Antimicrobial and Antioxidant Effects of Kappa-Carrageenan Coatings Enriched with Cinnamon Essential Oil in Pork Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of KC-Based Coatings
2.3. Coating Treatment of Pork Meat
2.4. Evaluation of Microbial Property
2.5. Measurement of Antioxidant Activity
2.6. Measurement of pH
2.7. Determination of Color
2.8. Statistical Analysis
3. Results and Discussion
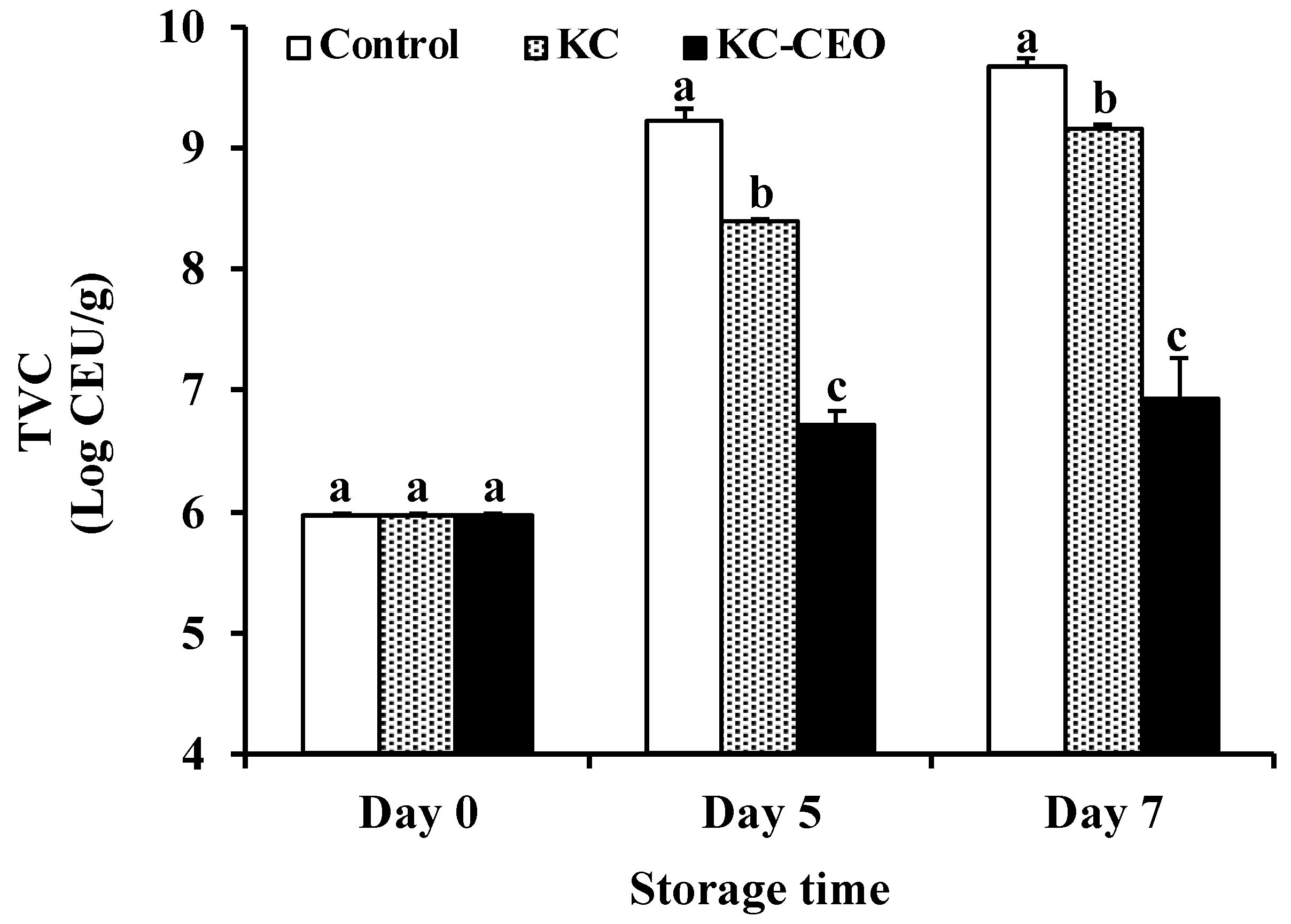
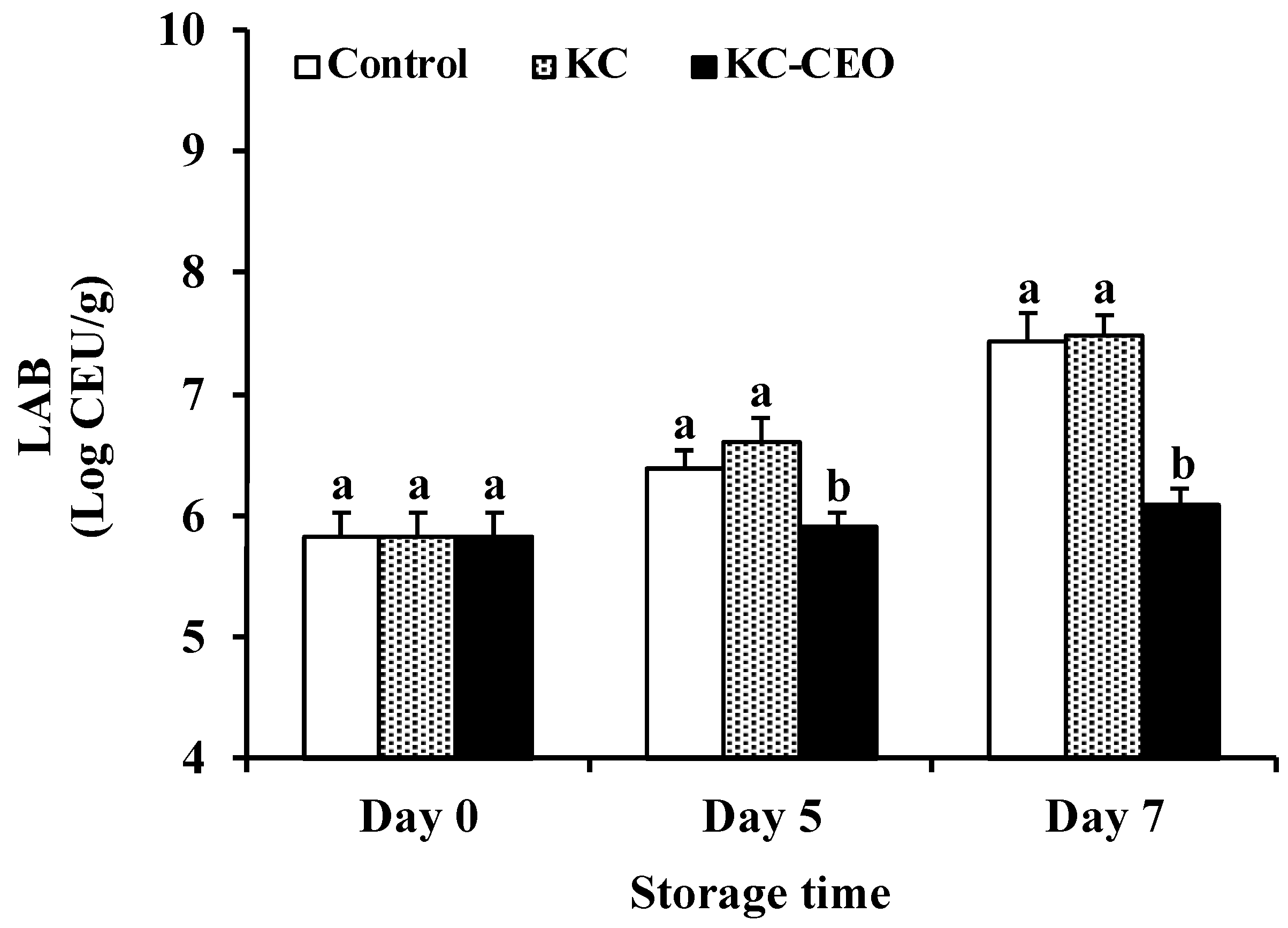
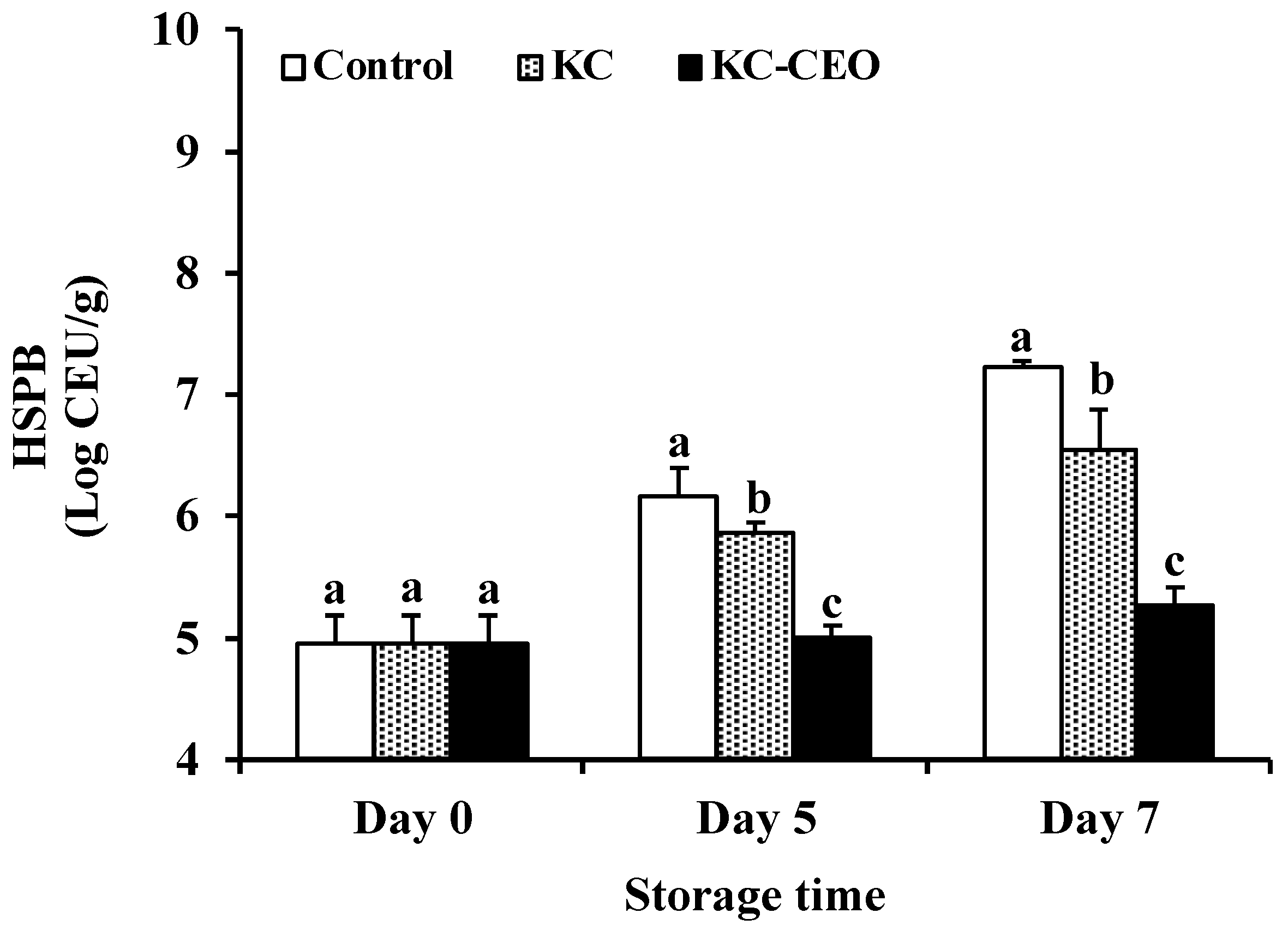
3.1. Impact of KC-Based Coatings on Microbial Properties of Pork Meat
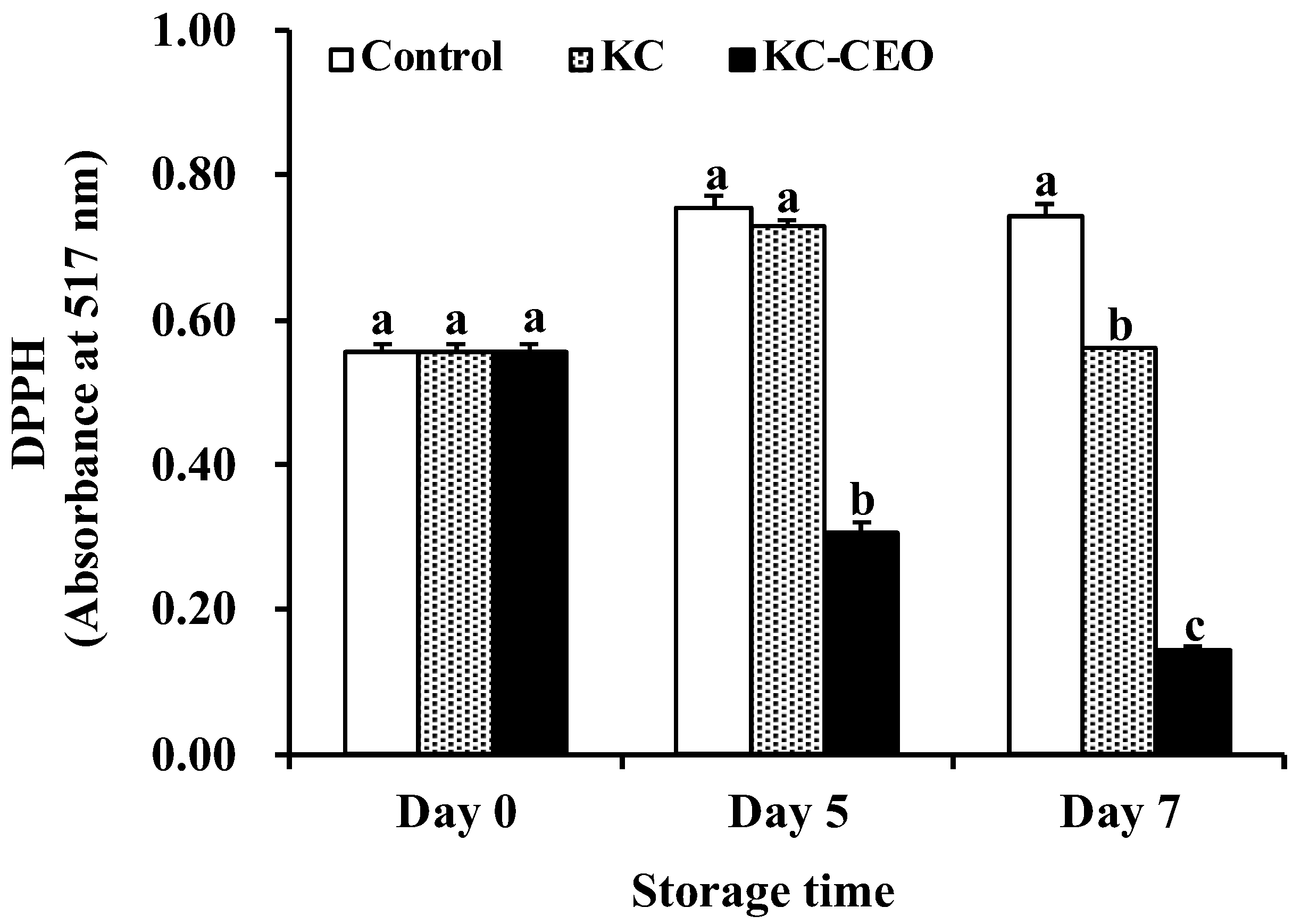
3.2. Impact of KC-Based Coatings on DPPH Contents of Pork Meat
3.3. Impact of KC-Based Coatings on pH Values of Pork Meat
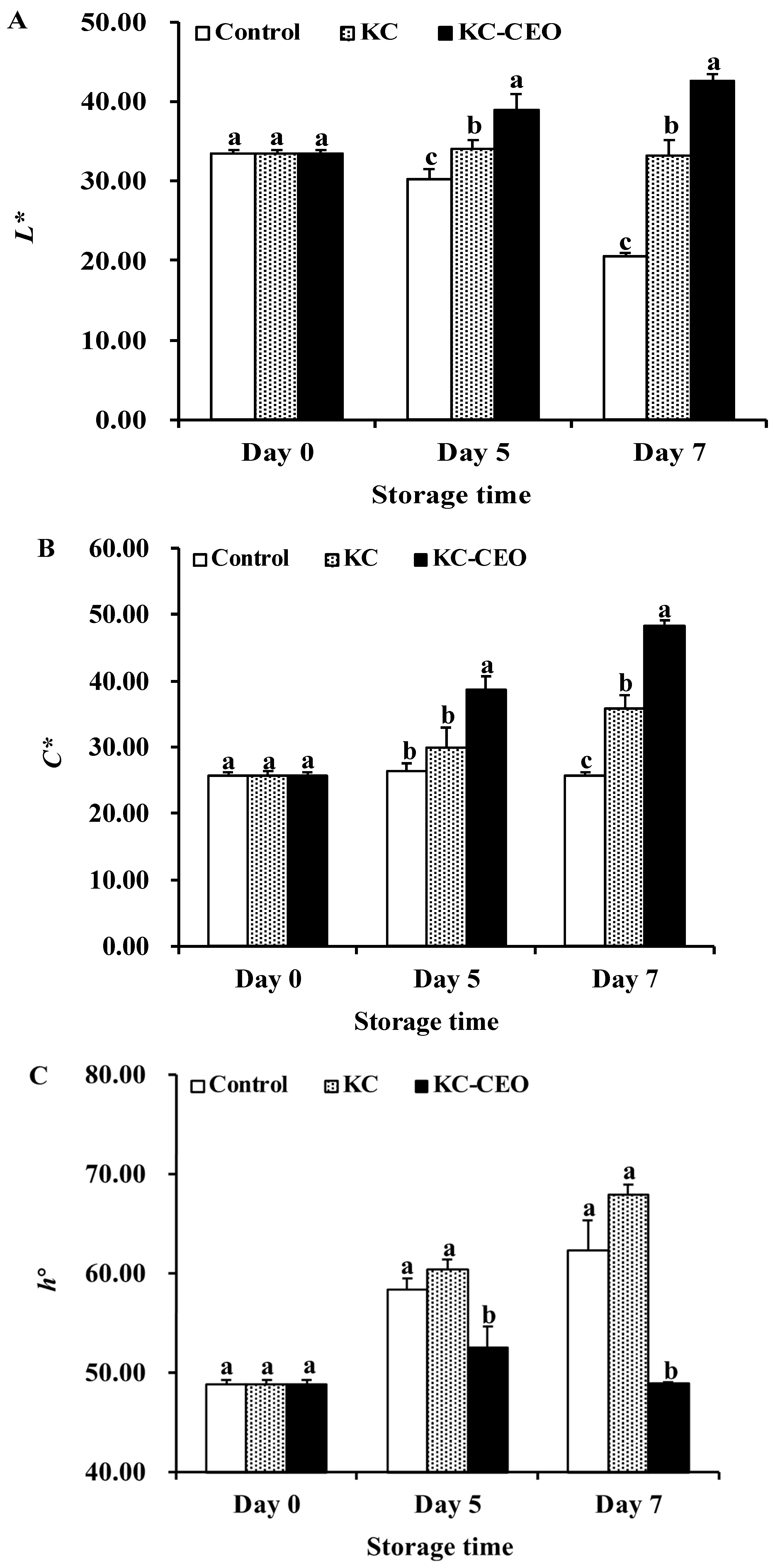
3.4. Impact of KC-Based Coatings on Color Attributes of Pork Meat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Zhang, L.; Ding, W. Eugenol nanocapsules embedded with gelatin-chitosan for chilled pork preservation. Int. J. Biol. Macromol. 2020, 158, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.M.; Sun, S.N.; Shi, L.Y.; Cheng, L.; Fan, Z.C. Application of antimicrobial peptide mytichitin-CB in pork preservation during cold storage. Food Control 2021, 125, 108041. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Ren, B.; Wu, W.; Soladoye, O.P.; Bak, K.H.; Fu, Y.; Zhang, Y. Application of biopreservatives in meat preservation: A review. Int. J. Food Sci. Technol. 2021, 56, 6124–6141. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-derived natural antioxidants in meat and meat products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Plasma enhanced-nutmeg essential oil solid liposome treatment on the gelling and storage properties of pork meat batters. J. Food Eng. 2020, 266, 109696. [Google Scholar] [CrossRef]
- Delgado-Pando, G.; Ekonomou, S.I.; Stratakos, A.C.; Pintado, T. Clean label alternatives in meat products. Foods 2021, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, H.; Abdel-Samie, M.A.; Siva, S.; Lin, L. Controlled-release casein/cinnamon essential oil nanospheres for the inactivation of Campylobacter jejuni in duck. Int. J. Food Microbiol. 2021, 341, 109074. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Al-Maqtari, Q.A.; Mohammed, J.K.; Al-Ansi, W.; Cui, H.; Lin, L. Enhancement of antioxidant activity, antifungal activity, and oxidation stability of Citrus reticulata essential oil nanocapsules by clove and cinnamon essential oils. Food Biosci. 2021, 43, 101226. [Google Scholar] [CrossRef]
- El-Hack, A.; Mohamed, E.; Alagawany, M.; Abdel-Moneim, A.M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- Ji, J.; Shankar, S.; Royon, F.; Salmieri, S.; Lacroix, M. Essential oils as natural antimicrobials applied in meat and meat products—A review. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaïssari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of essential oils as natural food antimicrobial agents: An overview. Appl. Sci. 2021, 11, 5778. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y.; Zhang, P.; Ye, L.; Wu, L.; He, S. Physical characterization and pork packaging application of chitosan films incorporated with combined essential oils of cinnamon and ginger. Food Bioprocess Technol. 2017, 10, 503–511. [Google Scholar] [CrossRef]
- Xing, Y.; Lin, H.; Cao, D.; Xu, Q.; Han, W.; Wang, R.; Che, Z.; Li, X. Effect of chitosan coating with cinnamon oil on the quality and physiological attributes of China jujube fruits. BioMed. Res. Int. 2015, 2015, 835151. [Google Scholar] [CrossRef]
- Andevari, G.T.; Rezaei, M. Effect of gelatin coating incorporated with cinnamon oil on the quality of fresh rainbow trout in cold storage. Int. J. Food Sci. Technol. 2011, 46, 2305–2311. [Google Scholar] [CrossRef]
- Yu, K.; Xu, J.; Zhou, L.; Zou, L.; Liu, W. Effect of chitosan coatings with cinnamon essential oil on postharvest quality of mangoes. Foods 2021, 10, 3003. [Google Scholar] [CrossRef]
- Basaglia, R.R.; Pizato, S.; Santiago, N.G.; de Almeida, M.M.M.; Pinedo, R.A.; Cortez-Vega, W.R. Effect of edible chitosan and cinnamon essential oil coatings on the shelf life of minimally processed pineapple (Smooth cayenne). Food Biosci. 2021, 41, 100966. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Lu, X.; Mao, Y.; Luo, X.; Liu, G.; Zhu, L.; Zhang, Y. Effect of chitosan coating incorporated with oregano or cinnamon essential oil on the bacterial diversity and shelf life of roast duck in modified atmosphere packaging. Food Res. Int. 2021, 147, 110491. [Google Scholar] [CrossRef]
- Pilmal, M.; Alizadeh Doughikollaee, E.; Yousef Elahi, M. Effect of edible chitosan coating containing cinnamon essential oil on the shelf life of silver carp fish finger during refrigerated storage. J. Fish. 2018, 71, 294–305. [Google Scholar]
- Singla, M.; Pareek, S.; Kumar, N.; Sagar, N.A.; Fawole, O.A. Chitosan-cinnamon oil coating maintains quality and extends shelf life of ready-to-use pomegranate arils under low-temperature storage. J. Food Qual. 2022, 2022, 3404691. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Maroufi, L.Y.; Ghorbani, M.; Tabibiazar, M.; Mohammadi, M.; Pezeshki, A. Advanced properties of gelatin film by incorporating modified kappa-carrageenan and zein nanoparticles for active food packaging. Int. J. Biol. Macromol. 2021, 183, 753–759. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-carrageenan films incorporated plant essential oils with improved antimicrobial activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Ojagh, S.M.; Hosseini, S.M.; Khaksar, R. Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int. J. Biol. Macromol. 2013, 52, 116–124. [Google Scholar] [CrossRef]
- Shojaee-Aliabadi, S.; Mohammadifar, M.A.; Hosseini, H.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Haghshenas, M.; Khaksar, R. Characterization of nanobiocomposite kappa-carrageenan film with Zataria multiflora essential oil and nanoclay. Int. J. Biol. Macromol. 2014, 69, 282–289. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite coating based on carrageenan and ZnO nanoparticles to maintain the storage quality of mango. Food Packag. Shelf Life 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Volpe, M.G.; Siano, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varricchio, E. Active edible coating effectiveness in shelf-life enhancement of trout (Oncorhynchusmykiss) fillets. LWT-Food Sci. Technol. 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Seol, K.H.; Lim, D.G.; Jang, A.; Jo, C.; Lee, M. Antimicrobial effect of κ-carrageenan-based edible film containing ovotransferrin in fresh chicken breast stored at 5 °C. Meat Sci. 2009, 83, 479–483. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Yang, Q.; Ren, X.; Zi, J.; Lu, S.; Wang, S.; Zhang, Y.; Wang, Y. Antimicrobial efficiency of chitosan solutions and coatings incorporated with clove oil and/or ethylenediaminetetraacetate. J. Food Saf. 2014, 34, 345–352. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Chattopadhyay, U.K.; Sherikar, A.T.; Waskar, V.S.; Paturkar, A.M.; Latha, C.; Munde, K.D.; Pathare, N.S. Chemical sprays as a method for improvement in microbiological quality and shelf-life of fresh sheep and goat meats during refrigeration storage (5–7 °C). Meat Sci. 2003, 63, 339–344. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Guo, X. Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality. Food Sci. Hum. Well. 2016, 5, 39–48. [Google Scholar] [CrossRef]
- Zhang, H.; He, P.; Kang, H.; Li, X. Antioxidant and antimicrobial effects of edible coating based on chitosan and bamboo vinegar in ready to cook pork chops. LWT-Food Sci. Technol. 2018, 93, 470–476. [Google Scholar] [CrossRef]
- Lee, J.S.; Chang, Y.; Lee, E.S.; Song, H.G.; Chang, P.S.; Han, J. Ascorbic acid-based oxygen scavenger in active food packaging system for raw meatloaf. J. Food Sci. 2018, 83, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Ekonomou, S.I.; Parlapani, F.F.; Kyritsi, M.; Hadjichristodoulou, C.; Boziaris, I.S. Preservation status and microbial communities of vacuum-packed hot smoked rainbow trout fillets. Food Microbiol. 2022, 103, 103959. [Google Scholar] [CrossRef]
- Hussain, Z.; Li, X.; Zhang, D.; Hou, C.; Ijaz, M.; Bai, Y.; Xiao, X.; Zheng, X. Influence of adding cinnamon bark oil on meat quality of ground lamb during storage at 4 °C. Meat Sci. 2021, 171, 108269. [Google Scholar] [CrossRef]
- Vogel, B.F.; Venkateswaran, K.; Satomi, M.; Gram, L. Identification of Shewanella baltica as the most important H2S-producing species during iced storage of Danish marine fish. Appl. Environ. Microbiol. 2005, 71, 6689–6697. [Google Scholar] [CrossRef]
- Sun, Y.; Lan, W.; Liu, S.; Guan, Y.; Zhu, S.; Xie, J. Preparation of chitosan grafted caffeic acid coating and its effect on pompano (Trachinotus ovatus) preservation. J. Sci. Food Agric. 2022, 102, 2835–2845. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, P.; Fang, S.; Liu, W.; Mei, J.; Xie, J. Preservative effects of gelatin active coating enriched with eugenol emulsion on Chinese seabass (Lateolabrax maculatus) during superchilling (−0.9 °C) storage. Coatings 2019, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Lan, W.; Zhao, X.; Wang, M.; Xie, J. Effects of chitosan and apple polyphenol coating on quality and microbial composition of large yellow croaker (Pseudosciaena crocea) during ice storage. J. Sci. Food Agric. 2022, 102, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Benjakul, S. Melanosis and quality changes of Pacific white shrimp (Litopenaeus vannamei) treated with catechin during iced storage. J. Agric. Food Chem. 2009, 57, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, Y.; Wang, H.; Liu, W.; Cheong, K.L.; Teng, B. Effect of sodium alginate-agar coating containing ginger essential oil on the shelf life and quality of beef. Food Control 2021, 130, 108216. [Google Scholar] [CrossRef]
- Dvorackova, E.; Snoblova, M.; Chromcova, L.; Hrdlicka, P. Effects of extraction methods on the phenolic compounds contents and antioxidant capacities of cinnamon extracts. Food Sci. Biotech. 2015, 24, 1201–1207. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Kang, H.; Peng, X. Antimicrobial and antioxidant effects of edible nanoemulsion coating based on chitosan and Schizonepeta tenuifolia essential oil in fresh pork. J. Food Process. Preserv. 2021, 45, e15909. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, Z.; Fan, L.; Dong, T.; Jin, Y.; Saldaña, M.D.A.; Sun, W. Sustained-release antibacterial pads based on nonwovens polyethylene terephthalate modified by β-cyclodextrin embedded with cinnamaldehyde for cold fresh pork preservation. Food Packag. Shelf Life 2020, 26, 100554. [Google Scholar] [CrossRef]
- Cui, H.; Yang, M.; Shi, C.; Li, C.; Lin, L. Application of xanthan-gum-based edible coating incorporated with Litsea cubeba essential oil nanoliposomes in salmon preservation. Foods 2022, 11, 1535. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Wang, Y. Antimicrobial and Antioxidant Effects of Kappa-Carrageenan Coatings Enriched with Cinnamon Essential Oil in Pork Meat. Foods 2022, 11, 2885. https://doi.org/10.3390/foods11182885
He S, Wang Y. Antimicrobial and Antioxidant Effects of Kappa-Carrageenan Coatings Enriched with Cinnamon Essential Oil in Pork Meat. Foods. 2022; 11(18):2885. https://doi.org/10.3390/foods11182885
Chicago/Turabian StyleHe, Shoukui, and Yifei Wang. 2022. "Antimicrobial and Antioxidant Effects of Kappa-Carrageenan Coatings Enriched with Cinnamon Essential Oil in Pork Meat" Foods 11, no. 18: 2885. https://doi.org/10.3390/foods11182885




