Spectroscopic Investigation of the Impact of Cold Plasma Treatment at Atmospheric Pressure on Sucrose and Glucose
Abstract
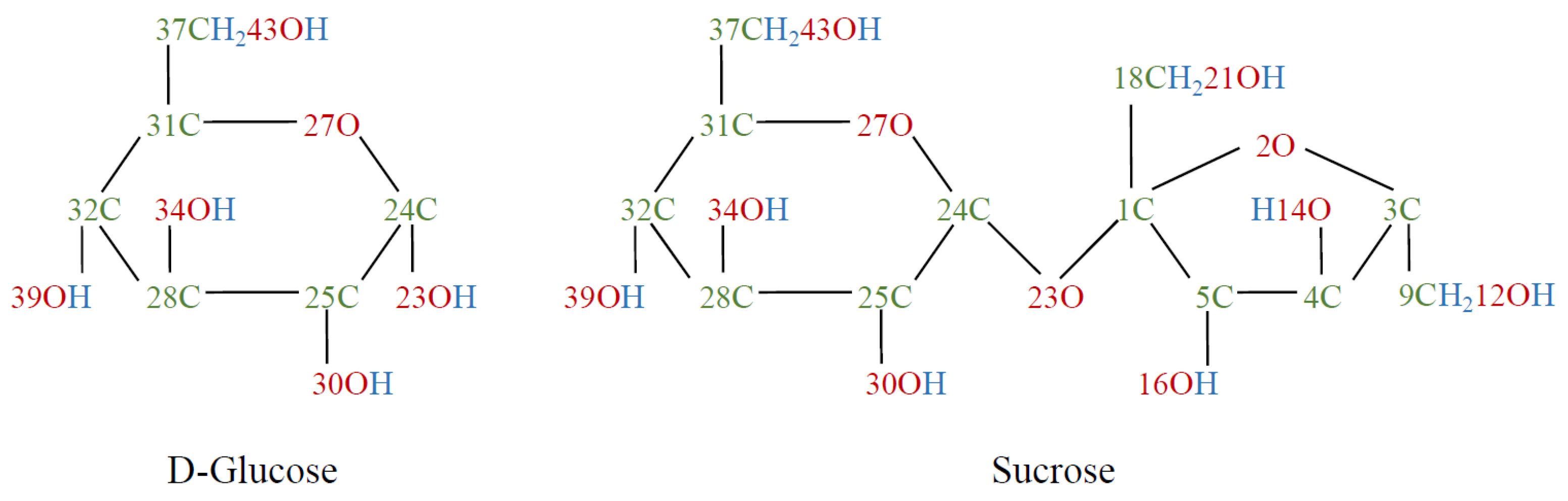
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Plasma Source and Treatment
2.3. Surface Chemistry
2.3.1. FTIR Measurements
2.3.2. XPS Measurements
3. Results
3.1. Measurements Via FTIR Spectroscopy
3.2. Measurements Via XPS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feizollahi, E.; Arshad, M.; Yadav, B.; Ullah, A.; Roopesh, M.S. Degradation of deoxynivalenol by atmospheric-pressure cold plasma and sequential treatments with heat and UV light. Food Eng. Rev. 2021, 13, 696–705. [Google Scholar] [CrossRef]
- Schmidt, M.; Zannini, E.; Arendt, E.K. Recent advances in physical post-harvest treatments for shelf-life extension of cereal crops. Foods 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Mohammadi Shad, Z.; Oduola, A.; Zhou, Z.; Jiang, H.; Carbonero, F.; Atungulu, G. Decontamination of mycotoxigenic fungi on shelled corn using selective infrared heating technique. Cereal Chem. 2021, 98, 31–43. [Google Scholar] [CrossRef]
- Lung, H.-M.; Cheng, Y.C.; Chang, Y.-H.; Huang, H.-W.; Yang, B.B.; Wang, C.-Y. Microbial decontamination of food by electron beam irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Zhao, F.J.; Adams, M.L.; Dumont, C.; McGrath, S.P.; Chaudri, A.M.; Nicholson, F.A.; Chambers, B.J.; Sinclair, A.H. Factors affecting the concentrations of lead in British wheat and barley grain. Environ. Pollut. 2004, 131, 461–468. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Rotolo, C.; Morano, M.; Minafra, A.; Ambrico, M.; Pollastro, S.; Gerin, D.; Faretra, F.; De Miccolis Angelini, R.M. Surface Dielectric Barrier Discharge plasma: A suitable measure against fungal plant pathogens. Sci. Rep. 2020, 10, 3673. [Google Scholar] [CrossRef]
- Avramidis, G.; Stüwe, B.; Wascher, R.; Bellmann, M.; Wieneke, S.; von Tiedemann, A.; Viöl, W. Fungicidal effects of an atmospheric pressure gas discharge and degradation mechanisms. Surf. Coat. Technol. 2010, 205, 405–408. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Understanding the role of plasma technology in food industry. Food Bioproc. Tech. 2016, 9, 734–750. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Cheng, J.-H.; Sun, D.-W. Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: Mechanisms and application advances. Crit. Rev. Food Sci. Nutr. 2020, 60, 2676–2690. [Google Scholar] [CrossRef]
- Charoux, C.M.G.; Patange, A.; Lamba, S.; O’Donnell, C.P.; Tiwari, B.K.; Scannell, A.G.M. Applications of nonthermal plasma technology on safety and quality of dried food ingredients. J. Appl. Microbiol. 2021, 130, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Patil, S. Non-thermal plasma: An advanced technology for food industry. Food Sci. Technol. Int. 2020, 26, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Anbarasan, R.; Jaspin, S.; Bhavadharini, B.; Pare, A.; Pandiselvam, R.; Mahendran, R. Chlorpyrifos pesticide reduction in soybean using cold plasma and ozone treatments. LWT 2022, 159, 113–193. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef]
- Kogelheide, F.; Kartaschew, K.; Strack, M.; Baldus, S.; Metzler-Nolte, N.; Havenith, M.; Awakowicz, P.; Stapelmann, K.; Lackmann, J.-W. FTIR spectroscopy of cysteine as a ready-to-use method for the investigation of plasma-induced chemical modifications of macromolecules. J. Phys. D 2016, 49, 8. [Google Scholar] [CrossRef]
- Katlafsky, B.; Keller, R.E. Attenuated Total Reflectance Infrared Analysis of Aqueous Solutions. J. Anal. Chem. 1963, 35, 1665–1670. [Google Scholar] [CrossRef]
- Wojciechowski, C.; Dupuy, N.; Ta, C.D.; Huvenne, J.-P.; Legrand, P. Quantitative analysis of water-soluble vitamins by ATR-FTIR spectroscopy. Food Chem. 1998, 63, 133–140. [Google Scholar] [CrossRef]
- Elderderi, S.; Leman-Loubiere, C.; Wils, L.; Henry, S.; Bertrand, D.; Byrne, H.J.; Chourpa, I.; Enguehard-Gueiffier, C.; Munnier, E.; Elbashir, A.A. ATR-IR spectroscopy for rapid quantification of water content in deep eutectic solvents. J. Mol. Liq. 2020, 311, 113361. [Google Scholar] [CrossRef]
- Heinze, S.; Vuillemin, B.; Giroux, P. Application of ATR-FTIR spectroscopy in quantitative analysis of deuterium in basic solutions. Analusis 1999, 27, 549–551. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Friedman, G.; Fridman, A.; Ji, H.-F. Decomposition of sugars under non-thermal dielectric barrier discharge plasma. Clin. Plasma Med. 2014, 2, 56–63. [Google Scholar] [CrossRef]
- Ozen, E.; Singh, R.K. Atmospheric cold plasma treatment of fruit juices: A review. Trends Food Sci. Technol. 2020, 103, 144–151. [Google Scholar] [CrossRef]
- Brizuela, A.B.; Bichara, L.C.; Romano, E.; Yurquina, A.; Locatelli, S.; Brandán, S.A. A complete characterization of the vibrational spectra of sucrose. Carbohydr. Res. 2012, 361, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Brückner, S.; Rösner, S.; Gerhard, C.; Wieneke, S.; Viöl, W. Plasmagestützte Ionisationsspektroskopie (PGIS) zur Materialanalyse. Mater. Test. 2011, 53, 639–642. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Y.; Chen, S.; Jiang, H.; Martin, P. Power calculation of pulse power-driven DBD plasma. IEEE Trans Plasma Sci. 2021, 49, 2210–2216. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X.; Kolobov, V.; Arslanbekov, R. Power consideration in the pulsed dielectric barrier discharge at atmospheric pressure. J. Appl. Phys. 2004, 96, 3028–3030. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alaam, M.; El-Haes, H.; Jalbout Abraham, F.; de Leon, A. Analysis of the structure and vibrational spectra of glucose and fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar] [CrossRef]
- Tajmir-Riahi, H.-A. Interaction of D-glucose with alkaline-earth metal ions. Synthesis, spectroscopic, and structural characterization of Mg (II)-and Ca (II)-D-glucose adducts and the effect of metal-ion binding on anomeric configuration of the sugar. Carbohyd. Res. 1988, 183, 35–46. [Google Scholar] [CrossRef]
- Čapková, P.; Matoušek, J.; Rejnek, J.; Bendlová, N.; Pavlík, J.; Kormunda, M.; Šplíchalová, L.; Pilařová, V. Effect of plasma treatment on structure and surface properties of montmorillonite. Appl. Clay Sci. 2016, 129, 15–19. [Google Scholar] [CrossRef]
- Strachan, C.J.; Rades, T.; Gordon, K.C. A theoretical and spectroscopic study of γ-crystalline and amorphous indometacin. Pharm. Pharmacol. 2007, 59, 261–269. [Google Scholar] [CrossRef]
- Vasko, P.D.; Blackwell, J.; Koenig, J.L. Infrared and Raman spectroscopy of carbohydrates: Part II: Normal coordinate analysis of α-D-glucose. Carbohyd. Res. 1972, 23, 407–416. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Ivanov, A.Y. Vibrational analysis of α-D-glucose trapped in Ar matrix. J. Phys. Chem. B 2009, 113, 2151–2159. [Google Scholar] [CrossRef]
- Tong, D.S.; Xia, X.; Luo, X.P.; Wu, L.M.; Lin, C.X.; Yu, W.H.; Zhou, C.H.; Zhong, Z.K. Catalytic hydrolysis of cellulose to reducing sugar over acid-activated montmorillonite catalysts. Appl. Clay Sci. 2013, 74, 147–153. [Google Scholar] [CrossRef]
- El-Hendawy, A.-N.A. Variation in the FTIR spectra of a biomass under impregnation, carbonization and oxidation conditions. J. Anal. Appl. Pyrolysis 2006, 75, 159–166. [Google Scholar] [CrossRef]
- Stevens, J.S.; Schroeder, S.L.M. Quantitative analysis of saccharides by X-ray photoelectron spectroscopy. Surf. Interface Anal. 2009, 41, 453–462. [Google Scholar] [CrossRef]
- Kotilainen, R.A.; Toivanen, T.-J.; Alén, R.J. FTIR monitoring of chemical changes in softwood during heating. J. Chem. Technol. Biot. 2000, 20, 307–320. [Google Scholar] [CrossRef]
- Thanki Paragkumar, N.; Edith, D.; Jean-Luc, S. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Study of the ageing behaviour of polymer films treated with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7847–7854. [Google Scholar] [CrossRef]
- Leutgoeb, R.A.; Heinrich, H. The electrolytic preparation of glucuronic acid. Am. Chem. Soc. 1939, 61, 870–873. [Google Scholar] [CrossRef]
- Popoff, T.; Theander, O. Formation of aromatic compounds from carbohydrates: Part 1. Reaction of D-glucuronic Acid, D-glacturonic Acid, D-xylose, and L-arabinose in slightly acidic, aqueous solution. Carbohyd. Res. 1972, 22, 135–149. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 12 July 2022).










| Sugar | [min] |
|---|---|
| Glucose | |
| Sucrose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hauswirth, A.; Köhler, R.; ten Bosch, L.; Avramidis, G.; Gerhard, C. Spectroscopic Investigation of the Impact of Cold Plasma Treatment at Atmospheric Pressure on Sucrose and Glucose. Foods 2022, 11, 2786. https://doi.org/10.3390/foods11182786
Hauswirth A, Köhler R, ten Bosch L, Avramidis G, Gerhard C. Spectroscopic Investigation of the Impact of Cold Plasma Treatment at Atmospheric Pressure on Sucrose and Glucose. Foods. 2022; 11(18):2786. https://doi.org/10.3390/foods11182786
Chicago/Turabian StyleHauswirth, Anna, Robert Köhler, Lars ten Bosch, Georg Avramidis, and Christoph Gerhard. 2022. "Spectroscopic Investigation of the Impact of Cold Plasma Treatment at Atmospheric Pressure on Sucrose and Glucose" Foods 11, no. 18: 2786. https://doi.org/10.3390/foods11182786





