Abstract
Essential oils (EOs) from aromatic plants seem to have the potential to control several fungal pathogens and food contaminants. Botrytis cinerea is the main strawberry fruit contaminant causing high losses during storage. Here, thirteen EOs applied in the vapor phase were evaluated for their potential to inhibit the growth of three different strains of B. cinerea isolated from strawberry fruits. Eight EOs (lemongrass, litsea, lavender, peppermint, mint, petitgrain, sage, and thyme) were able to completely inhibit the growth of B. cinerea for 7 days when applied at a concentration of 625 μL·L−1. Four EOs with the lowest minimal inhibition concentrations (thyme, peppermint, lemongrass, and litsea) have been tested on strawberry fruits intentionally inoculated by B. cinerea. All four EOs showed high inhibition at a concentration of 250 or 500 μL·L−1, but only peppermint EO was able to completely inhibit B. cinerea lesion development at a concentration of 125 μL·L−1. The sensory evaluation of strawberries treated by EOs at a concentration 125 μL·L−1 resulted in a statistically significant decrease in taste, aftertaste, aroma, and overall quality. Lemongrass and litsea EOs scored better than thyme and peppermint ones, thus forming two viable methods for B. cinerea suppression and the extension of packed strawberries’ shelf life.
1. Introduction
Strawberries are one of the most cultivated fruits in the world, and their cultivation is constantly growing [1,2,3]. Strawberry fruits have a very short shelf life, and significant post-harvest losses occur in the fresh-product supply chain. The quality of fruit is rapidly reduced over time, resulting in up to 40% losses [4]. The degradation of berries is mainly caused by fungal pathogens [2,5]. The most common cause of decay of strawberries is gray mold, also called Botrytis rot as it is caused by Botrytis cinerea. This species grows rapidly and destroys strawberry fruit within a few days. The disease can begin pre harvest, remain as a hidden infection, or start after harvest [6,7,8,9]. Up to 77% of strawberry fruit samples were contaminated by this fungal species [10].
Post-harvest rot control is traditionally achieved using chemical fungicides [11]. However, there is an increasing tendency among consumers to refuse chemical treatments. Furthermore, natural substances for the extension of the shelf life of food are preferred today. Essential oils (EOs) are considered suitable substitutes for chemical food preservatives [8,12,13,14,15,16,17,18]. They appear to be effective against various species of microorganisms that are resistant to other preservatives [19]. EOs and their components are also important because of their availability, their range of biological activities, and their low cost [20].
Depending on the source plant species, EOs contain various active ingredients such as phenols (thymol, carvacrol), alcohols (linalool, menthol), aldehydes (citral, cinnamaldehyde), and others. These compounds can efficiently stop the growth of microorganisms through the inhibition of microbial metabolism and gene expression, cell wall degradation, the blockage of DNA repair, and other mechanisms [21]. In addition, some EOs showed antioxidant properties that may help to extend the shelf life of fruits [22].
The effectiveness of the EO treatment in the management of fungal pathogens after harvest depends on the cultivar of the fruit, the composition and the concentration of the applied EO, the duration of storage, and the method of application [23].
There is growing evidence that the application of EOs in the vapor phase is an effective antimicrobial treatment and has advantages over liquid-phase EOs applications, such as increased activity, use at lower concentrations, and the ability to be used in a variety of environments [24]. Due to these advantages, many studies on the possible use of EOs in the vapor phase during fruit storage have been carried out in past years [25,26,27,28]. Nevertheless, there is no general uniformity regarding the effectiveness of EOs against various microorganisms, and therefore it is necessary to experimentally identify the activity of each EO vapor with regard to particular microbial species [29].
In recent years, the antifungal activity of EOs against B. cinerea has been examined by several studies [9,13,30,31,32,33,34,35,36,37], and some of EOs have shown promising properties regarding B. cinerea inhibition on various fruits or vegetables. Additionally some EOs like tea tree [38], thyme [39], lemon, cinnamon [40], oregano, and ziziphora [41] were evaluated for strawberry fruit preservation. Some studies have yielded results that do not correspond to other studies, although the same EOs are used. Thus, there is still a challenge to find candidate EOs for strawberry preservation due to differences in treatments, doses, and the origin of fungal isolates. Excellent EOs antifungal activity often does not correlate with the potential of real use. EOs have a strong flavor and scent that may not be accepted by consumers [42]. Thus, only some of the EOs are suitable for the preservation of particular foods.
The aim of the presented research was to measure the ability of selected EOs to inhibit the growth of B. cinerea strains, and to select the most effective EOs and evaluate their potential to control B. cinerea development directly on strawberries. Finally, the impact of these EOs on the sensory properties of strawberries was considered. We hypothesized that some of the EOs are able to effectively suppress B. cinerea and provide an extension of storage without negative effects on strawberry sensory traits.
2. Materials and Methods
2.1. Fungal Strains
Strains of Botrytis cinerea used in assays were isolated from moldy strawberries in 2020. Strain B. cinerea KMi-284 was isolated from packed strawberries obtained from supermarket (fruit origin–Spain). Strains KMi-507 and KMi-508 were isolated from strawberries obtained from local fresh market in Nitra (Western Slovakia) and Banská Bystrica (Middle Slovakia), respectively. Strains were identified using a polyphasic system involving micro- and macro-morphological traits and molecular methods. The internal transcribed spacer DNA sequences of the strains were deposited in GenBank under accession numbers ON318873-5. The strains of B. cinerea were deposited in the Collection of Microorganisms of the Institute of Biotechnology, Department of Microbiology, Faculty of Biotechnology and Food Science, SUA in Nitra, Slovakia.
For growth-inhibition assays, the strains were grown on potato-dextrose agar (PDA; HIMEDIA India) at 22 ± 1 °C for 7 days. Spores were collected by rinsing the colony with physiological saline solution supplemented with Tween 80 (0.5%). Conidial suspension with a concentration of 104 spores/mL was prepared for each fungal strain. The EVETM automatic cell counter (NanoEnTek, Seoul, Korea) was used to determine the number of spores.
2.2. EOs
Thirteen commercially available EOs from seven plant families were used in the assay. According to the information provided by the producers, the EOs were obtained by hydro-distillation. The semi-quantitative composition of the EO samples was determined by gas chromatography coupled with mass spectrometry (GC-MS) using an Agilent 7890B oven coupled with Agilent 5977A mass detector (Agilent Technologies Inc., Palo Alto, CA, USA) and CombiPal autosampler 120 (CTC Analytics AG, Zwingen, Switzerland). The methodology for the determination of EOs components was detailed in our previous study [43]. Details about the composition of the EOs are listed in Table 1.

Table 1.
Main compounds of essential oils (EOs) used in Botrytis cinerea inhibition assay determined by gas chromatography coupled with mass spectrometry (GC-MS). Only compounds representing more than 5% of particular EO are listed.
2.3. Fungal Growth Inhibition Assays
We used a multi-step selection strategy where only promising EOs were used in further steps for the complex evaluation of the inhibitory potential of the EOs. The first step involved testing the inhibitory effects in vitro using high doses of EOs, and the second step comprised in vitro testing of several concentrations of EOs and the determination of inhibitory concentrations. The in vivo assay with the strawberries infected by B. cinerea represented the third step. The final step was sensory analysis to evaluate the potential of EO use in strawberry packaging.
2.3.1. In Vitro Testing of Inhibitory Effect
The vapor-phase diffusion method was used to determine the inhibitory effect of EOs on B. cinerea growth. Strains were cultivated on PDA for 7 days in 22 ± 1 °C. Petri dishes with 9 cm diameter were filled by 15 mL of PDA medium. Five microliters of spore suspension prepared as mentioned in 2.1 were inoculated in the center of the media. A small piece of Whatman No.1 filter paper (1 cm × 1 cm) was placed in the center of the Petri dish cover and infused by 50 µL of concentrated EO. The Petri dish was sealed by parafilm M and cultivated in an upside-down position. The evaporated EO provided a concentration of 625 µL of EO in one liter of air. The experiment was carried out in triplicates. Fifty microliters of dimethylsulfoxide (DMSO) were used instead of EOs in a control treatment. The growth of colonies was observed on the 3rd, 4th, and 7th day of cultivation. The diameter of the colonies was evaluated using a digital caliper. The antifungal activity of the EOs was expressed by relative inhibition calculated using Equation (1), where RI is the relative inhibition in %, c is the diameter of the colony in the control, and t is the diameter of the colony treated by EO.
Equation (1):
RI = [(c − t)/c] × 100
2.3.2. Determination of Inhibitory Concentrations
Minimal inhibitory concentrations (MICs) were estimated only for EOs that showed a 100% inhibitory effect in the previous step with 625 μL·L−1 concentration. For this purpose, EOs were diluted in DMSO to a concentration that provided 500 μL·L−1 in vapor phase when the oil was applied to the paper in amounts of 50 μL. This concentration was serially diluted in DMSO to obtain concentrations of 250, 125, 62.5, 31.25, and 15.625 μL·L−1 in the vapor phase. Six replications were conducted for each dose. The presence of fungal growth was evaluated on the 3rd, 4th, and 7th day of cultivation and probit analysis was used for the estimation of inhibitory doses when 50% (IC50) or 90% (IC90) of the colonies were not able to grow.
2.3.3. In Vivo Evaluation of Antifungal Activity of EOs on Strawberries
The strawberries were purchased directly from the local Slovak grower. The experiment was established on the day of the collection and purchase of fruits. Fruits were selected to have the same weight without any signs of infection or mechanical damage. The strawberries were treated with a freshly prepared 1% sodium hypochlorite solution to minimize microbial contamination from the field. Further, the fruits were rinsed with sterile water and dried at room temperature. Cleaned strawberries were placed in small clear plastic containers. Four 1 mm wounds were made on each strawberry with a sterile tip in the equatorial plane. Then, 5 µL of B. cinerea spore suspension (104 spores in 1 mL) was added to the wound site with a micropipette. Containers with strawberries were placed in sealable glass jars with a volume of 500 mL. Whatman No. 1 filter papers (diameter 50 mm) were placed in the cup closures and EOs were applied. Thyme, litsea, peppermint, and lemongrass were used in the assay. EOs were prepared in 3 concentrations (100%, 50% and 25%) using DMSO as solvent, and 250 μL of solution was applied to filter paper providing EO vapor concentrations of 500, 250, and 125 μL·L−1. DMSO (250 µL) was applied instead of EO as a control. All variants had three replicates. The glasses were covered with foil to prevent access to light and stored at room temperature (21 ± 1 °C). The growth of B. cinerea was monitored on the 3rd, 4th, 5th, 6th, and 7th day after the inoculation. The number of lesions (0–12) developed in 12 inoculating points (4 points in each replication), was scored in each treatment.
2.4. Sensory Analysis
The strawberries were placed in sealable transparent glass cups with a volume 500 mL. Strawberries (5 pieces) were selected to have the same weight. The EOs at a concentration 125 µL·dm−3 were applied to Whatman No. 1 filter papers in a cup cap. The glasses were stored in a refrigerator at 3 ± 1 °C for 5 days. All variants had three replicates.
Sensory quality was rated on a 9-point scale (1–2 represented extreme dislike; 3–5 fair; 6–8 good; and 9 excellent) for appearance, aroma, taste, aftertaste, and overall acceptability. At the beginning of the experiment, 5 panelists were trained to evaluate the relevant characteristics of the fruit. Sensory evaluation was performed in a sensory laboratory equipped with separate sensor boxes.
2.5. Statistical Analysis
Data from fungal inhibition analysis and sensory analysis were evaluated using one-way variance analysis (ANOVA) followed by a post hoc Tukey HSD test. Inhibitory concentrations of IC50 and IC90 were estimated using probit analysis [44]. All statistical analyses were carried out in an R environment [45].
3. Results
3.1. Evaluation of EOs Inhibitory Properties
Among thirteen evaluated EOs, nine oils demonstrated absolute inhibition of B. cinerea growth in the first step of our multi-level evaluation when 625 μL·L−1 concentration of EO vapor was tested (Table 2). The antifungal activity of EOs expressed as the relative inhibition of fungal growth is summarized in Supplementary Table S1. EOs from lemongrass, litsea, lavender, peppermint, mint, petitgrain, sage, and thyme inhibited the growth of all strains during the whole period of 7 days. After eucalyptus EO treatment, the growth of a single strain, i.e., KMi-507, was detected on the 4th and 7th day, while other strains remained completely inhibited. Grapefruit EO caused a delay in the growth of fungal colonies as there was no measurable growth on the second day. However, colony growth was detected on the third day, and finally on 7th day fungus overgrew the whole Petri plate, similarly to the colonies in the untreated control. Ginger EO acted similarly, but the inhibitory level was lower. Jasmine EO did not significantly slow down growth, and it even stimulated the growth of strain KMi-284 on the 2nd day. The last four mentioned EOs have been removed from further testing.

Table 2.
Average diameter of Botrytis cinerea colonies on potato dextrose agar (22 ± 1 °C) after treatment by essential oils in vapor phase (625 μL·L−1).
3.2. Inhibitory Concentrations of EOs
The lowest MICs were observed for thyme, litsea, and peppermint EOs. These EOs completely inhibited the growth of any strain of B. cinerea on the 7th or 14th days at a concentration of 250 μL·L−1. The EOs concentration of 500 μL·L−1 inhibited growth when lemongrass and lavender EOs were used. Cardamom, mint, petitgrain, and sage EOs inhibited growth completely only when used in the highest dose (625 μL·L−1).
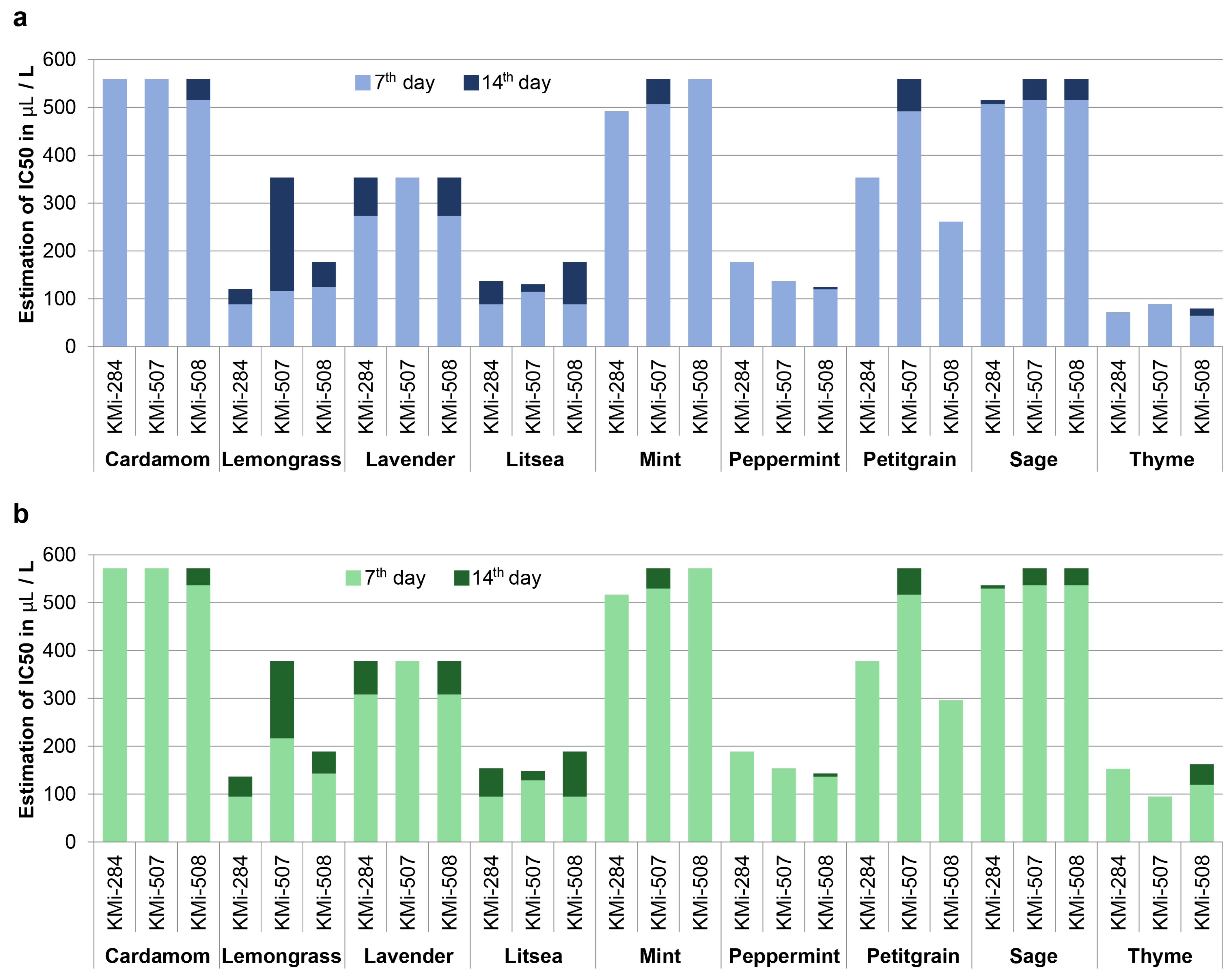
We estimated concentrations that inhibited B. cinerea growth in 50% or 90% of cases (IC50 and IC90) by probit analysis for each fungal strain and EO (Figure 1). The values of IC50 varied greatly among EOs, as well as among strains, which possess different reactions of particular strain to EO treatment. For example, the strain KMi-507 was the most sensitive to the presence of thyme EO (IC90 = 94.61 μL·L−1), but it was the most resistant to litsea EO treatment (IC90 = 128.65 μL·L−1) on the 7th day. IC90 values were not very different from IC50, suggesting that the threshold for growth inhibition is relatively sharp and the cessation of growth occurs relatively shortly after the specific concentration of EO in the vapor phase is reached. The lowest values of IC50 were detected for thyme EO, followed by litsea, mint, and lemongrass. They were substantially more effective than lavender, petitgrain, and sage EOs.
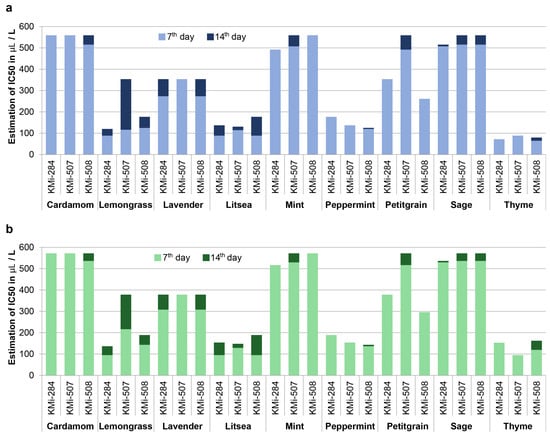
Figure 1.
Graph of estimated concentration of essential oils that inhibited growth of B. cinerea on 50% (a) and 90% (b) inoculated plates.
3.3. Inhibition of B. cinerea on Strawberries
The real ability of previously selected EOs to suppress B. cinerea during the storage of strawberries was tested in three concentrations 125, 250, and 500 μL·L−1 (Table 3). The development of B. cinerea lesions was observed in all 12 inoculation points on control strawberry fruits. On the other hand, lesions were not developed in any 500 μL·L−1 treatment. The EOs applied at a concentration of 250 μL·L−1 inhibited B. cinerea development in all cases except lemongrass EO against the KMi-508 strain and litsea EO against strains KMi-284 and KMi-508. The lowest tested concentration also inhibited lesion development despite the fact its action was not sufficient in case of litsea, lemongrass, or thyme. Peppermint EO at a concentration of 125 μL·L−1 did not allow for the development of the B. cinerea lesion at all.

Table 3.
Development of B. cinerea lesions on strawberries treated by the essential oils in vapor phase.
3.4. Sensory Analysis of Strawberries Treated by EOs
Samples of strawberries treated with the 125 μL·L−1 concentrations of EOs were also evaluated from a sensory point of view after 5 day storage. Higher concentrations of EOs, e.g., 250 and 500 μL·L−1, were evaluated as unacceptable in the preliminary assay.
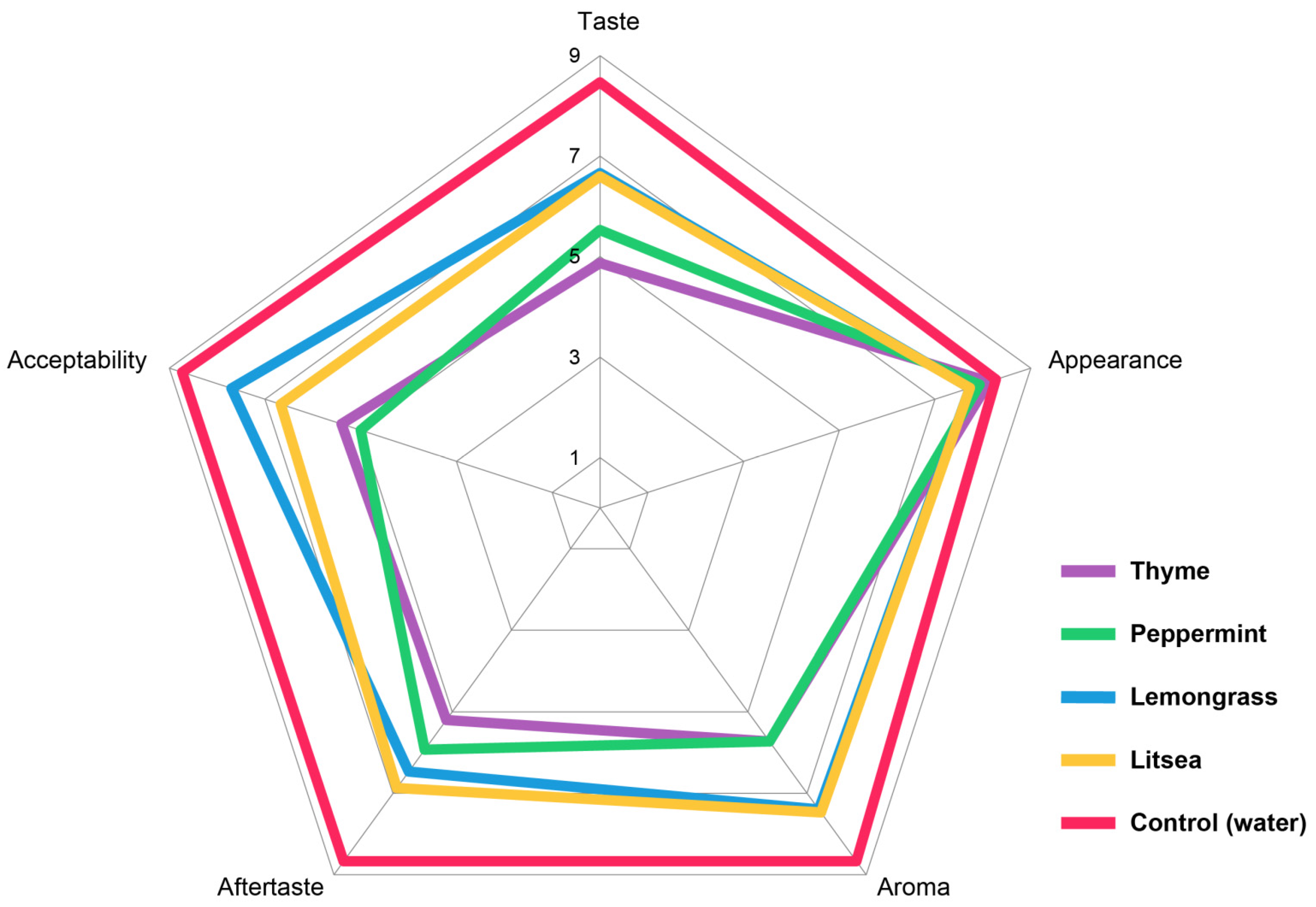
In terms of statistical significance (p < 0.05), the best sensory quality in terms of taste, aftertaste, and overall acceptability was achieved by the control sample (Supplementary Table S2). The treatment of samples with EOs did not show a statistically significant change in the appearance of the samples. All of the samples had a fresh appearance, the fruits were shiny, and the stem of the fruit was green and unadulterated. Sample treatment by EOs resulted in statistically significant differences in taste, aftertaste, aroma, and overall quality (Figure 2). In the aroma trait, the best results were achieved by samples treated with lemongrass EO. It was characterized by a dominant strawberry aroma. In the samples treated with peppermint and thyme EOs, the scores dropped to 5.73 and the evaluators described the aroma of these samples as pleasant, but the typical strawberry aroma was overpowered by the EO.
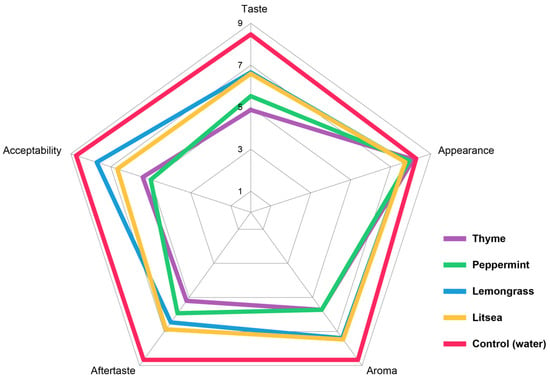
Figure 2.
Radar plot of the sensory evaluation of strawberries treated by essential oils in vapor phase.
There was a decrease in taste and aftertaste scores by 1.86–3.60 points. The highest scores were achieved by samples treated with lemongrass, litsea, and peppermint EOs. The taste of the samples was considered to be good. The taste and aftertaste were, statistically speaking, significantly worse in the samples treated with thyme EO. The taste of these samples was acceptable, but the flavor of the used EO dominated over the natural strawberry flavor.
4. Discussion
In this study, we assessed the effect of EOs from 13 different plant species against B. cinerea. The quality and composition of used EOs are crucial in evaluating their effects, because the quality can significantly affect the results. Many studies [46,47,48,49] confirmed variation in the composition of EOs depending on the growing season, the nature of plant parts, and different stages of plant growth and climatic conditions. According to GC-MS analysis, used commercially available EOs had compositions in line with other published studies, and the main components were in the typical range for certain types of EOs.
According to the results, EOs were divided into three groups. The first group consisted of EOs that had a weak inhibitory effect on the strains of B. cinerea. This group included grapefruit, jasmine, and ginger EOs. The efficiency of grapefruit and ginger EOs declined rapidly. The least effective EO was the jasmine one, which even stimulated the growth of fungal colonies. However, the inhibitory effect of these EOs on microscopic fungi was reported in the research of other authors. According to Viuda-Martos et al. [50], grapefruit EO was the best in terms of the growth reduction in Penicillium chrysogenum and Penicillium verrucosum when several citrus EOs were tested. Kujur et al. [51] showed significant protection of maize seeds against fungal infection after treatment by nano-encapsulated jasmine EO. Jasmine EO also showed the antibiofilm activity of Candida albicans [52]. According to Nerilo et al. [53], only a low concentration of ginger EO was needed to curb the production of aflatoxin B1 by Aspergillus flavus.
The second group consisted of effective EOs that inhibited the growth of the strains tested: eucalyptus and cardamom EOs. Eucalyptus EO 100% inhibited the growth of two of the three B. cinerea strains. Davari et Ezazi [54] reported an inhibitory effect of eucalyptus EO on B. cinerea from 0 to 84.88, depending on the concentration used. They rated this EO as moderately or poorly effective. Similarly, other authors report that eucalyptus EO is effective against fungi but only in higher concentrations [55].
In our essay, Botrytis cinerea growth in the presence of cardamom EO was recorded in only one strain (KMi-284) and only in the last measurement (day 7). The effectiveness of inhibition at this time point was high, 83.33%. Antibacterial action and the suppression of biofilm formation was previously recorded for cardamom extracts [56]. Traditional medicine has been using the cardamom EO for a long time, and it is a promising compound in the fight against acute campylobacteriosis [57,58]. Cardamom EO effectively inhibited A. flavus growth in peanuts and reduced aflatoxin production [59].
The third group of EOs in our study comprised thyme, litsea, peppermint, lemongrass, lavender, petitgrain, mint, and sage EOs. These completely inhibited the growth of B. cinerea strains at a concentration 625 μL·L−1. Out of them, thyme, litsea, peppermint, and lemongrass showed the best antifungal properties in further testing.
In correspondence with our results, a significant antifungal effect on Fusarium species (F. avenaceum, F. culmorum, F. graminearum, and F. oxysporum) has been reported after treatment by thyme, litsea, lemongrass, and verbena EOs. Their effect was comparable to a synthetic pesticide Funaben T [60]. Litsea EO applied by the agar dilution method at a concentration of 1.0% resulted in the complete inhibition of B. cinerea growth [36]. Amiri et al. [35] showed that the use of the peppermint EO and savory EO applied in the vapor phase was more effective at inhibiting the growth of B. cinerea than the liquid application. These EOs had a significant impact on the growth of B. cinerea at reasonably low concentrations. Moreover, the application of EOs in the vapor phase is easily adaptable for the food industry.
In the experiment carried out by Reang et al. [37], thyme was the best evaluated in terms of the growth inhibition of B. cinerea among five EOs (clove, thyme, lavender, lemongrass, and peppermint). All of these EOs inhibited the growth of B. cinerea when tested by the agar dilution method, but the efficiency of inhibition varied between the EOs and the concentration used. At a concentration of 1.5%, thyme EO inhibited the growth of mycelia by 50%, clove 44.65%, lemongrass 40.89%, lavender 40.35%, and peppermint 38.39%. Thyme EO significantly (by 64%) reduced the colonization of detached tomato leaves by B. cinerea when applied by foliar spraying. [61].
The best evaluated EOs have different main components, and their mode action in fungal inhibition is not the same. For example, thyme EO affected the growth of fungus Mycosphaerella graminicola through the regulation of the expression of genes involved in cell development and detoxification [62]. Citral affected the cell membrane [63], but its mechanism of action does not involve the cell wall or ergosterol [64]. Detailed knowledge of the mode of action is still lacking for most of the EOs compounds. Moreover, the interaction of particular compounds within an EO plays an important role [65].
Ansarifar et Moradinezhad [39] showed promising ways to preserve strawberries using thyme EO encapsulated in zein nanofibers. This packaging led to a decrease in bacterial and fungal development, while acidity, total phenol content, and antioxidant activity were maintained. Despite the authors examining the appearance of fruit, they did not report changes in flavor or taste.
As mentioned earlier, the strong organoleptic properties of EOs are a complication for their use in the preservation of food. For this reason, we also evaluated the sensory properties of strawberries after the application of EO. In our essay, the aroma, flavor, and aftertaste of the strawberries were significantly overpowered by peppermint EO. In terms of overall acceptability, strawberries samples treated with lemongrass EO received the highest score. The fruits retained typical strawberry properties, and the EO did not interfere with the character of the aroma and flavor. Of all the EOs tested, the panelist describes lemongrass EO as the most compatible with strawberries in sensory traits. Citral, which is the main component of lemongrass EO, was recently positively evaluated for Rhizopus oryzae control on table grapes [25]. After application at a dose of 0.0125 µL·cm−3, the panelists still recognized the odor of citral in grape samples. However, the authors of the study declared the odor diminishing 30 min after the package opening.
Yanzhen et al. [38] treated strawberries after harvesting with tea tree EO at a concentration of 0.3–0.9 g·L−1 air; then, they left the fruit for 3 h in the EO environment. After 3 days of storage, all treatments significantly (p < 0.05) maintained higher sensory scores than those of control groups, which indicated that all these treatments help to maintain the color, aroma quality, and overall acceptability of strawberry fruit. The treatment of strawberries with Solidago canadensis EO in the vapor phase effectively suppressed the growth of B. cinerea and preserved the postharvest quality. Additionally, the sensory acceptance of the strawberries was higher than the control in the 2nd, 3rd, and 4th day [66]. However, a sensory analysis of the same treatment can result in contrary results, e.g., Shehata et al. [67] described the increase of sensory traits after strawberry treatment by lemon EO, while Perdones et al. [34] evaluated the effect of the same oil in the combination with chitosan negatively. Gol et al. [5] treated strawberries only with chitosan after harvesting. After 8 and 12 days, they found significantly better sensory quality in the coated samples than in the untreated samples.
It is important to note that our strawberries in sensory tests were not decayed and did not show any microbial damage in control and treated samples at the time of the test (on the 5th day). Authors of some studies [66,67] used the conditions (long storage or higher temperature) in which fruits in the untreated control decayed, while samples treated by EOs scored better due to EOs antimicrobial activity. Although a test in these conditions reflects reality, it can also hide the negative sensory effects of EOs. Minor changes of aroma or flavor caused by EOs are significantly lower than changes due to microbial activity [67]. Such tests partially lack relevance because consumers are not supposed to buy or consume decayed fruit. The evaluation should be carried out in non-decayed fruits to reveal the true impact of EOs on sensory traits.
Despite the fact thyme EO showed the best inhibitory action in our essay, we cannot recommend its use due to the changed sensory values. Small fruits are exceptionally challenging in this because consumers have high demand for the natural and original sensory properties of the fruit. Based on a complex view of all of the tested EOs, considering their ability to preserve strawberries along with their drawbacks in terms of potential customer acceptance, lemongrass EO and (to some extent) litsea EO are good candidates for use in the food industry. In recent years, encapsulation, nanoparticles, and substances capable of creating an edible coating with high preservation ability, such as chitosan, have gained attention [68,69,70,71,72]. The combination of the high effective EOs with these technologies in active packaging can improve the shelf life of many foods, including fruits such as strawberries. As EOs are natural substances with the potential ability to extend the shelf-life of fruits, they can be a healthier choice for consumers than the use of inorganic substances in active packaging [73].
5. Conclusions
Four of thirteen evaluated EOs showed promising levels of B. cinerea growth inhibition and decreased lesion development in packed strawberries. However, EOs more or less changed the sensory quality of strawberries. Lemongrass and litsea EOs seem to be acceptable for consumers when applied at 125 μL·L−1 concentration. The effect may be strengthened by storing them at lower temperatures. However, the selection of EOs with good inhibition properties and without negative sensory effects is desirable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11192945/s1, Table S1: The relative inhibition of Botrytis cinerea colony growth after treatment by essential oils in vapor phase; Table S2: The statistical analysis of strawberry sensory traits after treatment by essential oils in vapor phase.
Author Contributions
Conceptualization, methodology, funding acquisition, project administration, supervision, and writing—original draft, D.T.; investigation Z.M., A.M., D.F. and Z.B.; formal analysis, validation, and writing—review and editing, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scientific Grant Agency of the Ministry of Education and Science of the Slovak Republic and the Slovak Academy of Sciences (VEGA) grant number 0517/21.
Data Availability Statement
All data are available on correspondence author upon request.
Acknowledgments
The authors would thank to Eva Sádovská for her work in laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mezzetti, B.; Giampieri, F.; Zhang, Y.-T.; Zhong, C.-F. Status of strawberry breeding programs and cultivation systems in Europe and the rest of the world. J. Berry Res. 2018, 8, 205–221. [Google Scholar] [CrossRef]
- Parvez, S.; Wani, I.A. Postharvest biology and technology of strawberry. In Postharvest Biology and Technology of Temperate Fruits; Mir, S., Shah, M., Mir, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 331–348. [Google Scholar] [CrossRef]
- Simpson, D. The economic importance of strawberry crops. In The Genomes of Rosaceous Berries and Their Wild Relatives; Springer: Cham, Germany, 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Trinetta, V.; McDaniel, A.; Batziakas, K.G.; Yucel, U.; Nwadike, L.; Pliakoni, E. Antifungal packaging film to maintain quality and control postharvest diseases in strawberries. Antibiotics 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Salami, P.; Ahmadi, H.; Keyhani, A.; Sarsaifee, M. Strawberry post-harvest energy losses in Iran. Researcher 2010, 2, 67–73. [Google Scholar]
- Petrasch, S.; Knapp, S.J.; van Kan, J.A.L.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Chen, R.-Y.; Chou, J.-Y. Screening and Evaluation of Yeast Antagonists for Biological Control of Botrytis cinerea on Strawberry Fruits. Mycobiology 2018, 46, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, H.; Chen, K.; Feng, J.; Zhang, Y. Antifungal Activities and Mode of Action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum Essential Oil Vapors against Botrytis cinerea and Their Potential Application to Control Postharvest Strawberry Gray Mold. Foods 2021, 10, 2451. [Google Scholar] [CrossRef]
- Tournas, V.; Katsoudas, E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int. J. Food Microbiol. 2005, 105, 11–17. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay control in the postharvest system: Role of microbial and plant volatile organic compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Adeyinka, A.; Richard, F. Application of phytochemical extracts and essential oils in food products: A review. Int. J. Biotechnol. Food Sci. 2015, 3, 31–35. [Google Scholar]
- Aguilar-González, A.E.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Herman, R.A.; Ayepa, E.; Shittu, S.; Fometu, S.S.; Wang, J. Essential oils and their applications—A mini review. Adv. Nutr. Food Sci. 2019, 4, 1–13. [Google Scholar]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Bhandari, B.; Mujumdar, A.S. Edible flower essential oils: A review of chemical compositions, bioactivities, safety and applications in food preservation. Food Res. Int. 2021, 139, 109809. [Google Scholar] [CrossRef]
- Anupama, G.; Netravathi, D.; Avinash, M. Essential oils: A novel source for food preservation. J. Pharmacogn. Phytochem. 2019, 8, 2098–2101. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: An updated review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Lopez-reyes, J.G.; Spadaro, D.; Prelle, A.; Garibaldi, A.; Gullino, M.L. Efficacy of Plant Essential Oils on Postharvest Control of Rots Caused by Fungi on Different Stone Fruits In Vivo. J. Food Prot. 2013, 76, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Jurado, F.; Navarro-Cruz, A.R.; Ochoa-Velasco, C.E.; Palou, E.; López-Malo, A.; Ávila-Sosa, R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Fancello, F.; Caputo, L.; Sorrentino, A.; Zara, S.; Lippolis, V.; Cervellieri, S.; Fanelli, F.; Corvino, A.; Pace, B.; et al. Effect of Gaseous Citral on Table Grapes Contaminated by Rhizopus oryzae ITEM 18876. Foods 2022, 11, 2478. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-J.; Lin, Y.-L.; Huang, B.-B.; Lin, Y.-T.; Li, H.-K.; Lu, W.-J.; Lin, T.-C.; Tsui, Y.-C.; Lin, H.-T.V. Solid- and vapour-phase antifungal activities of six essential oils and their applications in postharvest fungal control of peach (Prunus persica L. Batsch). LWT 2022, 156, 113031. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Kuganov, R.; Obistioiu, D.; Popescu, I.; Radulov, I.; Alexa, E.; Negrea, M.; Salimzoda, A.F.; Sumalan, R.L.; Cocan, I. Assessment of Mint, Basil, and Lavender Essential Oil Vapor-Phase in Antifungal Protection and Lemon Fruit Quality. Molecules 2020, 25, 1831. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Rousos, C.; Xylia, P.; Tzortzakis, N. Vapour Application of Sage Essential Oil Maintain Tomato Fruit Quality in Breaker and Red Ripening Stages. Plants 2021, 10, 2645. [Google Scholar] [CrossRef]
- Laird, K.; Phillips, C. Vapour phase: A potential future use for essential oils as antimicrobials? Lett. Appl. Microbiol. 2012, 54, 169–174. [Google Scholar] [CrossRef]
- Banani, H.; Olivieri, L.; Santoro, K.; Garibaldi, A.; Gullino, M.L.; Spadaro, D. Thyme and savory essential oil efficacy and induction of resistance against Botrytis cinerea through priming of defense responses in apple. Foods 2018, 7, 11. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Valiuškaitė, A. Application of plant extracts to control postharvest gray mold and susceptibility of apple fruits to B. cinerea from different plant hosts. Foods 2020, 9, 1430. [Google Scholar] [CrossRef]
- Aouadi, G.; Grami, L.K.; Taibi, F.; Bouhlal, R.; Elkahoui, S.; Zaagueri, T.; Jallouli, S.; Chaanbi, M.; Hajlaoui, M.R.; Mediouni Ben Jemâa, J. Assessment of the efficiency of Mentha pulegium essential oil to suppress contamination of stored fruits by Botrytis cinerea. J. Plant Dis. Prot. 2022, 129, 881–893. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef] [PubMed]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Amiri, A.; Sourestani, M.M.; Mortazavi, S.M.H.; Kiasat, A.R.; Ramezani, Z. Efficiency of chemical composition of some essential oils against Botrytis cinerea, the pathogen of post-harvest strawberry fruits. J. Food Meas. Charact. 2022, 16, 66–75. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Deng, J.; Liu, X.; Zhou, J.; Li, X. Antibacterial activity of Litsea cubeba essential oil and its mechanism against Botrytis cinerea. RSC Adv. 2019, 9, 28987–28995. [Google Scholar] [CrossRef] [PubMed]
- Reang, S.P.; Mishra, J.; Prasad, R. In vitro antifungal activities of five plant essential oils against Botrytis cinerea causing gray mold of orange. J. Pharmacogn. Phytochem. 2020, 9, 1046–1048. [Google Scholar]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The combined effects of tea tree oil and hot air treatment on the quality and sensory characteristics and decay of strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Preservation of strawberry fruit quality via the use of active packaging with encapsulated thyme essential oil in zein nanofiber film. Int. J. Food Sci. Technol. 2021, 56, 4239–4247. [Google Scholar] [CrossRef]
- Freche, E.; Gieng, J.; Pignotti, G.; Ibrahim, S.A.; Feng, X. Applications of lemon or cinnamon essential oils in strawberry fruit preservation: A review. J. Food Process. Preserv. 2022, 46, e16526. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Panfilova, O.; Kesimci, T.G.; Bozhüyük, A.U.; Gürbüz, R.; Alptekin, H. Control of Postharvest Gray Mold at Strawberry Fruits Caused by Botrytis cinerea and Improving Fruit Storability through Origanum onites L. and Ziziphora clinopodioides L. Volatile Essential Oils. Agronomy 2022, 12, 389. [Google Scholar] [CrossRef]
- Kaliamurthi, S.; Selvaraj, G.; Hou, L.; Li, Z.; Wei, Y.; Gu, K.; Wei, D. Synergism of essential oils with lipid based nanocarriers: Emerging trends in preservation of grains and related food products. Grain Oil Sci. Technol. 2019, 2, 21–26. [Google Scholar] [CrossRef]
- Tančinová, D.; Hlebová, M.; Foltinová, D.; Mašková, Z.; Barboráková, Z. Influence of Eight Chosen Essential Oils in the Vapor Phase on the Growth of Rhizopus stolonifer and Rhizopus lyococcus. Slovak J. Food Sci. 2021, 15, 378–386. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Tilaoui, M.; Ait Mouse, H.; Jaafari, A.; Zyad, A. Comparative Phytochemical Analysis of Essential Oils from Different Biological Parts of Artemisia herba alba and Their Cytotoxic Effect on Cancer Cells. PLoS ONE 2015, 10, e0131799. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, M.; Jordán, M.J.; Chaouch-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Phenophase effects on sage (Salvia officinalis L.) yield and composition of essential oil. J. Appl. Res. Med. Aromat. Plants 2016, 3, 87–93. [Google Scholar] [CrossRef]
- Dušková, E.; Dušek, K.; Indrák, P.; Smékalová, K. Postharvest changes in essential oil content and quality of lavender flowers. Ind. Crops Prod. 2016, 79, 225–231. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Novak, J.; Sponza, S.; Herrero, B.; Asensio-S-Manzanera, M.C. Variability in essential oil composition of wild populations of Labiatae species collected in Spain. Ind. Crops Prod. 2016, 79, 18–28. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Kujur, A.; Kumar, A.; Singh, P.P.; Prakash, B. Fabrication, Characterization, and Antifungal Assessment of Jasmine Essential Oil-Loaded Chitosan Nanomatrix against Aspergillus flavus in Food System. Food Bioprocess Technol. 2021, 14, 554–571. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.; El-Ganiny, A.M. Back to nature: Combating candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef]
- Nerilo, S.B.; Rocha, G.H.O.; Tomoike, C.; Mossini, S.A.G.; Grespan, R.; Mikcha, J.M.G.; Machinski Jr, M. Antifungal properties and inhibitory effects upon aflatoxin production by Zingiber officinale essential oil in Aspergillus flavus. Int. J. Food Sci. Technol. 2016, 51, 286–292. [Google Scholar] [CrossRef]
- Davari, M.; Ezazi, R. Chemical composition and antifungal activity of the essential oil of Zhumeria majdae, Heracleum persicum and Eucalyptus sp. against some important phytopathogenic fungi. J. Mycol. Médicale 2017, 27, 463–468. [Google Scholar] [CrossRef]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Souissi, M.; Azelmat, J.; Chaieb, K.; Grenier, D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe 2020, 61, 102089. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Mousavi, S.; Weschka, D.; Bereswill, S. Anti-Pathogenic and Immune-Modulatory Effects of Peroral Treatment with Cardamom Essential Oil in Acute Murine Campylobacteriosis. Microorganisms 2021, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Ingok, A.; Karbancioglu-Guler, F. Cardamom, cumin, and dill weed essential oils: Chemical compositions, antimicrobial activities, and mechanisms of action against Campylobacter spp. Molecules 2017, 22, 1191. [Google Scholar] [CrossRef]
- Achar, P.N.; Quyen, P.; Adukwu, E.C.; Sharma, A.; Msimanga, H.Z.; Nagaraja, H.; Sreenivasa, M.Y. Investigation of the antifungal and anti-aflatoxigenic potential of plant-based essential oils against Aspergillus flavus in peanuts. J. Fungi 2020, 6, 383. [Google Scholar] [CrossRef]
- Krzyśko-Łupicka, T.; Sokół, S.; Piekarska-Stachowiak, A. Evaluation of fungistatic activity of eight selected essential oils on four heterogeneous fusarium isolates obtained from cereal grains in Southern Poland. Molecules 2020, 25, 292. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef]
- Ben Jabeur, M.; Somai-Jemmali, L.; Hamada, W. Thyme essential oil as an alternative mechanism: Biofungicide-causing sensitivity of Mycosphaerella graminicola. J. Appl. Microbiol. 2017, 122, 932–939. [Google Scholar] [CrossRef]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef]
- Leite, M.C.; Bezerra, A.P.; de Sousa, J.P.; Guerra, F.Q.; Lima Ede, O. Evaluation of Antifungal Activity and Mechanism of Action of Citral against Candida albicans. Evid. Based Complement. Altern. Med. eCAM 2014, 2014, 378280. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shao, X.; Wei, Y.; Li, Y.; Xu, F.; Wang, H. Solidago canadensis L. Essential Oil Vapor Effectively Inhibits Botrytis cinerea Growth and Preserves Postharvest Quality of Strawberry as a Food Model System. Front. Microbiol. 2016, 7, 1179. [Google Scholar] [CrossRef] [PubMed]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of some citrus essential oils on post-harvest shelf life and physicochemical quality of strawberries during cold storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of essential oils in packaging films for the preservation of fruits and vegetables: A review. Food Chem. 2022, 375, 131810. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Sharma, L.; Maity, T. Chapter 34—Enrichment of edible coatings and films with plant extracts or essential oils for the preservation of fruits and vegetables. In Biopolymer-Based Formulations; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W.-P., Dubey, N.K., Majumder, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 859–880. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Wang, D.; Yang, H.; Lu, X.; Wu, Y.; Blasi, F. The Inhibitory Effect of Chitosan Based Films, Incorporated with Essential Oil of Perilla frutescens Leaves, against Botrytis cinerea during the Storage of Strawberries. Processes 2022, 10, 706. [Google Scholar] [CrossRef]
- Tian, Q.; Zhou, W.; Cai, Q.; Ma, G.; Lian, G. Concepts, processing, and recent developments in encapsulating essential oils. Chin. J. Chem. Eng. 2021, 30, 255–271. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Harnkarnsujarit, N. Migration, aggregations and thermal degradation behaviors of TiO2 and ZnO incorporated PBAT/TPS nanocomposite blown films. Food Packag. Shelf Life 2022, 33, 100901. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).