CrossRef]
- Drozdzik, M.; Groer, C.; Penski, J.; Lapczuk, J.; Ostrowski, M.; Lai, Y.R.; Prasad, B.; Unadkat, J.D.; Siegmund, W.; Oswald, S. Protein Abundance of Clinically Relevant Multidrug Transporters along the Entire Length of the Human Intestine. Mol. Pharm. 2014, 11, 3547–3555. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouche, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Regnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPAR alpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Okamura, A.; Koyanagi, S.; Dilxiat, A.; Kusunose, N.; Chen, J.J.; Matsunaga, N.; Shibata, S.; Ohdo, S. Bile Acid-regulated Peroxisome Proliferator-activated Receptor-alpha (PPAR alpha) Activity Underlies Circadian Expression of Intestinal Peptide Absorption Transporter PepT1/Slc15a1. J. Biol. Chem. 2014, 289, 25296–25305. [Google Scholar] [CrossRef]
- Qiao, L.-S.; Chen, Y.-K.; Luo, G.-G.; Lu, F.; Liu, S.-J.; Li, G.-Y.; Zhang, Y.-L. Synergistic mechanism of traditional Chinese medicine based on target combination of PepT1 and PPARalpha. Chin. J. Chin. Mater. Med. 2017, 42, 2146–2151. [Google Scholar]
- Shimizu, T.; Shibuya, N.; Narukawa, Y.; Oshima, N.; Hada, N.; Kiuchi, F. Synergistic effect of baicalein, wogonin and oroxylin A mixture: Multistep inhibition of the NF-kappa B signalling pathway contributes to an anti-inflammatory effect of Scutellaria root flavonoids. J. Nat. Med. 2018, 72, 181–191. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K.D. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Zhao, C.F.; Lei, D.J.; Song, G.H.; Zhang, H.; Xu, H.; Yu, L.J. Characterisation of water-soluble proanthocyanidins of Pyracantha fortuneana fruit and their improvement in cell bioavailable antioxidant activity of quercetin. Food Chem. 2015, 169, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.; Ottaviani, J.I.; Rodriguez-Mateos, A.; Heinen, Y.; Noske, D.; Spencer, J.P.; Crozier, A.; Merx, M.W.; Kelm, M.; Schroeter, H.; et al. Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: Randomized, double-masked controlled studies. Am. J. Clin. Nutr. 2017, 105, 352–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efferth, T.; Koch, E. Complex Interactions between Phytochemicals. The Multi-Target Therapeutic Concept of Phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Fernandez, M.; Pico, Y.; Manes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary Administration of High Doses of Pterostilbene and Quercetin to Mice Is Not Toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.L.; Yang, J.P.; Yang, C.L.; Guo, M.Q. Screening and characterisation of potential antioxidant, hypoglycemic and hypolipidemic components revealed in Portulaca oleracea via multi-target affinity ultrafiltration LC-MS and molecular docking. PhytoChem. Anal. 2021, 33, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. Methods Mol. Biol. 2008, 443, 365–382. [Google Scholar]
- Zhang, H.; Chen, G.L.; Zhang, Y.L.; Yang, M.; Chen, J.M.; Guo, M.Q. Potential hypoglycemic, hypolipidemic, and anti-inflammatory bioactive components in Nelumbo nucifera leaves explored by bioaffinity ultrafiltration with multiple targets. Food Chem. 2022, 375, 131856. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Ma, N.; Karam, I.; Liu, X.W.; Kong, X.J.; Qin, Z.; Li, S.H.; Jiao, Z.H.; Dong, P.C.; Yang, Y.J.; Li, J.Y. UPLC-Q-TOF/MS-based urine and plasma metabonomics study on the ameliorative effects of aspirin eugenol ester in hyperlipidemia rats. Toxicol. Appl. Pharm. 2017, 332, 40–51. [Google Scholar] [CrossRef]
- Chen, M.; Guo, W.L.; Li, Q.Y.; Xu, J.X.; Cao, Y.J.; Liu, B.; Yu, X.D.; Rao, P.F.; Ni, L.; Lv, X.C. The protective mechanism ofLactobacillus plantarumFZU3013 against non-alcoholic fatty liver associated with hyperlipidemia in mice fed a high-fat diet. Food Funct. 2020, 11, 3316–3331. [Google Scholar] [CrossRef]
- Wang, F.R.; Wang, J.; Cai, H.D.; Yuan, L.X.; Sun, C.L.; Peng, X.; Yan, W.W.; Zhang, J.L. Network pharmacology combined with metabolomics to investigate the anti-hyperlipidemia mechanism of a novel combination. J. Funct. Foods 2021, 87, 104848. [Google Scholar] [CrossRef]






| Category | Phytochemical Combinations | Ratios and Concentration | Experimental Model | Effect or Mechanisms | Reference |
|---|---|---|---|---|---|
| Flavonoids | quercetin and kaempferol | 1:1, 15 μM each | HepG2 cells | Improved LDL-C uptake more effectively | [29] |
| Flavonoids | acacetin and apigenin | 1:1, 10 μM each | 3T3-L1 cells | Promoted the phosphorylation of AMPK and ACC | [30] |
| Flavonoids | rutin and epicatechin | 1:3, 10 mg/kg, 30 mg/kg | Alloxan-induced diabetic mice | Showed a very significant improvement in body weight and a potent antihyperglycemic activity | [31] |
| Flavonoids | quercetin, catechin, hesperidin and isorhamnetin | 2:2:2:1, 40 μM, 20 μM | HL7702 cells | Down-regulated the mRNA expression of SREBP-2 and LDLR. | [32] |
| Polysaccharides | high molecular weight dextran and low molecular weight heteropolysaccharide | 1:1, 50 μg/mL each | RAW264.7 macrophage cell | Demonstrated stronger inhibitory effect on NO, TNF- α, and IL- 6 production | [33] |
| Polysaccharides | soluble dietary fiber and insoluble dietary fiber | 1:1, 0.15 g/kg each | Sprague–Dawley rats | The mRNA expression levels of lipid synthesis genes SREBP-1c and FAS were significantly down-regulated | [34] |
| Polyphenols | oligomeric proanthocyanidins and pterostilbene | 5:3, 50 mg/kg, 30 mg/kg | Male albino rabbits | The LDL/HDL ratio and atherogenic index were suppressed by 59.3% and 25% | [35] |
| Polyphenols | catechin, hesperidin, ferulic acid and quercetin | 20:9.3:4.3:2 20, 9.3, 4.3, 2 μMol/L | Human plasma | Effects of polyphenols protecting LDL from oxidation were observed | [36] |
| Amides | zanthoxylum and capsaicin | 3:6 3 mg/kg, 6 mg/kg | Sprague–Dawley rats | Reduced the serum levels of TC, TG, and LDL-C | [37] |
| Category | Phytochemical Combinations | Ratios and Concentration | Experimental Model | Effect or Mechanisms | Reference |
|---|---|---|---|---|---|
| Flavonoids with Polyphenols | quercetin and resveratrol | 2:1, 30 mg/kg/d, 15 mg/kg/d | Rats fed an HFD | May suppress obesity and associated inflammation via the AMPKα1/SIRT1 signaling pathway | [68] |
| Flavonoids with polyphenols | genistein, quercetin and resveratrol | 1:2:2, 6.25 ΜM, 12.5 ΜM, 12.5 μM | Human primary adipocytes and 3T3-L1 mouse adipocytes | Lipid accumulation was reduced by 80.3%; resulted in a significant decrease in lipid accumulation | [69] |
| Flavonoids with polyphenols and terpenoids | quercetin, crocin, chlorogenic acid and geniposide | 10:1:30:10 10 μmol/L,1 μmol/L, 30 μmol/L, 10 μmol/L | HepG2 cells | Increased ABCA1, CYP7A1, and AMPKα2 mRNA expression, decreased SREBP2, and LXRα mRNA expression | [24] |
| Flavonoids with polyphenols | quercetin, hyperoside, rutin and chlorogenic acid | 6:9:2:1 | Inhibitory activity of HMG-CoA reductase | Increased the inhibitory activity of HMG-CoA reductase by 58.9% | [70] |
| Flavonoids with aldehydes | kaempferol and cinnamaldehyde | 39: 58, 39 mg/kg, 58 mg/kg | Eight-week-old male Kunming mice | Ameliorated glucose and lipid metabolism disorders by enhancing lipid metabolism via the activation of AMPK | [71] |
| Polysaccharides with polyphenols | tea polysaccharide and polyphenols | 1:1, 400 mg/ kg each | Sprague–Dawley rats fed with high-fat diet | Reduced rat serum leptin levels, inhibited the absorption of fatty acids, reduced the expression levels of IL-6, TNF-α gene | [72] |
| Polysaccharides with flavonoids | pumpkin polysaccharides and puerarin | 2:1, 400 mg/kg, 200 mg/kg | Male Kunming mice | Up-regulated the expression of the critical proteins in the Nrf2/HO-1 and PI3K/Akt signaling pathways. | [73] |
| Polysaccharides with phytosterols | oat β-glucan and phytosterols | 3:2, 3 g/d, 2 g/d | Healthy adults aged 18–70 years old | TC: HDL-C ratio was significantly reduced | [74] |
| Polyphenols with polysaccharides | pectin and polyphenols | 1:2 5 g/100 g, 10 g/100 g | Male Wistar rats | Significantly lowered plasma cholesterol and triglycerides | [75] |
| Polyphenols with alkaloids | epigallocatechin-3-gallate and caffeine | 2:1, 40 mg/kg/d,20 mg/kg/d | Four-week-old Sprague–Dawley male rats | Improved gut microbiota, inhibited fat accumulation, increased expression of hepatic TGR5 | [76] |
| Polyphenols with carotenoids | epigallocatechin-3-gallate and lipophilic lycopene | 3:1, 30 mg/kg, 10 mg/kg | Healthy 4-week-old male Sprague–Dawley rats | Triggered the pathways of HMGCR, LDLR, PPAR and AMPK | [77] |
| Polyphenols with amino acids | curcuminoid, S-methyl cysteine | 1:1, 50 mg/kg each | Rats with cholesterol metabolism abnormality | Increased the conversion of cholesterol into the feces as much as 3 times | [78] |
| Terpenoids with acetyl compounds | ursolic acid and artesunate | 1:1, 12.5 mg/kg each | Rabbit fed with Western-type diet | Significantly decreased the plasma cholesterol and triglyceride | [79] |
| Others | policosanol and 10-dehydrocongerdione | 1:1, 10 mg/kg each | Adult male albino rabbits | Resulted in a CETP inhibitory activity, increasing HDL-C level | [80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, C.; Kou, X.; Wang, Y.; Yu, Y.; Zhen, N.; Jiang, J.; Zhaxi, P.; Xue, Z. Synergistic Hypolipidemic Effects and Mechanisms of Phytochemicals: A Review. Foods 2022, 11, 2774. https://doi.org/10.3390/foods11182774
Liu Y, Liu C, Kou X, Wang Y, Yu Y, Zhen N, Jiang J, Zhaxi P, Xue Z. Synergistic Hypolipidemic Effects and Mechanisms of Phytochemicals: A Review. Foods. 2022; 11(18):2774. https://doi.org/10.3390/foods11182774
Chicago/Turabian StyleLiu, Yazhou, Chunlong Liu, Xiaohong Kou, Yumeng Wang, Yue Yu, Ni Zhen, Jingyu Jiang, Puba Zhaxi, and Zhaohui Xue. 2022. "Synergistic Hypolipidemic Effects and Mechanisms of Phytochemicals: A Review" Foods 11, no. 18: 2774. https://doi.org/10.3390/foods11182774
APA StyleLiu, Y., Liu, C., Kou, X., Wang, Y., Yu, Y., Zhen, N., Jiang, J., Zhaxi, P., & Xue, Z. (2022). Synergistic Hypolipidemic Effects and Mechanisms of Phytochemicals: A Review. Foods, 11(18), 2774. https://doi.org/10.3390/foods11182774






