Antibacterial Regularity Mining Beneath the Systematic Activity Database of Lipopeptides Brevilaterins: An Instructive Activity Handbook for Its Food Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Brevilaterins Stock Solution
2.2. Indicator Bacteria and Medium
2.2.1. Standard Bacteria
2.2.2. Isolated Bacteria from Spoilage Food
2.2.3. Isolated Bacteria to Resist Antibiotics
2.3. MIC and MBC Determination and Quality-Control Test
2.4. Relative Antibacterial Activity Affected by Food Compositions
2.5. Surface Hydrophobicity
2.6. Circular Dichroism Spectrum
2.7. Surface Plasmon Resonance
3. Results and Discussion
3.1. Quality Control of MIC Determination System
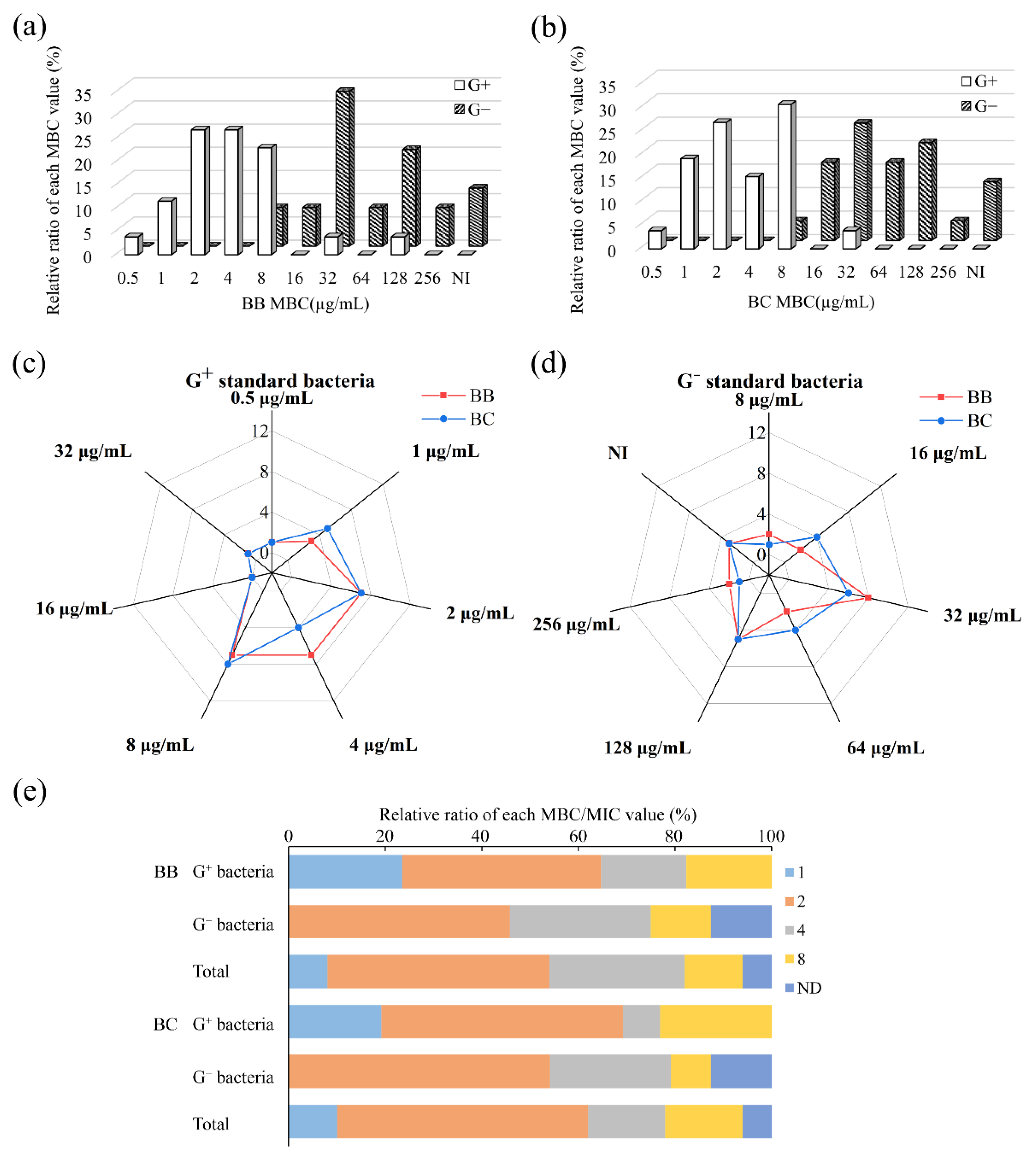
3.2. Antibacterial Regularity against Standard Bacteria
3.2.1. Inhibitory Property of Brevilaterins
3.2.2. Bactericidal Property of Brevilaterins
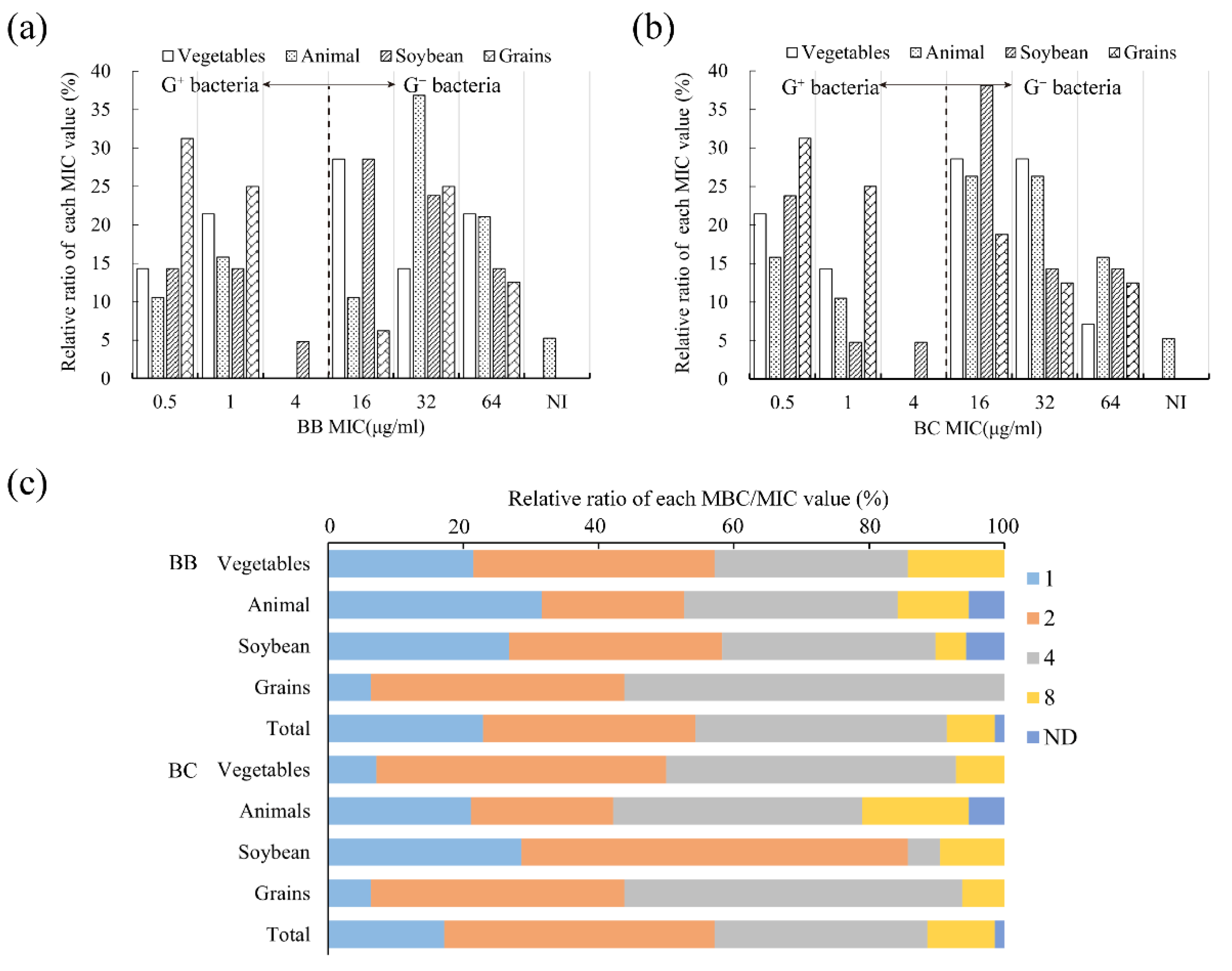
3.3. Verification of Antibacterial Regularity on Isolated Bacteria
3.3.1. Antibacterial Property against Food Spoilage Bacteria
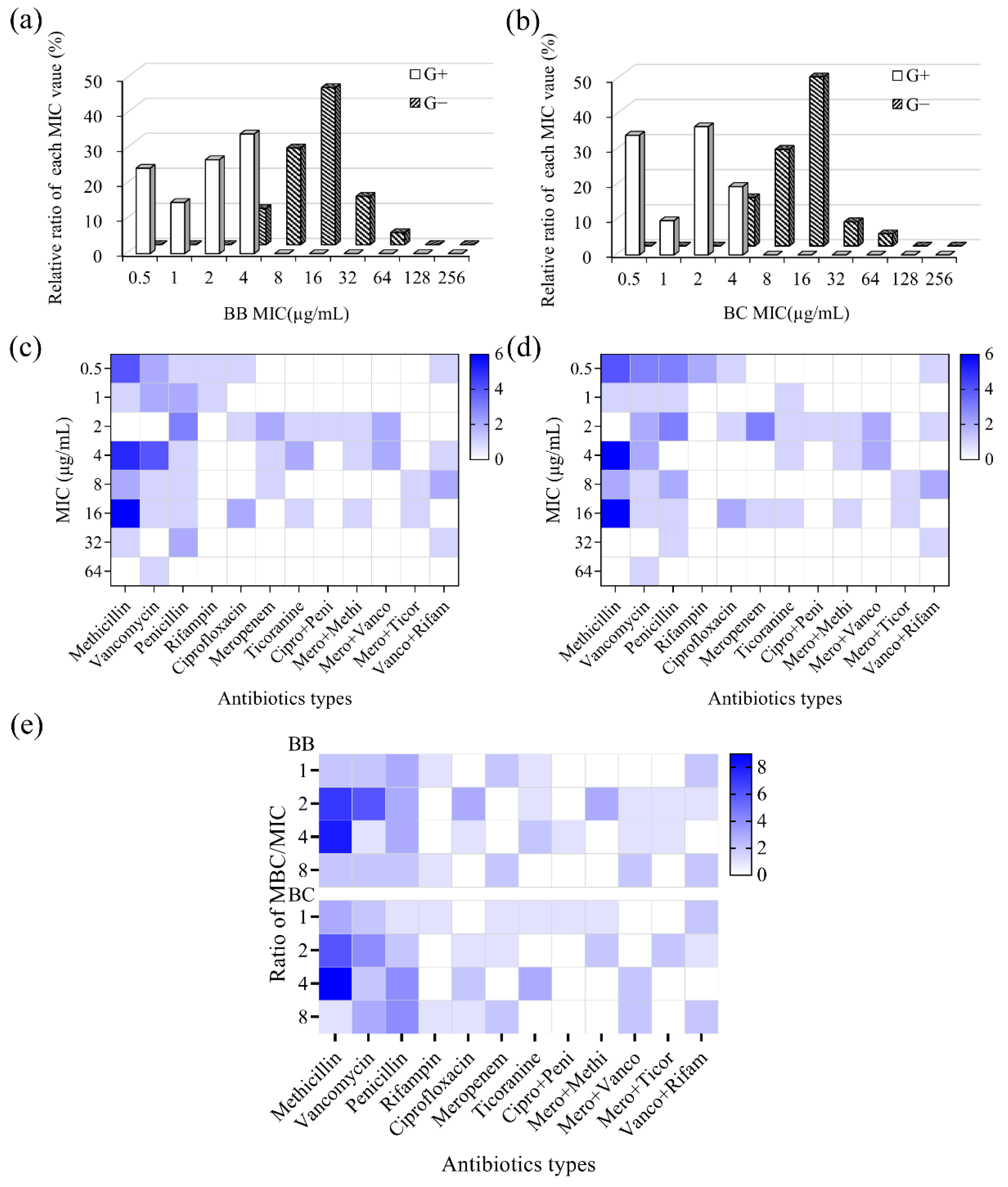
3.3.2. Antibacterial Property against Antibiotic-Resistant Bacteria
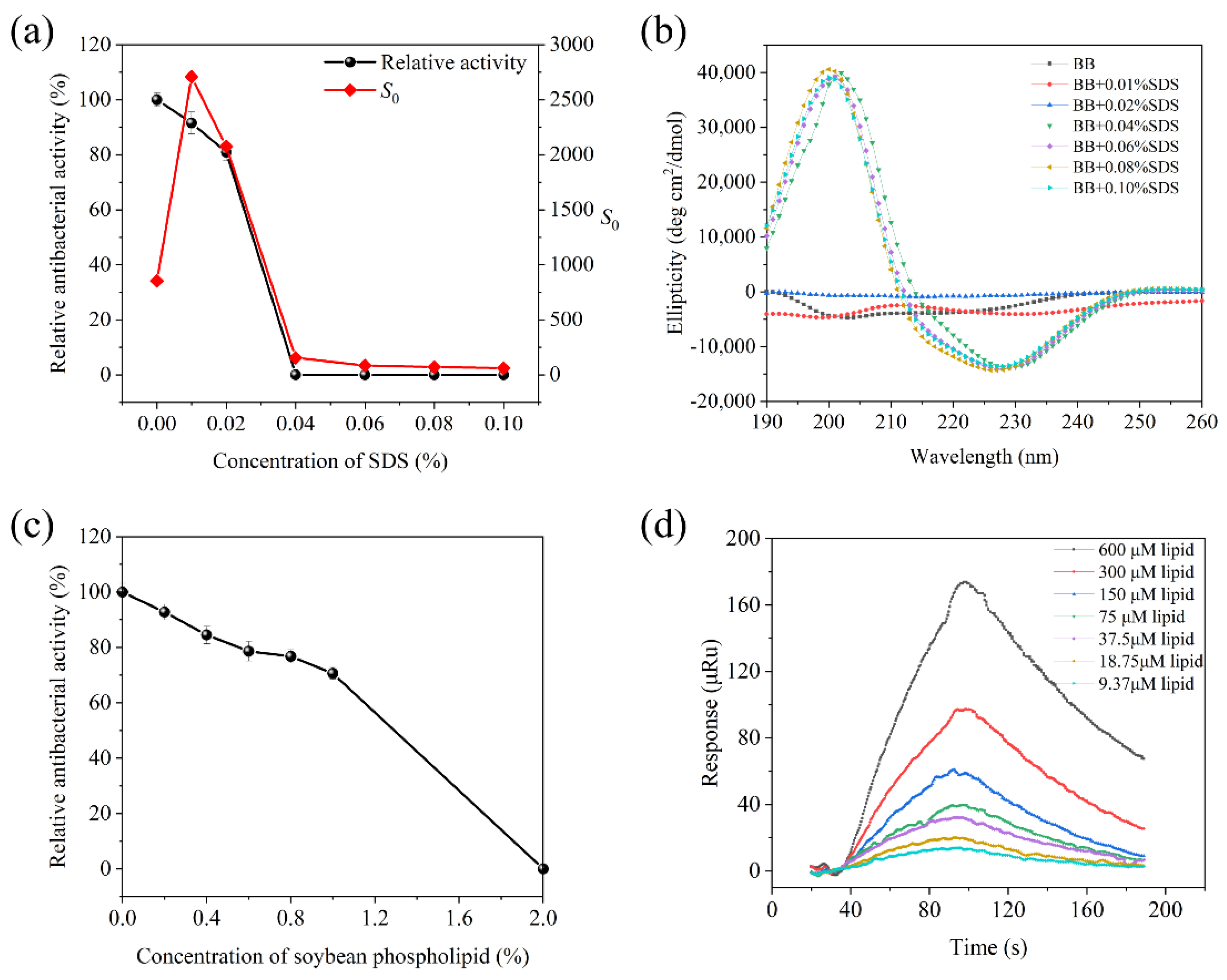
3.4. Effect of Food Compositions on Brevilaterins
3.4.1. Interaction with SDS
3.4.2. Interaction with Soybean Phospholipid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; den Besten, H.M.W.; Böhnlein, C.; Gareis, M.; Zwietering, M.H.; Fusco, V. Microbial food safety in the 21st century: Emerging challenges and foodborne pathogenic bacteria. Trends Food Sci. Technol. 2018, 81, 155–158. [Google Scholar] [CrossRef]
- Liu, X.Y.; Hu, Q.; Xu, F.; Ding, S.Y.; Zhu, K. Characterization of Bacillus cereus in dairy products in China. Toxins 2020, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Rajmohan, S.; Dodd, C.E.R.; Waites, W.M. Enzymes from isolates of Pseudomonas fluorescens involved in food spoilage. J. Appl. Microbiol. 2002, 93, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef]
- Wai, G.Y.; Tang, J.Y.H.; New, C.Y.; Son, R. A review on Listeria monocytogenes in food: Prevalence, pathogenicity, survivability and antibiotic resistance. Food Res. 2020, 4, 20–27. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhong, Z.; Zhang, W.P.; Qian, P.Y. Discovery of cationic nonribosomal peptides as Gram-negative antibiotics through global genome mining. Nat. Commun. 2018, 9, 3273. [Google Scholar] [CrossRef]
- Li, Z.; de Vries, R.H.; Chakraborty, P.; Song, C.; Zhao, X.; Scheffers, D.J.; Roelfes, G.; Kuipers, O.P. Novel modifications of nonribosomal peptides from Brevibacillus laterosporus MG64 and investigation of their mode of action. Appl. Environ. Microbiol. 2020, 86, 1–14. [Google Scholar] [CrossRef]
- Barsby, T.; Warabi, K.; Sørensen, D.; Zimmerman, W.T.; Kelly, M.T.; Andersen, R.J. The bogorol family of antibiotics: Template-based structure elucidation and a new approach to positioning enantiomeric pairs of amino acids. J. Org. Chem. 2006, 71, 6031–6037. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, X.; Shukla, R.; Kumar, R.; Weingarth, M.; Breukink, E.; Kuipers, O.P. Brevibacillin 2V, a novel antimicrobial lipopeptide with an exceptionally low hemolytic activity. Front. Microbiol. 2021, 12, 693725. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yousef, A.E. Antimicrobial peptides produced by Brevibacillus spp.: Structure, classification and bioactivity: A mini review. World J. Microbiol. Biotechnol. 2018, 34, 57. [Google Scholar] [CrossRef]
- Zhao, X.; Kuipers, O.P. Brevicidine B, a new menmber of the Brevicidine family, displays an extended target specificity. Front. Microbiol. 2021, 12, 693117. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chakraborty, P.; de Vries, R.H.; Song, C.; Zhao, X.; Roelfes, G.; Scheffers, D.J.; Kuipers, O.P. Characterization of two relacidines belonging to a novel class of circular lipopeptides that act against Gram-negative bacterial pathogens. Environ. Microbiol. 2020, 22, 5125–5136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Han, P.; Liu, Y.; Hong, D.; Li, S.; Ma, A.; Jia, Y. Discovery of novel antimicrobial peptides, Brevilaterin V, from Brevibacillus laterosporus S62-9 after regulated by exogenously-added L-valine. LWT 2022, 155, 112962. [Google Scholar] [CrossRef]
- Ning, Y.; Han, P.; Ma, J.; Liu, Y.; Fu, Y.; Wang, Z.; Jia, Y. Characterization of brevilaterins, multiple antimicrobial peptides simultaneously produced by Brevibacillus laterosporus S62-9, and their application in real food system. Food Biosci. 2020, 42, 104743. [Google Scholar] [CrossRef]
- Han, P.; Chen, Z.; Liu, Y.; Ma, A.; Li, S.; Jia, Y. Structural organization of Brevilaterin biosynthesis in Brevibacillus laterosporus S62-9: A novel MbtH-Independent cationic antimicrobial peptide synthetase system. J. Agric. Food Chem. 2022, 70, 7471–7478. [Google Scholar] [CrossRef]
- McInnes, C.; Kondejewski, L.H.; Hodges, R.S.; Sykes, B.D. Development of the structural basis for antimicrobial and hemolytic activities of peptides based on gramicidin S and design of novel analogs using NMR spectroscopy. J. Biol. Chem. 2000, 275, 14287–14294. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, L.; Zeng, H.; Yang, X.; Yuan, J.; Shi, H.; Xiong, Y.; Chen, M.; Han, L.; Qiu, D. Purification and characterization of a novel antimicrobial peptide from Brevibacillus laterosporus strain A60. Peptides 2012, 33, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, M.; Jovanovic, S.; O’Connor, P.M.; Mirkovic, N.; Jovcic, B.; Filipic, B.; Dinic, M.; Studholme, D.J.; Fira, D.; Cotter, P.D.; et al. Brevibacillus laterosporus strains BGSP7, BGSP9 and BGSP11 isolated from silage produce broad spectrum multi-antimicrobials. PLoS ONE 2019, 14, e0216773. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qin, C.; Zhang, R.; Niu, W.; Shang, X. Solid-phase synthesis and antibiotic activities of cyclodecapeptides on the scaffold of naturally occurring Laterocidin. Bioorgan. Med. Chem. Lett. 2010, 20, 164–167. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Food Additives Permitted for Direct Addition to Food for Human Consumption. Sodium Lauryl Sulfate. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-I/section-172.822 (accessed on 30 August 2022).
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; ISBN 1-56238-738-9. [Google Scholar]
- Liu, Y.; Ma, A.; Han, P.; Chen, Z.; Jia, Y. Antibacterial mechanism of brevilaterin B: An amphiphilic lipopeptide targeting the membrane of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2020, 104, 10531–10539. [Google Scholar] [CrossRef]
- Alizadeh-Pasdar, N.; Li-Chan, E.C.Y. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food Chem. 2000, 48, 328–334. [Google Scholar] [CrossRef]
- Turnidge, J.; Paterson, D.L. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 2007, 20, 391–408. [Google Scholar] [CrossRef]
- Duport, C.; Jobin, M.; Schmitt, P. Adaptation in Bacillus cereus: From stress to disease. Front. Microbiol. 2016, 7, 1550. [Google Scholar] [CrossRef]
- Yu, S.; Yu, P.; Wang, J.; Li, C.; Guo, H.; Liu, C.; Kong, L.; Yu, L.; Wu, S.; Lei, T.; et al. A study on prevalence and characterization of Bacillus cereus in ready-to-eat foods in China. Front. Microbiol. 2020, 10, 3043. [Google Scholar] [CrossRef]
- Rodrigo, D.; Rosell, C.M.; Martinez, A. Risk of bacillus cereus in relation to rice and derivatives. Foods 2021, 10, 302. [Google Scholar] [CrossRef]
- Liu, H.; Pei, H.; Han, Z.; Feng, G.; Li, D. The antimicrobial effects and synergistic antibacterial mechanism of the combination of ε-Polylysine and nisin against Bacillus subtilis. Food Control 2015, 47, 444–450. [Google Scholar] [CrossRef]
- Jangra, M.; Kaur, M.; Nandanwar, H. In-vitro studies on a natural lantibiotic, paenibacillin: A new-generation antibacterial drug candidate to overcome multi-drug resistance. Int. J. Antimicrob. Agents 2019, 53, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Beaz-Hidalgo, R.; Agüeria, D.; Latif-Eugenín, F.; Yeannes, M.I.; Figueras, M.J. Molecular characterization of Shewanella and Aeromonas isolates associated with spoilage of Common carp (Cyprinus carpio). FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Flint, S.H.; Palmer, J.S.; Gagic, D.; Fletcher, G.C.; On, S.L.W. Global expansion of Vibrio parahaemolyticus threatens the seafood industry: Perspective on controlling its biofilm formation. LWT 2022, 158, 113182. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Wang, H.; Wu, Q.; Ding, Y.; Xu, T.; Ma, G.; Zhong, Y.; Zhang, J.; Chen, M.; et al. Occurrence, molecular characterization, and antimicrobial susceptibility of Yersinia enterocolitica isolated from retail food samples in China. LWT 2021, 150, 111876. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Tahoun, A.B.M.B.; Abou Elez, R.M.M.; Abd El-Hamid, M.I.; Abd Ellatif, S.S. Prevalence of Yersinia enterocolitica in milk and dairy products and the effects of storage temperatures on survival and virulence gene expression. Int. Dairy J. 2019, 94, 16–21. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Patrzykat, A. Clinical development of cationic antimicrobial peptides: From natural to novel antibiotics. Curr. Drug Targets—Infect. Disord. 2002, 2, 79–83. [Google Scholar] [CrossRef]
- Berridge, N.J.; Newton, G.G.; Abraham, E.P. Purification and nature of the antibiotic nisin. Biochem. J. 1952, 52, 529–535. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- French, G.L. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Sader, H.S.; Fritsche, T.R.; Jones, R.N. Daptomycin bactericidal activity and correlation between disk and broth microdilution method results in testing of Staphylococcus aureus strains with decreased susceptibility to vancomycin. Antimicrob. Agents Chemother. 2006, 50, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- May, J.; Shannon, K.; King, A.; French, G. Glycopeptide tolerance in Staphylococcus aureus. J. Antimicrob. Chemother. 1998, 42, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; De Bellis, P.; Di Biase, M.; Lonigro, S.L.; Giussani, B.; Visconti, A.; Lavermicocca, P.; Sisto, A. Diversity of spore-forming bacteria and identification of Bacillus amyloliquefaciens as a species frequently associated with the ropy spoilage of bread. Int. J. Food Microbiol. 2012, 156, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhao, Y. Antimicrobial efficiency of essential oil and freeze-thaw treatments against Escherichia coli O157:H7 and Salmonella enterica Ser. enteritidis in Strawberry juice. J. Food Sci. 2009, 74, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Federico, B.; Pinto, L.; Quintieri, L.; Carito, A.; Calabrese, N.; Caputo, L. Efficacy of lactoferricin B in controlling ready-to-eat vegetable spoilage caused by Pseudomonas spp. Int. J. Food Microbiol. 2015, 215, 179–186. [Google Scholar] [CrossRef]
- Artursson, K.; Schelin, J.; Thisted Lambertz, S.; Hansson, I.; Olsson Engvall, E. Foodborne pathogens in unpasteurized milk in Sweden. Int. J. Food Microbiol. 2018, 284, 120–127. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef]
- Ning, Y.; Su, D.; Fu, Y.; Liu, Y.; Wang, Z.; Jia, Y. Antibacterial mechanism of antimicrobial peptide brevilaterin combined with citric acid against Escherichia coli. Food Sci. China 2020, 41, 31–37. [Google Scholar] [CrossRef]
- Qin, G.; Jia-hui, C.; Cheng-wan, R.; Yun-ting, L.; Muhammad, A.F.; Bin, X. A new strategy for the shelf life extension of fresh noodles by accurately targeting specific microbial species. Food Control 2022, 138, 109037. [Google Scholar]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Hansford, K.A.; Butler, M.S.; Jia, Z.; Mark, A.E.; Cooper, M.A. Developments in glycopeptide antibiotics. ACS Infect. Dis. 2018, 4, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Wolfson, J.S.; Ng, E.Y.; Swartz, M.N. Mechanisms of action of and resistance to ciprofloxacin. Am. J. Med. 1987, 82, 12–20. [Google Scholar] [PubMed]
- Campbell, E.A.; Korzheva, N.; Mustaev, A.; Murakami, K.; Nair, S.; Goldfarb, A.; Darst, S.A. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 2001, 104, 901–912. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Dekker, M.; Fogliano, V.; Heising, J. Modelling the effect of food composition on antimicrobial compound absorption and degradation in an active packaging. J. Food Eng. 2021, 300, 110539. [Google Scholar] [CrossRef]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Bezrodnykh, E.A.; Antonov, Y.A.; Berezin, B.B.; Kulikov, S.N.; Tikhonov, V.E. Molecular features of the interaction and antimicrobial activity of chitosan in a solution containing sodium dodecyl sulfate. Carbohydr. Polym. 2021, 270, 118352. [Google Scholar] [CrossRef]
- Liu, W.; Ye, A.; Liu, C.; Liu, W.; Singh, H. Structure and integrity of liposomes prepared from milk- or soybean-derived phospholipids during in vitro digestion. Food Res. Int. 2012, 48, 499–502. [Google Scholar] [CrossRef]
- Schmidtchen, A.; Pasupuleti, M.; Malmsten, M. Effect of hydrophobic modifications in antimicrobial peptides. Adv. Colloid Interface Sci. 2014, 205, 265–274. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zasloff, M. A database view of naturally occurring antimicrobial peptides: Nomenclature, classification and amino acid sequence analysis. In Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; CABI: Wallingford, UK, 2010; pp. 1–21. [Google Scholar]
- Gänzle, M.G.; Weber, S.; Hammes, W.P. Effect of ecological factors on the inhibitory spectrum and activity of bacteriocins. Int. J. Food Microbiol. 1999, 46, 207–217. [Google Scholar] [CrossRef]

| QC Strains | MIC (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Test Results | QC Range | ||||||
| BB | BC | PB | Da | Van | PB | Da | |
| S. aureus subsp. aureus ATCC 29213 | 1 | 1 | - | 1 | 1 | - | 0.12~1 |
| E. faecalis ATCC 29212 | 1 | 1 | - | 4 | 1 | - | 1~4 |
| P. aeruginosa ATCC 27853 | 32 | 32 | 2 | - | - | 0.25~2 | - |
| E. coli ATCC 25922 | 16 | 16 | 2 | - | - | 0.5~4 | - |
| Genera | Species | MIC (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Brevilaterin B | Brevilaterin C | ||||||
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | ||
| Gram-positive bacteria | |||||||
| Bacillus | Bacillus coagulans (CICC 20138) | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| Bacillus megaterium (CICC 10448) | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 1 | |
| Bacillus megaterium | 1 | 1 | 1 | 1 | 1 | 1 | |
| Bacillus cereus (ATCC 11778) | 1 | 2 | 2 | 1 | 1 | 1 | |
| Bacillus pumilus (CICC 10900) | 1 | 8 | 8 | 1 | 8 | 8 | |
| Bacillus fusiformis (CICC 20463) | 2 | 4 | 2 | 2 | 4 | 2 | |
| Bacillus subtilis (CICC 10275) | 2 | 4 | 2 | 2 | 4 | 2 | |
| Bacillus subtilis subsp. Subtilis (ATCC 6051) | 2 | 2 | 1 | 1 | 1 | 1 | |
| Staphylococcus | Staphylococcus cohnii subsp. cohnii (CICC 20742) | 1 | 2 | 2 | 1 | 2 | 2 |
| Staphylococcus aureus (CICC 10001) | 2 | 4 | 2 | 2 | 4 | 2 | |
| Staphylococcus epidermidis (CICC 10436) | 2 | 8 | 4 | 1 | 8 | 8 | |
| Staphylococcus aureus (ATCC 25923) | 1 | 4 | 4 | 1 | 2 | 2 | |
| Staphylococcus aureus (ATCC 29213) | 1 | 4 | 4 | 1 | 2 | 2 | |
| Staphylococcus aureus subsp. aureus (ATCC 6538) | 2 | 2 | 1 | 2 | 2 | 1 | |
| Methicillin-resistant and oxacillin-resistant Staphylococcus aureus (ATCC 43300) | 4 | 8 | 2 | 4 | 8 | 2 | |
| Listeria | Listeria monocytogenes (10403s) | 1 | 4 | 4 | 1 | 8 | 8 |
| Listeria monocytogenes (ATCC 19115) | 1 | 8 | 8 | 1 | 8 | 8 | |
| Enterococcus | Vancomycin resistant enterococcus (ATCC 51299) | 1 | 2 | 2 | 1 | 2 | 2 |
| Enterococcus faecalis (ATCC 29212) | 1 | 8 | 8 | 1 | 8 | 8 | |
| Enterococcus faecalis (CICC 10396) | 2 | 8 | 4 | 1 | 8 | 8 | |
| Paenibacillus | Paenibacillus polymyxa (CICC 20128) | 16 | 32 | 2 | 16 | 32 | 2 |
| Micrococcus | Micrococcus luteus (CICC 10269) | 0.5 | 1 | 2 | 0.5 | 1 | 2 |
| Streptococcus | Streptococcus gallolyticus (CICC 10203) | 1 | 4 | 4 | 1 | 4 | 4 |
| Lactococcus | Lactococcus lactis (CICC 20711) | 1 | 2 | 2 | 1 | 2 | 2 |
| Leuconosto | Leuconostoc mesenteroides (CICC 20074) | 2 | 8 | 4 | 2 | 8 | 4 |
| Lactobacillus | Lactobacillus buchneri (CICC 20015) | 1 | 2 | 2 | 1 | 2 | 2 |
| Gram-negative bacteria | |||||||
| Acinetobacter | Acinetobacter baumannii (CICC 10980) | 4 | 16 | 4 | 4 | 16 | 4 |
| Alcaligenes | Alcaligenes faecalis (CICC 10981) | 4 | 32 | 8 | 8 | 16 | 2 |
| Shewanella | Shewanella putrefaciens (CICC 22940) | 4 | 8 | 2 | 4 | 8 | 2 |
| Pseudomonas | Pseudomonas maltophilia (CICC 20702) | 16 | 32 | 2 | 8 | 16 | 2 |
| Pseudomonas aeruginosa (ATCC 9027) | 32 | 128 | 4 | 16 | 128 | 8 | |
| Pseudomonas aeruginosa (ATCC 27853) | 32 | 128 | 4 | 32 | 128 | 4 | |
| Pseudomonas fluorescens (ATCC 13525) | 128 | 256 | 2 | 128 | 256 | 2 | |
| Shigella | Shigella dysenteriae (CICC 23829) | 8 | 32 | 4 | 8 | 16 | 2 |
| Shigella flexneri (CICC 10865) | 16 | 64 | 4 | 16 | 64 | 4 | |
| Shigella sonnei (CICC 21535) | 16 | 32 | 2 | 16 | 64 | 4 | |
| Escherichia | Escherichia coli (ATCC 25922) | 8 | 16 | 2 | 8 | 32 | 4 |
| Escherichia coli (CMCC 44752) | 16 | 32 | 2 | 16 | 32 | 2 | |
| Klebsiella | Klebsiella pneumoniae (CICC 10870) | 16 | 32 | 2 | 16 | 32 | 2 |
| Cronobacter | Cronobacter sakazakii (CICC 21560) | 16 | 128 | 8 | 16 | 128 | 8 |
| Vibrio | Vibrio parahaemolyticus (CICC 21528) | 16 | 32 | 2 | 16 | 32 | 2 |
| Vibrio cholerae (CICC 23794) | 16 | 32 | 2 | 16 | 32 | 2 | |
| Citrobacter | Citrobacter freundii (CICC 10404) | 32 | 128 | 4 | 32 | 128 | 4 |
| Yersinia | Yersinia enterocolitica (CICC 21565) | 32 | 256 | 8 | 32 | 64 | 2 |
| Salmonella | Salmonella typhimurium (CICC 21484) | 32 | 128 | 4 | 16 | 32 | 2 |
| Salmonella enterica subsp. enterica serovar typhimurium (ATCC 14028) | 32 | 64 | 2 | 32 | 64 | 2 | |
| Enterobacter | Enterobacter aerogenes (CICC 10293) | 64 | 128 | 2 | 64 | 128 | 2 |
| Serratia | Serratia marcescens (CICC 10898) | NI | NI | - | NI | NI | - |
| Proteus | Proteus mirabilis (CICC 21516) | NI | NI | - | NI | NI | - |
| Proteus vulgaris (CICC 10866) | NI | NI | - | NI | NI | - | |
| Brevilaterin | Standard Indicators | MIC50 | MIC90 | MICR | MBC50 | MBC90 | MBCR |
|---|---|---|---|---|---|---|---|
| μg/mL | |||||||
| BB | Gram-positive (n = 26) | 1 | 2 | 0.5~16 | 2 | 8 | 0.5~128 |
| Gram-negative (n = 24) | 16 | >128 | 4~128 | 32 | >128 | 8~256 | |
| Total (n = 50) | 4 | 32 | 0.5~128 | 8 | 128 | 0.5~256 | |
| BC | Gram-positive (n = 26) | 1 | 2 | 0.5~16 | 4 | 8 | 0.5~32 |
| Gram-negative (n = 24) | 16 | >128 | 4~128 | 64 | >128 | 8~128 | |
| Total (n = 50) | 4 | 32 | 0.5~128 | 8 | 128 | 0.5~256 | |
| Brevilaterin | Isolation Source | MIC50 | MIC90 | MICR | MBC50 | MBC90 | MBCR |
|---|---|---|---|---|---|---|---|
| μg/mL | |||||||
| BB | Vegetables and fruits (n = 14) | 16 | 64 | 0.5~64 | 32 | 128 | 1~128 |
| Animal products (n = 19) | 32 | 64 | 0.5~>256 | 64 | 256 | 2~>256 | |
| Soybean products (n = 21) | 16 | 32 | 0.5~64 | 32 | 128 | 0.5~128 | |
| Grain products(n = 16) | 1 | 64 | 0.5~64 | 4 | 128 | 1~256 | |
| Total (n = 70) | 16 | 64 | 0.5~>256 | 32 | 128 | 0.5~>256 | |
| BC | Vegetables and fruits (n = 14) | 16 | 32 | 0.5~64 | 32 | 256 | 1~256 |
| Animal products (n = 19) | 16 | 64 | 0.5~>256 | 64 | 256 | 2~>256 | |
| Soybean products (n = 21) | 16 | 64 | 0.5~64 | 32 | 128 | 0.5~128 | |
| Grain products (n = 16) | 1 | 64 | 0.5~64 | 4 | 128 | 1~256 | |
| Total (n = 70) | 16 | 64 | 0.5~>256 | 32 | 128 | 0.5~>256 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Han, P.; Jia, Y.; Chen, Z.; Li, S.; Ma, A. Antibacterial Regularity Mining Beneath the Systematic Activity Database of Lipopeptides Brevilaterins: An Instructive Activity Handbook for Its Food Application. Foods 2022, 11, 2991. https://doi.org/10.3390/foods11192991
Liu Y, Han P, Jia Y, Chen Z, Li S, Ma A. Antibacterial Regularity Mining Beneath the Systematic Activity Database of Lipopeptides Brevilaterins: An Instructive Activity Handbook for Its Food Application. Foods. 2022; 11(19):2991. https://doi.org/10.3390/foods11192991
Chicago/Turabian StyleLiu, Yangliu, Panpan Han, Yingmin Jia, Zhou Chen, Siting Li, and Aijin Ma. 2022. "Antibacterial Regularity Mining Beneath the Systematic Activity Database of Lipopeptides Brevilaterins: An Instructive Activity Handbook for Its Food Application" Foods 11, no. 19: 2991. https://doi.org/10.3390/foods11192991





