Comparison of Different Varieties on Quality Characteristics and Microbial Activity of Fresh-Cut Pineapple during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Minimal Processing
2.3. Measurement of the Weight Loss, L* Value and Firmness
2.4. Determination of the TSS, TA, and AA Acid Content
2.5. MDA Content
2.6. PPO and POD Activity
2.7. Microbiological Analysis
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss, L* Value, b Value, and Firmness
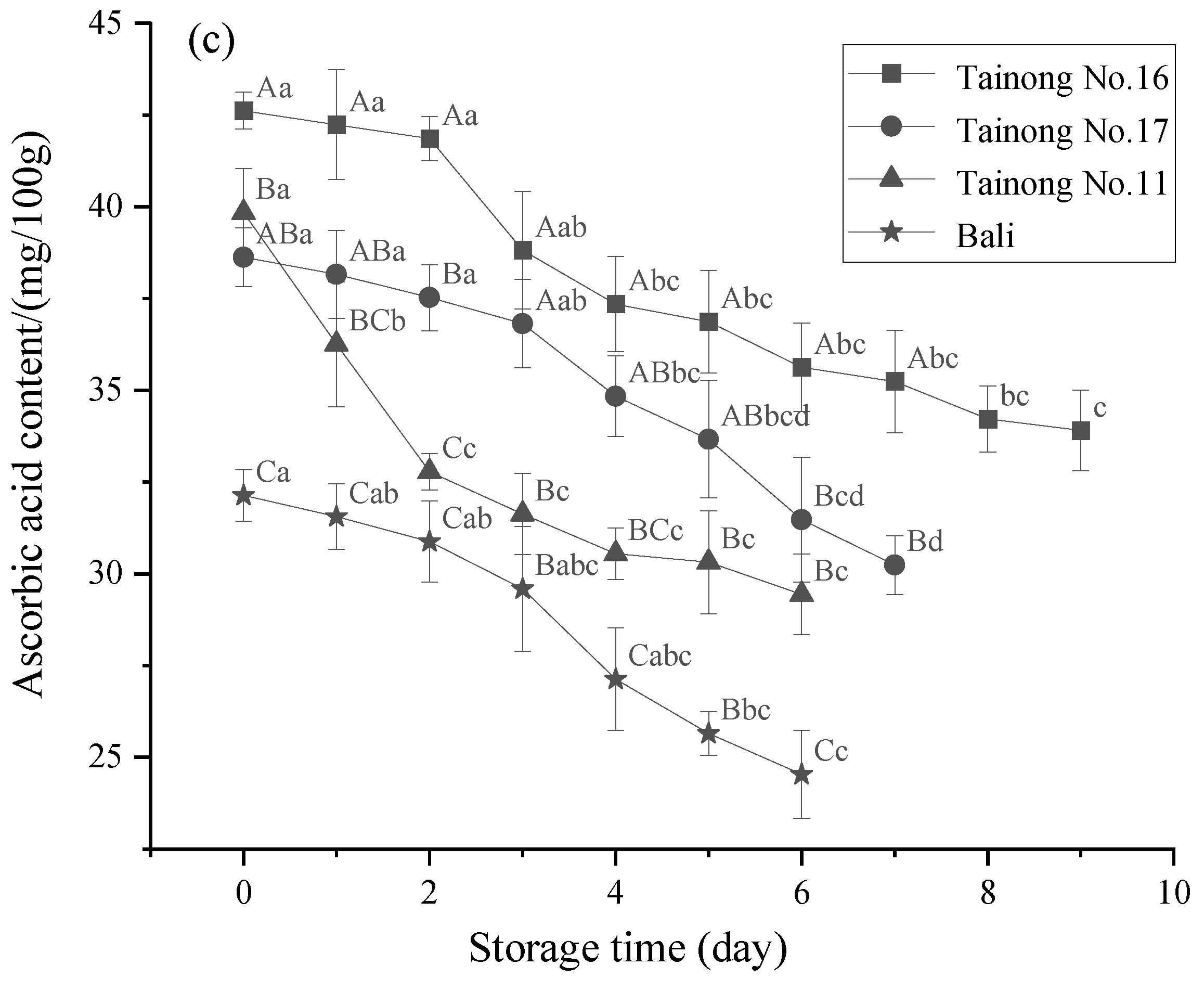
3.2. TSS, TA, and AA Content
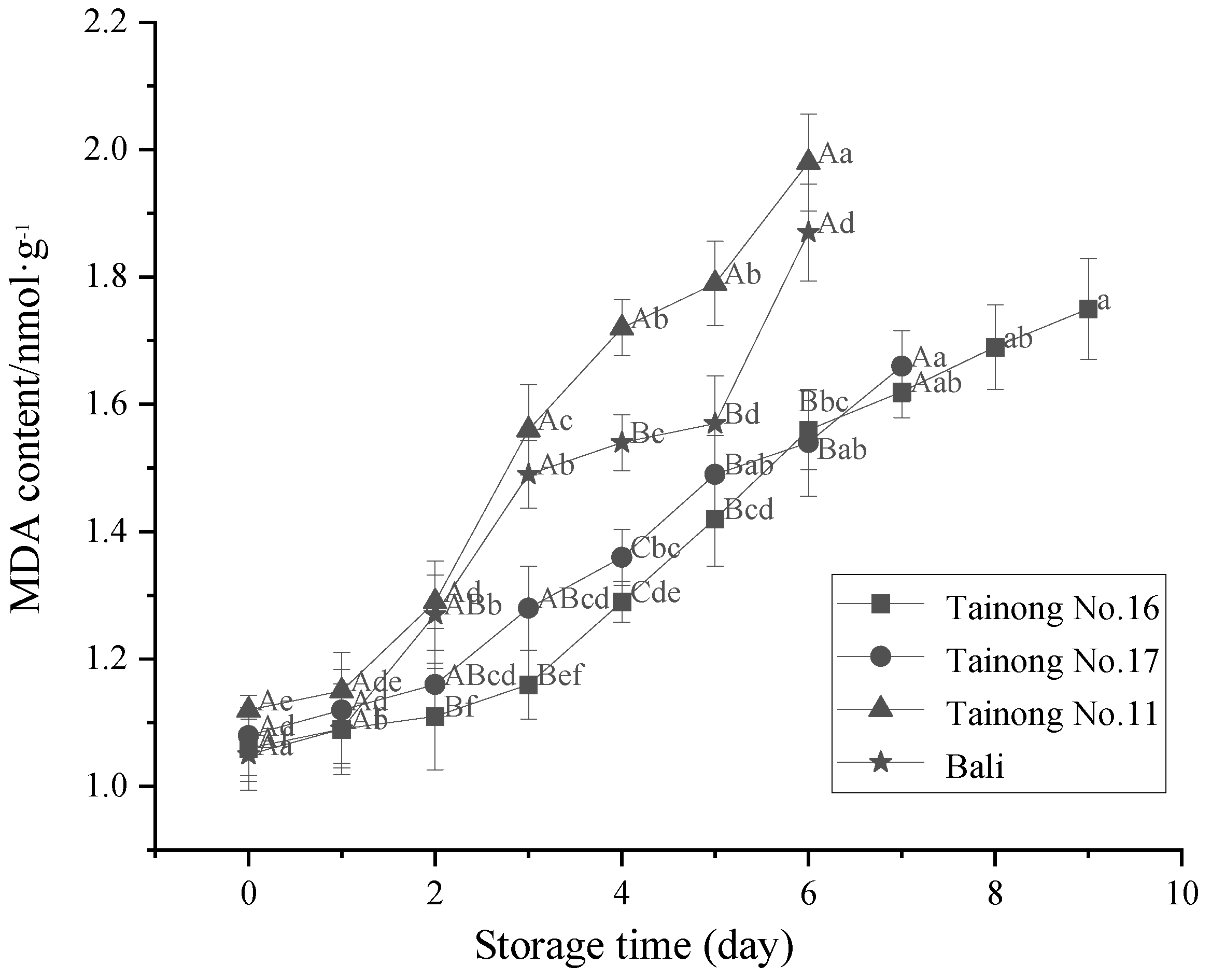
3.3. MDA Content
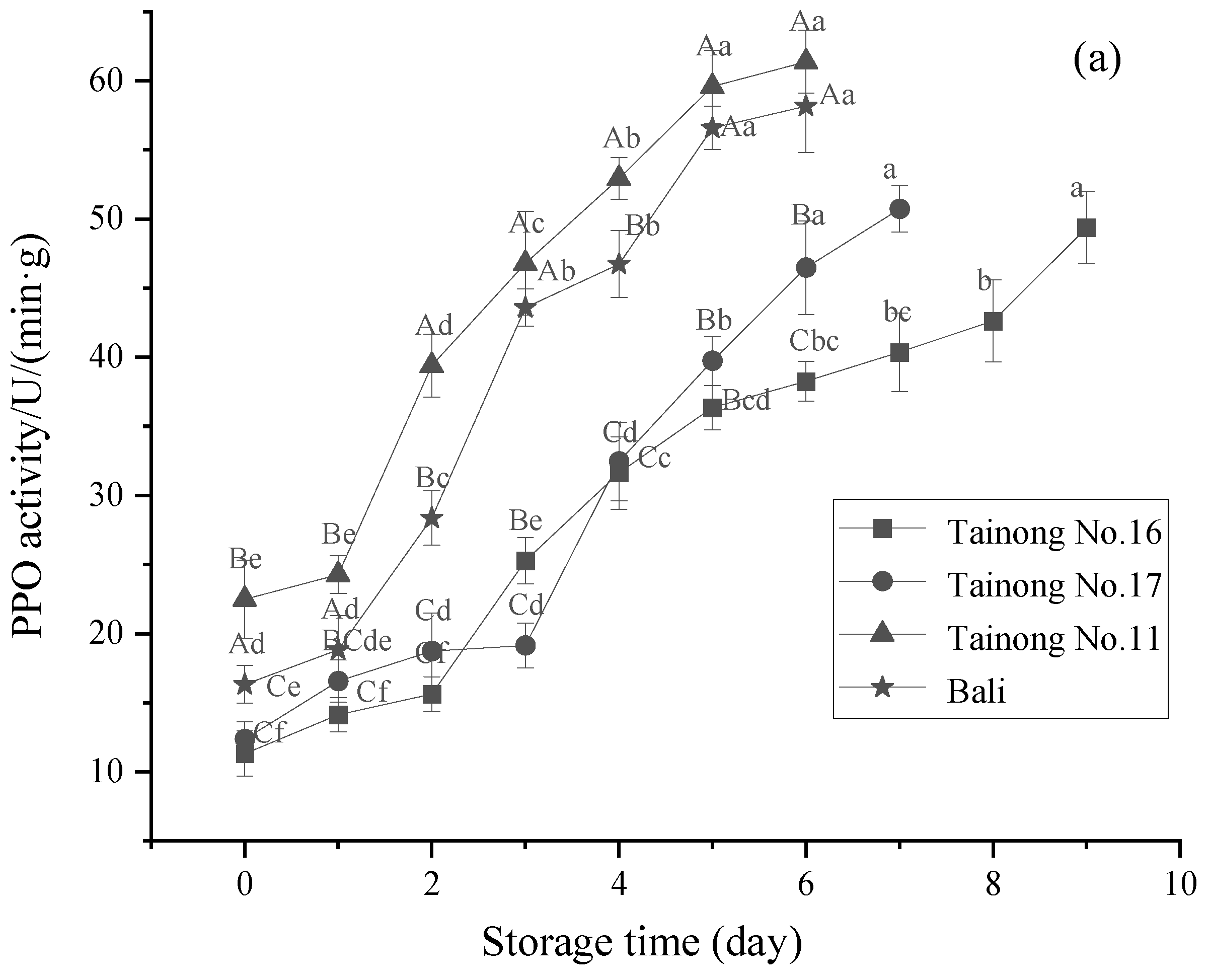
3.4. PPO and POD Activity
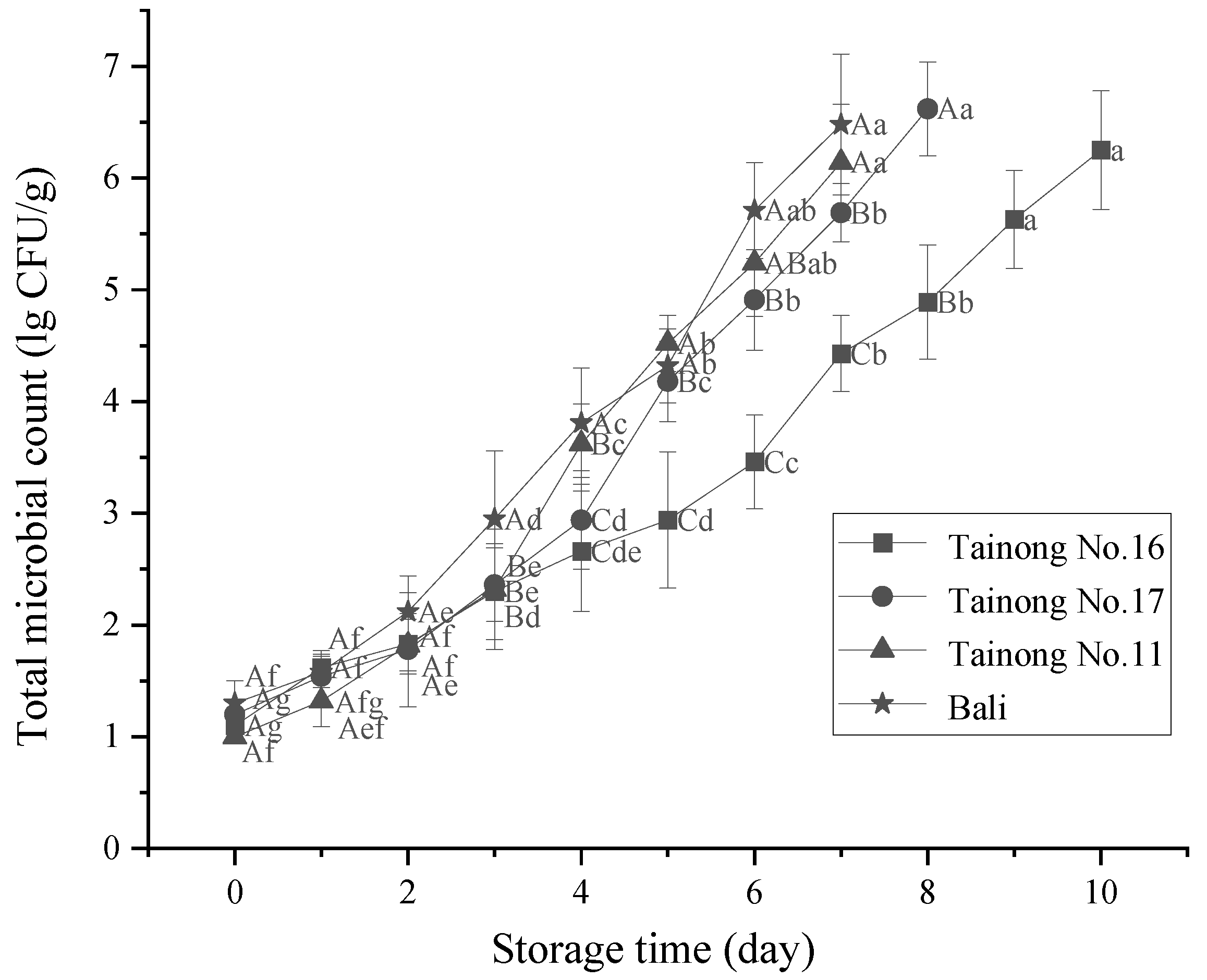
3.5. Microbiological Analysis
3.6. Evaluation of Sensory
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Pan, X.; Liu, S.; Lin, W.; Li, Y.; Zhang, X. Genome-wide analysis of AP2/ERF transcription factors in pineapple reveals functional divergence during flowering induction mediated by ethylene and floral organ development. Genomics 2021, 113, 474–489. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kumar, V.; Chandel, S.; Vaishali; Kumar, S. Pineapple (Ananas cosmosus) product processing: A review. J. Pharmacogn. Phytochem. 2019, 8, 4642–4652. [Google Scholar]
- Ikram, M.; Mizuno, R.; Putri, S.P.; Fukusaki, E. Comparative metabolomics and sensory evaluation of pineapple (Ananas comosus) reveal the importance of ripening stage compared to cultivar. J. Biosci. Bioeng. 2021, 132, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Nga, H.N.; Thao, P.; Quoc, B.; Duyen, K.; Ngoc, D.; Quyen, C.; Son, T.; Phung, K.L. Heat and sound insulation applications of pineapple aerogels from pineapple waste—ScienceDirect. Mater. Chem. Phys. 2020, 242, 122267. [Google Scholar]
- Lim, Z.E.; Thai, Q.B.; Le, D.K.; Luu, T.P.; Nguyen, P.T.T.; Do, N.H.N.; Le, P.K.; Phan-Thien, N.; Goh, X.Y.; Duong, H.M. Functionalized pineapple aerogels for ethylene gas adsorption and nickel (II) ion removal applications. J. Environ. Chem. Eng. 2020, 8, 104524. [Google Scholar] [CrossRef]
- Deng, C.; Yuping, L.I.; Liang, W.; Lu, Y.E. Present Situation and Countermeasures of Pineapple Industry in China. J. Agric. Sci. 2018, 46, 1031–1034. [Google Scholar]
- Yousuf, B.; Srivastava, A.K. Impact of honey treatments and soy protein isolate-based coating on fresh-cut pineapple during storage at 4 °C. Food Packag. Shelf Life 2019, 21, 100361. [Google Scholar] [CrossRef]
- Treviño-Garzaa, M.Z.; Garcíab, S.; Herediab, N.; Alanís-Guzmán, M.G.; Arévalo-Niño, K. Layer-by-layer edible coatings based on mucilages, pullulan and chitosan and its effect on quality and preservation of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Technol. 2017, 128, 63–75. [Google Scholar] [CrossRef]
- Azarakhsh, N.; Osman, A.; Ghazali, H.M.; Tan, C.P.; Adzahan, N.M. Effects of Gellan-Based Edible Coating on the Quality of Fresh-Cut Pineapple During Cold Storage. Food Bioprocess Technol. 2014, 7, 2144–2151. [Google Scholar] [CrossRef]
- Giannakourou, M.C.; Tsironi, T.N. Application of Processing and Packaging Hurdles for Fresh-Cut Fruits and Vegetables Preservation. Foods 2021, 10, 830. [Google Scholar] [CrossRef]
- De Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef]
- Wu, Z.S.; Min, Z.; Adhikari, B. Application of high pressure argon treatment to maintain quality of fresh-cut pineapples during cold storage. J. Food Eng. 2012, 110, 395–404. [Google Scholar] [CrossRef]
- Finnegan, E.; O’Beirne, D. Characterising and tracking deterioration patterns of fresh-cut fruit using principal component analysis—Part I. Postharvest Biol. Technol. 2015, 100, 73–80. [Google Scholar] [CrossRef]
- Finnegan, E.; O’Beirne, D. Characterising deterioration patterns in fresh-cut fruit using principal component analysis. II: Effects of ripeness stage, seasonality, processing and packaging. Postharvest Biol. Technol. 2015, 100, 91–98. [Google Scholar] [CrossRef]
- Baltazari, A.; Mtui, H.D.; Mwatawala, M.W.; Chove, L.M.; Msogoya, T.; Samwel, J.; Subramanian, J. Effects of Storage Conditions, Storage Duration and Post-Harvest Treatments on Nutritional and Sensory Quality of Orange (Citrus sinensis (L.) Osbeck) Fruits. Int. J. Fruit Sci. 2019, 20, 737–749. [Google Scholar] [CrossRef]
- Shoda, M.; Urasaki, N.; Sakiyama, S.; Terakami, S.; Hosaka, F.; Shigeta, N.; Nishitani, C.; Yamamoto, T. DNA profiling of pineapple cultivars in Japan discriminated by SSR markers. Breed. Sci. 2012, 62, 352–359. [Google Scholar] [CrossRef]
- Liu, C.H.; He, H.; He, X.G.; Shao, X.H.; Lai, D.; Kuang, S.Z.; Xiao, W.Q. Pineapple Cultivar Structure and Extension of New Independent Breeding Pineapple Cultivars in China. China Trop. Agric. 2021, 4, 13–15. [Google Scholar]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O.O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar]
- Lu, X.H.; Sun, D.Q.; Wu, Q.S.; Liu, S.H.; Sun, G.M. Physico-chemical properties, antioxidant activity and mineral contents of pineapple genotypes grown in China. Molecules 2014, 19, 8518–8532. [Google Scholar] [CrossRef]
- Zou, Y.; Yu, Y.; Cheng, L.; Li, L.; Xu, Y. Effects of curcumin-based photodynamic treatment on quality attributes of fresh-cut pineapple. LWT Food Sci. Technol. 2021, 141, 110902. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Technol. 2019, 59, 102256. [Google Scholar] [CrossRef]
- Tao, R.; Zhang, F.; Tang, Q.-J.; Xu, C.-S.; Ni, Z.-J.; Meng, X.-H. Effects of curcumin-based photodynamic treatment on the storage quality of fresh-cut apples. Food Chem. 2019, 274, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, M.; Jiang, F. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochem. 2019, 54, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, C.; Sun, J.; Xin, M.; Yi, P.; He, X.; Sheng, J.; Zhou, Z.; Ling, D.; Zheng, F.; et al. Synergistic effects of ultraviolet light irradiation and high-oxygen modified atmosphere packaging on physiological quality, microbial growth and lignification metabolism of fresh-cut carrots. Postharvest Biol. Technol. 2021, 173, 111365. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Li, D.; Li, L.; Luo, Z. Effect of high carbon dioxide treatment on reactive oxygen species accumulation and antioxidant capacity in fresh-cut pear fruit during storage. Sci. Hortic. 2021, 281, 109925. [Google Scholar] [CrossRef]
- Yousuf, B.; Srivastava, A.K. A novel approach for quality maintenance and shelf life extension of fresh-cut Kajari melon: Effect of treatments with honey and soy protein isolate. LWT Food Sci. Technol. 2017, 79, 568–578. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, M.A. Modied atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, Y.; Che, Z.; Guan, T.; Zhang, L.; Bai, Y. Effect of Chitosan Coating and Oil Fumigation on the Microbiological and Quality Safety of Fresh-Cut Pear. J. Food Saf. 2013, 33, 179–189. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Pereira, L.M.; Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Hubinger, M.D. Cassava Starch Coating and Citric Acid to Preserve Quality Parameters of Fresh-Cut “Tommy Atkins” Mango. J. Food Sci. 2010, 75, E297–E304. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T. Responses of fresh-cut products of four mango cultivars under two different storage conditions. J. Food Sci. Technol. 2017, 54, 1689–1702. [Google Scholar] [CrossRef]
- Oltenacu, N.; Oltenacu, C.V.; Neagu, C.; Lascar, E. Research on the behaviour during the storage of some apple varieties from the grown assortments in the south-east area of Romania. Sci. Pap. Ser. Manag. Eco. Eng. Agric. Rural Dev. 2020, 20, 359–363. [Google Scholar]
- Samira, A.; Woldetsadik, K.; Workneh, T.S. Postharvest quality and shelf life of some hot pepper varieties. J. Food Sci. Technol. 2013, 50, 842–855. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, X.; Zheng, J.; Wang, T.; You, X.; Liu, H.; Liu, X. The effect of ultrasonic on reducing anti-browning minimum effective concentration of purslane extract on fresh-cut potato slices during storage. Food Chem. 2020, 343, 128401. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chanasut, U.; Kumpoun, W. Enzymatic browning and its amelioration in fresh-cut tropical fruits. Fresh-Cut Fruits Veg. 2020, 3, 51–76. [Google Scholar]
- Montero-Calderón, M.; Rojas-Graü, M.A.A.; Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Marti’n-Belloso, O. Influence of Modified Atmosphere Packaging on Volatile Compounds and Physicochemical and Antioxidant Attributes of Fresh-Cut Pineapple (Ananas comosus). J. Agric. Food Chem. 2010, 58, 5042–5049. [Google Scholar] [CrossRef]
- Marrero, A.; Kader, A.A. Optimal temperature and modified atmosphere for keeping quality of fresh-cut pineapples. Postharvest Biol. Technol. 2006, 39, 163–168. [Google Scholar] [CrossRef]
- Yang, X.Y.; Cai, Y.B.; Sun, G.M. Relationship between color formation and change in composition of carotenoids in flesh of pineapple fruit. J. Fruit Sci. 2010, 27, 135–139. [Google Scholar]
- Bi, X.; Dai, Y.; Zhou, Z.; Xing, Y.; Che, Z. Combining natamycin and 1-methylcyclopropene with modified atmosphere packaging to evaluate plum (Prunus salicina cv. ‘Cuihongli’) quality. Postharvest Biol. Technol. 2022, 183, 111749. [Google Scholar] [CrossRef]
- Toivonen, P.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Saftner, R.A.; Abbott, J.A.; Bhagwat, A.A.; Vinyard, B.T. Quality Measurement of Intact and Fresh-cut Slices of Fuji, Granny Smith, Pink Lady, and GoldRush Apples. J. Food Sci. 2005, 70, S317–S324. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, J.; Li, S.; Hamzah, S.S.; Lin, S. Curcumin-Based Photodynamic Sterilization for Preservation of Fresh-Cut Hami Melon. Molecules 2019, 24, 2374. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, V.; Giacalone, G. Anthocyanins, phenolics and antioxidant capacity after fresh storage of blueberry treated with edible coatings. Int. J. Food Sci. Nutr. 2015, 66, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Rana, M.M.; Kimura, Y.; Roslan, H.A. Changes in biochemical characteristics and activities of ripening associated enzymes in mango fruit during the storage at different temperatures. BioMed Res. Int. 2014, 2014, 232969. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yang, S.; Xu, Q.; Xu, L.; Zhu, D.; Li, X.; Shui, Y.; Liu, X.; Bi, X. Effect of Chitosan/Nano-TiO2 Composite Coating on the Postharvest Quality of Blueberry Fruit. Coatings 2021, 11, 512. [Google Scholar] [CrossRef]
- Padrón-Mederos, M.; Rodríguez-Galdón, B.; Díaz-Romero, C.; Lobo-Rodrigo, M.G.; Rodríguez-Rodríguez, E. Quality evaluation of minimally fresh-cut processed pineapples. LWT Food Sci. Technol. 2020, 129, 109607. [Google Scholar] [CrossRef]
- Fagundes, C.; Carciofi, B.A.M.; Monteiro, A.R. Estimate of respiration rate and physicochemical changes of fresh-cut apples stored under different temperatures. Food Sci. Technol. 2013, 33, 60–67. [Google Scholar] [CrossRef]
- Fuentes-Pérez, M.C.; Nogales-Delgado, S.; Ayuso, M.C.; Bohoyo-Gil, D. Study of different peach cultivars and their suitability for minimal processing. Czech J. Food Sci. 2014, 32, 413–421. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Fuentes-Perez, M.D.C.; Ayuso-Yuste, C.; Bohoyo-Gil, D. Study of different nectarine cultivars and their suitability for fresh-cut processing. Int. J. Food Sci. Technol. 2013, 49, 114–120. [Google Scholar] [CrossRef]
- George, D.S.; Razali, Z.; Somasundram, C. Physiochemical Changes during Growth and Developmen t of Pineapple (Ananas comosus L. Merr. cv. Sarawak). J. Agric. Sci. Technol. 2016, 18, 491–503. [Google Scholar]
- Gonzalez-Aguilar, G.A.; Celis, J.; Sotelo-Mundo, R.R.; Rosa, L.A.; Alvarez-Parrilla, E. Physiological and biochemical changes of different fresh-cut mango cultivars stored at 5 °C. Int. J. Food Sci. Technol. 2008, 43, 91–101. [Google Scholar] [CrossRef]
- Gil, M.I.; Aguayo, E.; Kader, A.A. Quality Changes and Nutrient Retention in Fresh-Cut versus Whole Fruits during Storage. J. Agric. Food Chem. 2006, 54, 4284–4296. [Google Scholar] [CrossRef]
- Inglese, P.; Todaro, A.; Farina, V.; Allegra, A. Changes in Ascorbic Acid Content in Fresh Cut Sicilian Yellow-Flesh Peaches. Acta Hortic. 2015, 1084, 777–780. [Google Scholar]
- Wang, H.; Xiao, X.; Yang, M.; Gao, Z.; Zang, J.; Fu, X.; Chen, Y. Effects of salt stress on antioxidant defense system in the root of Kandelia candel. Bot. Stud. 2014, 55, 57. [Google Scholar] [CrossRef]
- Wu, Z.; Tu, M.; Yang, X.; Xu, J.; Yu, Z. Effect of cutting on the reactive oxygen species accumulation and energy change in postharvest melon fruit during storage. Sci. Hortic. 2019, 257, 108752. [Google Scholar] [CrossRef]
- Carvajal, F.; Martinez, C.; Jamilena, M.; Garrido, D. Differential response of zucchini varieties to low storage temperature. Sci. Hortic. 2011, 130, 90–96. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Gutiérrez-Cortez, E.; Real, A.D.; González-Reza, R.; Galindo-Pérez, M.; Quintanar-Guerrero, D. Fresh-cut Red Delicious apples coating using tocopherol/mucilage nanoemulsion: Effect of coating on polyphenol oxidase and pectin methylesterase activities. Food Res. Int. 2014, 62, 974–983. [Google Scholar] [CrossRef]
- Mishra, B.; Gautam, S.; Sharma, A. Free phenolics and polyphenol oxidase (PPO): The factors affecting post-cut browning in eggplant (Solanum melongena). Food Chem. 2013, 139, 105–114. [Google Scholar] [CrossRef]
- Mishra, B.B.; Gautam, S.; Sharma, A. Browning of fresh-cut eggplant: Impact of cutting and storage. Postharvest Biol. Technol. 2012, 67, 44–51. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.). Postharvest Biol. Technol. 2011, 61, 117–123. [Google Scholar] [CrossRef]
- Denoya, G.I.; Vaudagna, S.R.; Chamorro, V.C.; Godoy, M.F.; Budde, C.O.; Polenta, G.A. Suitability of different varieties of peaches for producing minimally processed peaches preserved by high hydrostatic pressure and selection of process parameters. LWT Food Sci. Technol. 2017, 78, 367–372. [Google Scholar] [CrossRef]
- Wang, X.; Kong, D.; Ma, Z.; Zhao, R. Effect of carrot puree edible films on quality preservation of fresh-cut carrots. Ir. J. Agric. Food Res. 2015, 54, 64–71. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Zhou, J.; Kong, X.; Han, P. Quality Changes of Different Varieties of Fresh-Type Potatoes during Storage. IOP Conf. Ser. Mater. Sci. Eng. 2019, 562, 012123. [Google Scholar] [CrossRef]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K.; Yildiz, F. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Prakash, A.; Baskaran, R.; Vellingeri, V. Citral nanoemulsion incorporated edible coating to extend the shelf life of fresh cut pineapples. LWT 2020, 118, 108851. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Samapundo, S.; Pothakos, V.; Baenst, I.D.; Noseda, B.; Devlieghere, F. Effect of atmospheres combining high oxygen and carbon dioxide levels on microbial spoilage and sensory quality of fresh-cut pineapple. Postharvest Biol. Technol. 2013, 86, 73–84. [Google Scholar] [CrossRef]
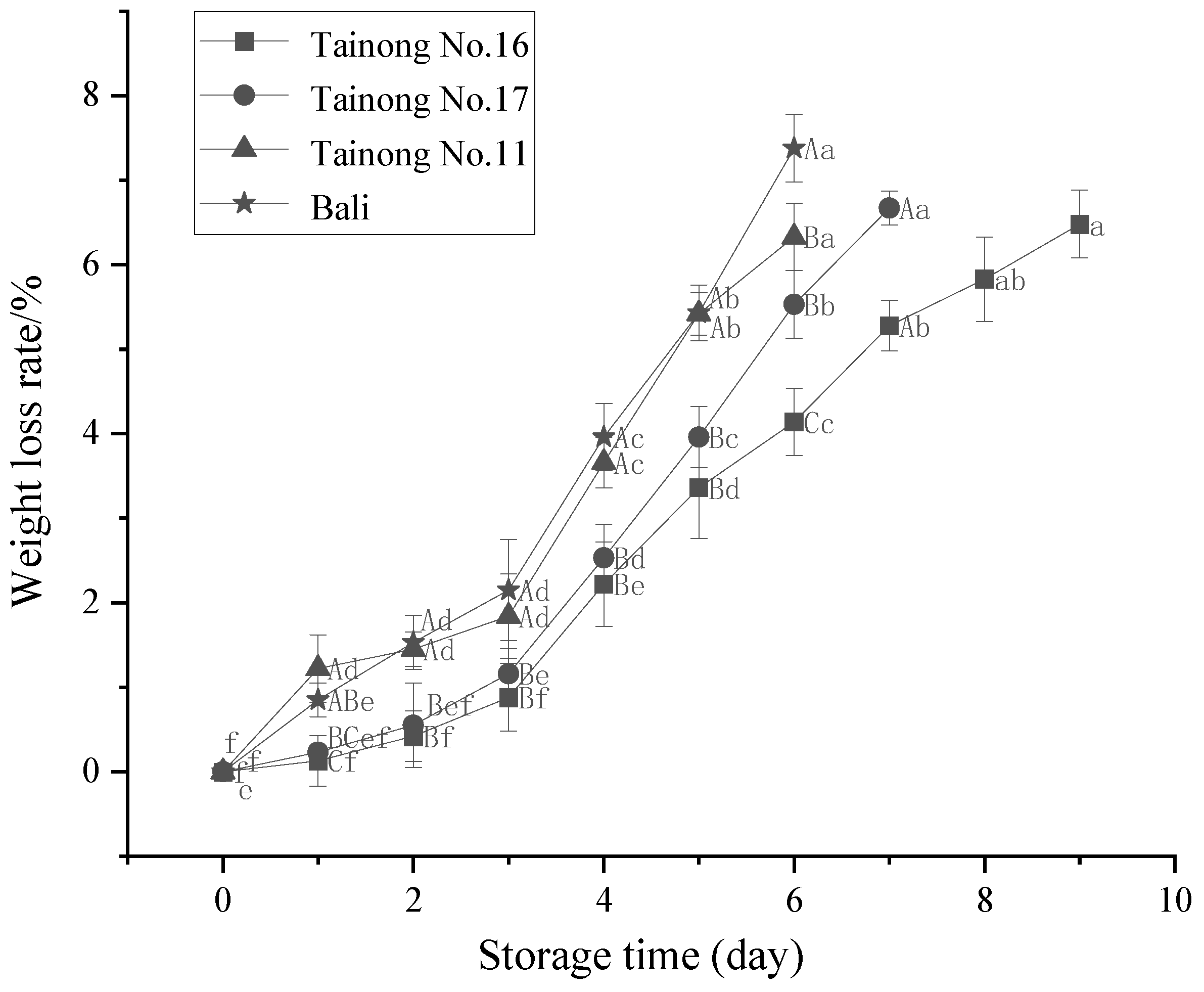

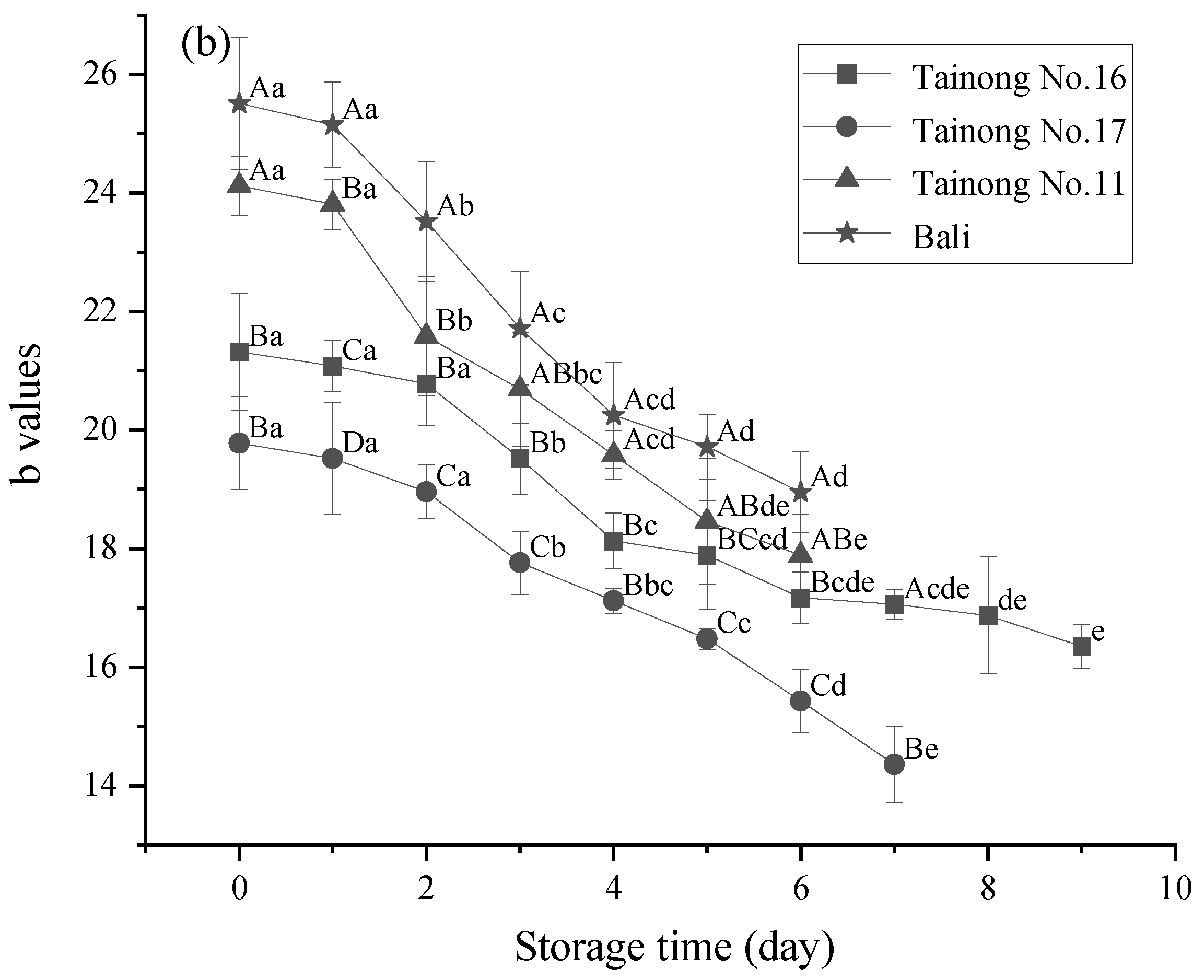
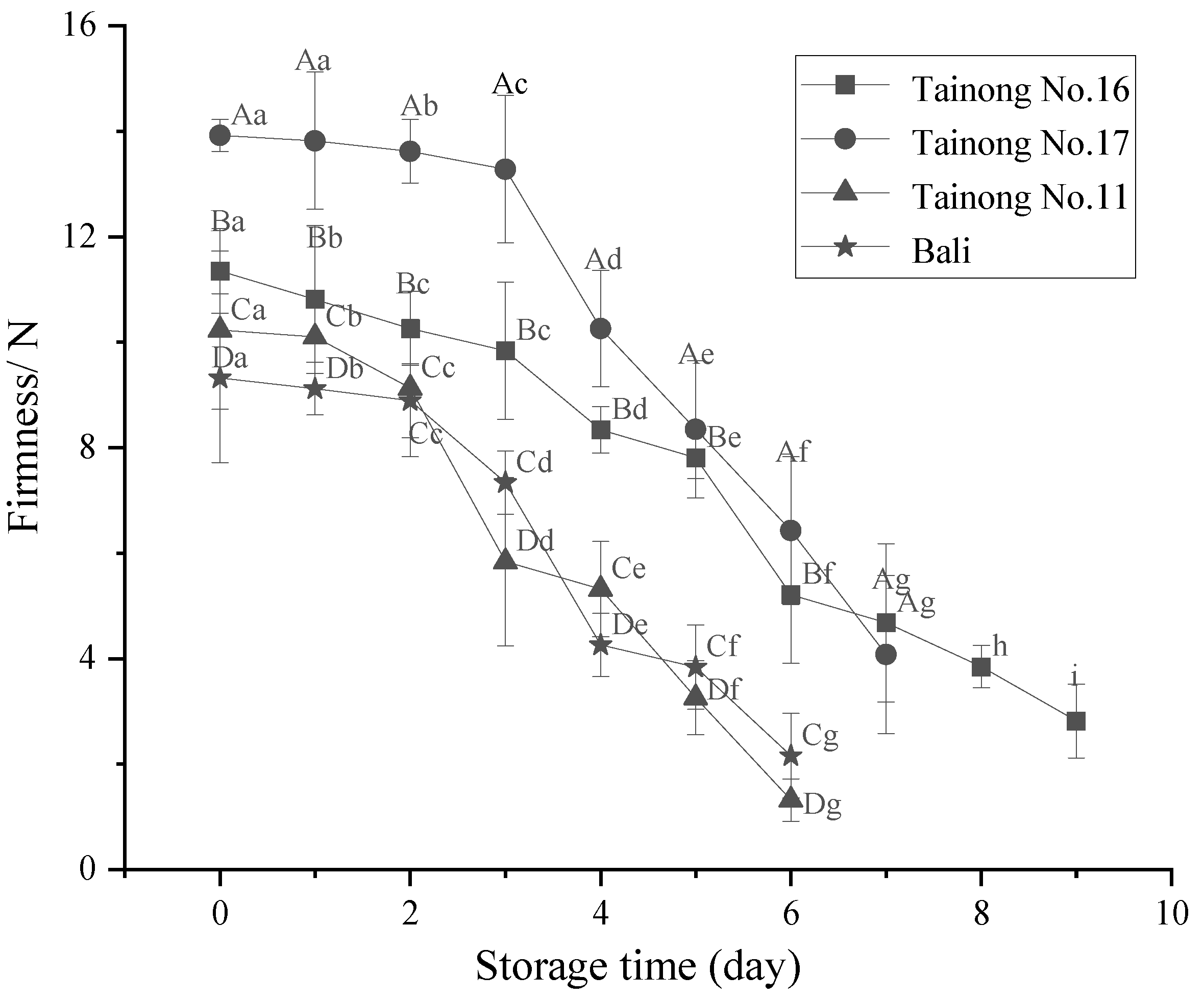
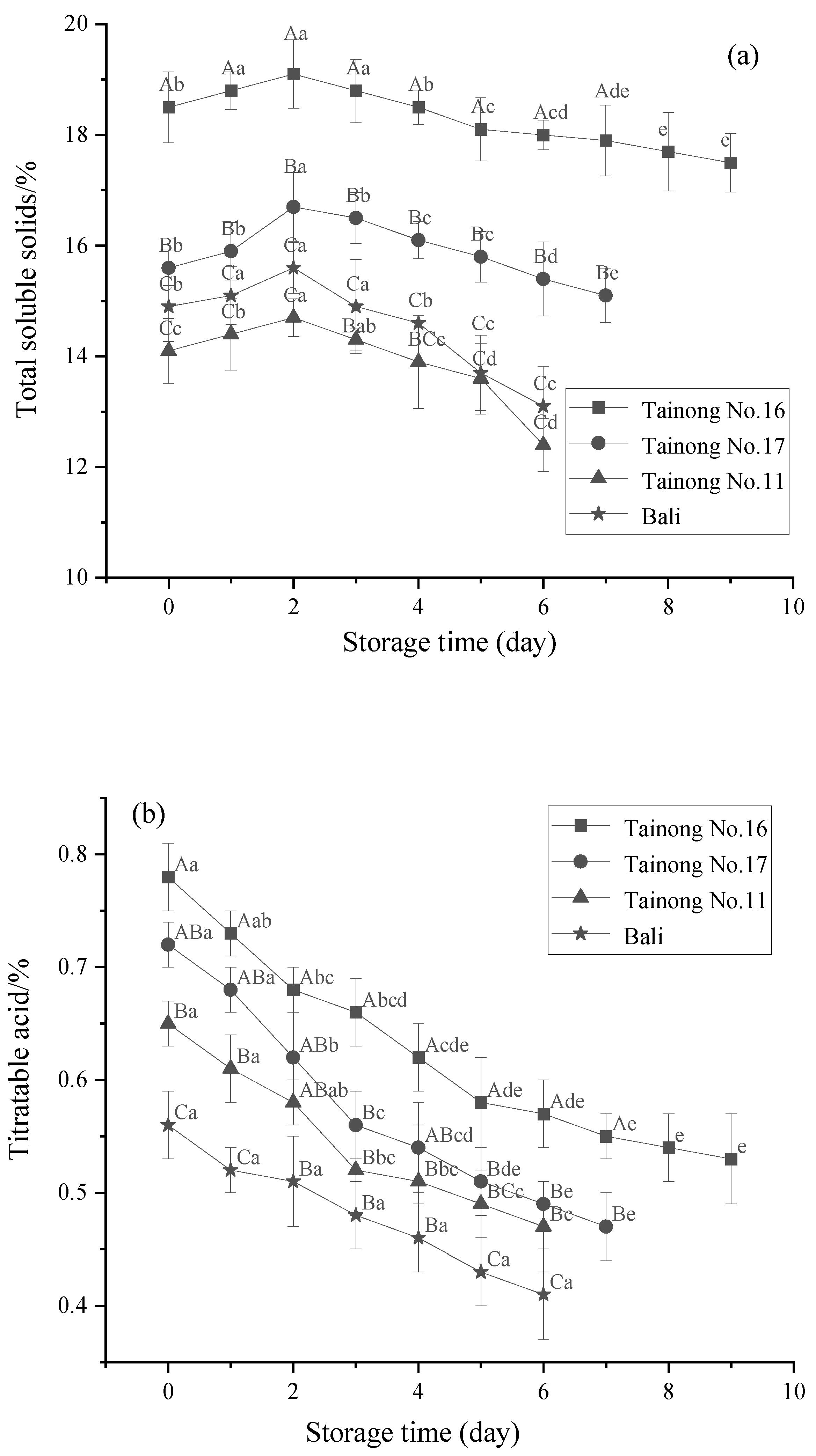

| Quality Properties | Grade I | Grade II | Grade III |
|---|---|---|---|
| Taste (30 points) | Crunchy mouthfeel, moderately sweet and sour (20–30 points) | Taste brittle, sweet and sour discordant (10–19 points) | Soft, no obvious sweet and sour pineapple taste (0–9 points) |
| Smell (30 points) | Pineapple-specific aroma is strong and fresh smelling (20–30 points) | Lighter pineapple clear flavor, insufficient aroma (10–19 points) | Pineapple aroma is very light, with a peculiar smell (0–9 points) |
| Color (20 points) | Bright yellow, lustrous (14–20 points) | Light yellow, slightly shiny (7–13 points) | Yellowish brown or dark brown, no luster (0–6 points) |
| Texture (20 points) | Plenty of moisture and hard tissue (14–20 points) | Surface slightly dry and soft tissue (7–13 points) | Slightly putrid and soft (0–6 points) |
| Variables | Cultivar | Storage Time | Cultivar·Storage Time | |
|---|---|---|---|---|
| Weight loss | p-value | 0.012 | 0.000 | 0.008 |
| Partial eta square | 0.721 | 0.977 | 0.517 | |
| L* value | p-value | 0.011 | 0.002 | 0.001 |
| Partial eta square | 0.860 | 0.767 | 0.668 | |
| b value | p-value | 0.024 | 0.006 | 0.003 |
| Partial eta square | 0.881 | 0.792 | 0.691 | |
| firmness | p-value | 0.000 | 0.001 | 0.000 |
| Partial eta square | 0.935 | 0.858 | 0.957 | |
| TSS | p-value | 0.031 | 0.001 | 0.009 |
| Partial eta square | 0.923 | 0.912 | 0.400 | |
| TA | p-value | 0.000 | 0.024 | 0.969 |
| Partial eta square | 0.755 | 0.806 | 0.120 | |
| AA | p-value | 0.000 | 0.001 | 0.790 |
| Partial eta square | 0.781 | 0.726 | 0.175 | |
| MDA | p-value | 0.006 | 0.000 | 0.003 |
| Partial eta square | 0.622 | 0.912 | 0.429 | |
| PPO | p-value | 0.005 | 0.001 | 0.000 |
| Partial eta square | 0.910 | 0.972 | 0.660 | |
| POD | p-value | 0.035 | 0.000 | 0.000 |
| Partial eta square | 0.890 | 0.981 | 0.671 | |
| Microorganism | p-value | 0.000 | 0.004 | 0.000 |
| Partial eta square | 0.764 | 0.986 | 0.742 | |
| Evaluation of sensory | p-value | 0.007 | 0.000 | 0.023 |
| Partial eta square | 0.841 | 0.867 | 0.634 | |
| Pineapple Cultivars | Parameter | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tainong No.16 | Taste | 29.42 ± 0.55 Aa | 29.11 ± 0.63 Aa | 28.61 ± 1.02 Aa | 28.24 ± 0.74 Aab | 27.21 ± 0.89 Ab | 23.26 ± 1.33 Ac | 21.88 ± 1.67 c | — | — | — |
| Smell | 29.34 ± 0.55 Aa | 29.42 ± 0.49 Aa | 29.24 ± 0.75 Aa | 28.71 ± 0.41 Aab | 27.64 ± 0.48 Ab | 24.68 ± 1.02 Ac | 23.65 ± 0.48 Bd | 21.43 ± 0.48 Ad | 18.61 ± 1.02 d | 16.82 ± 0.75 e | |
| Color | 19.71 ± 0.41 Aa | 19.48 ± 0.49 Aa | 19.23 ± 0.41 Aa | 18.94 ± 0.20 Aa | 16.87 ± 0.40 Ab | 13.82 ± 1.12 Ac | 13.24 ± 0.74 Bc | 13.26 ± 0.63 Ac | 11.66 ± 1.37 d | 11.48 ± 0.63 d | |
| Texture | 19.21 ± 0.75 Aa | 19.22 ± 0.75 Aa | 19.01 ± 0.63 Aab | 18.81 ± 0.41 Aab | 17.92 ± 0.49 Ab | 14.46 ± 1.20 Ac | 13.78 ± 0.80 Bde | 13.24 ± 0.65 Ad | 12.31 ± 1.08 de | 11.85 ± 0.42 e | |
| Score | 97.66 ± 0.76 Aa | 97.23 ± 0.90 Aa | 96.01 ± 1.26 Aab | 94.62 ± 0.49 Ab | 89.35 ± 1.17 Ac | 76.14 ± 1.10 Bd | 71.67 ± 2.45 Be | 47.91 ± 0.63 Ae | 41.59 ± 1.99 f | 38.33 ± 1.20 g | |
| Tainong No.17 | Taste | 28.21 ± 0.74 Ba | 28.15 ± 0.63 Aa | 27.44 ± 0.49 Bab | 26.62 ± 0.82 Bbc | 25.89 ± 0.98 Ac | 20.26 ± 1.33 Bd | — | — | — | — |
| Smell | 28.62 ± 0.48 Aa | 28.63 ± 1.03 Aa | 27.56 ± 0.45 Ba | 27.45 ± 0.48 Ba | 25.81 ± 0.98 Bb | 24.83 ± 0.75 Abc | 19.26 ± 0.75 Ac | 16.47 ± 1.50 Ad | — | — | |
| Color | 19.23 ± 0.42 ABab | 19.65 ± 0.36 Aa | 18.67 ± 0.48 ABab | 18.35 ± 1.10 Ab | 16.36 ± 1.41 Ac | 15.73 ± 1.09 Bcde | 14.42 ± 1.02 Ade | 13.62 ± 1.02 Bf | — | — | |
| Texture | 19.64 ± 0.48 Aa | 19.21 ± 0.47 Aa | 18.89 ± 0.74 Aa | 17.26 ± 0.75 Bb | 15.82 ± 0.75 Bc | 16.37 ± 1.25 Abc | 15.86 ± 0.75 Ac | 13.43 ± 1.34 Bd | — | — | |
| Score | 95.66 ± 1.01 ABa | 95.43 ± 1.49 Aa | 92.35 ± 1.07 Bb | 89.28 ± 1.72 Bc | 83.45 ± 2.80 Bd | 75.67 ± 2.61 Ae | 49.59 ± 0.81 Af | 43.77 ± 2.25 Bg | — | — | |
| Tainong No.11 | Taste | 27.53 ± 0.63 BCa | 26.66 ± 1.02 Ba | 26.24 ± 0.74 Ca | 23.25 ± 1.33 Cb | 19.82 ± 0.67 Bc | — | — | — | — | — |
| Smell | 29.32 ± 1.09 Aa | 28.62 ± 1.34 Aa | 28.42 ± 0.83 ABa | 26.53 ± 0.89 Cb | 23.86 ± 0.74 Cc | 19.20 ± 1.38 Bd | 16.63 ± 0.86 Cd | — | — | — | |
| Color | 18.84 ± 0.74 Ba | 17.44 ± 0.86 Bb | 17.45 ± 1.03 BCb | 16.22 ± 1.17 Bb | 13.68 ± 1.65 Bc | 12.52 ± 0.63 Cd | 11.28 ± 0.74 Cd | — | — | — | |
| Texture | 18.66 ± 0.48 Aa | 17.96 ± 0.9 c2 Bab | 18.48 ± 0.82 ABa | 16.65 ± 1.02 Bb | 12.42 ± 1.62 Bc | 11.18 ± 0.66 Bcd | 10.43 ± 1.06 Cd | — | — | — | |
| Score | 93.49 ± 1.62 Ba | 90.59 ± 1.73 Ba | 90.40 ± 1.36 Ba | 82.12 ± 2.28 Cb | 69.64 ± 2.42 Cc | 42.52 ± 2.49 Cd | 37.68 ± 2.24 Cd | — | — | — | |
| Bali | Taste | 26.83 ± 1.17 Ca | 26.51 ± 0.63 Ba | 26.52 ± 0.63 Ca | 23.61 ± 1.28 Cb | 18.62 ± 1.85 Bc | — | — | — | — | — |
| Smell | 26.86 ± 1.33 Ba | 26.44 ± 1.01 Ba | 26.62 ± 0.89 Ca | 25.45 ± 1.23 Ca | 22.54 ± 0.89 Db | 18.81 ± 0.75 Bc | 15.56 ± 0.89 Cc | — | — | — | |
| Color | 18.58 ± 1.45 Ba | 18.29 ± 0.75 Ba | 17.88 ± 0.76 Ca | 15.97 ± 0.66 Bb | 14.47 ± 1.02 Bc | 12.63 ± 1.06 BCd | 11.22 ± 0.75 Ce | — | — | — | |
| Texture | 18.82 ± 0.75 Aa | 18.64 ± 0.78 Ba | 17.42 ± 1.02 Bab | 16.83 ± 1.09 Bbc | 15.21 ± 0.25 Cc | 12.35 ± 1.66 Bd | 12.11 ± 0.66 Bd | — | — | — | |
| Score | 90.94 ± 2.42 Ca | 89.28 ± 0.74 Bab | 87.29 ± 2.71 Bb | 80.99 ± 2.76 Cc | 70.71 ± 3.68 Cd | 42.83 ± 1.72 Ce | 38.39 ± 1.40 Ce | — | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Liao, X.; Wu, H.; Qiu, J.; Wan, R.; Wang, X.; Yi, R.; Xu, Q.; Liu, X. Comparison of Different Varieties on Quality Characteristics and Microbial Activity of Fresh-Cut Pineapple during Storage. Foods 2022, 11, 2788. https://doi.org/10.3390/foods11182788
Xing Y, Liao X, Wu H, Qiu J, Wan R, Wang X, Yi R, Xu Q, Liu X. Comparison of Different Varieties on Quality Characteristics and Microbial Activity of Fresh-Cut Pineapple during Storage. Foods. 2022; 11(18):2788. https://doi.org/10.3390/foods11182788
Chicago/Turabian StyleXing, Yage, Xingmei Liao, Haijun Wu, Jiamin Qiu, Rufeng Wan, Xiaomin Wang, Rumeng Yi, Qinglian Xu, and Xiaocui Liu. 2022. "Comparison of Different Varieties on Quality Characteristics and Microbial Activity of Fresh-Cut Pineapple during Storage" Foods 11, no. 18: 2788. https://doi.org/10.3390/foods11182788
APA StyleXing, Y., Liao, X., Wu, H., Qiu, J., Wan, R., Wang, X., Yi, R., Xu, Q., & Liu, X. (2022). Comparison of Different Varieties on Quality Characteristics and Microbial Activity of Fresh-Cut Pineapple during Storage. Foods, 11(18), 2788. https://doi.org/10.3390/foods11182788





