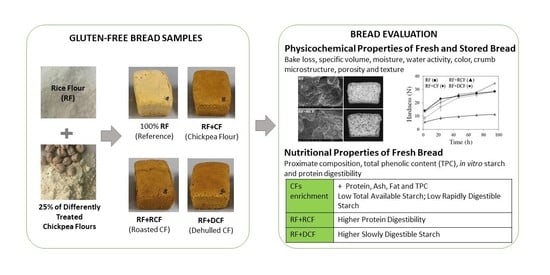
Impact of Raw, Roasted and Dehulled Chickpea Flours on Technological and Nutritional Characteristics of Gluten-Free Bread
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Flour Characterization
2.2.2. Bread Preparation
2.2.3. Technological Properties of GF Bread
2.2.4. Nutritional Properties of GF Bread
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Flour Properties
3.2. Technological Properties of Bread
3.3. Nutritional Properties of Bread
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Viti, M.; Montuori, M.; La Vecchia, A.; Cipolletta, E.; Calvani, L.; Bonamico, M. The gluten-free diet: A nutritional risk factor for adolescents with celiac disease? J. Pediatr. Gastroenterol. Nutr. 1998, 27, 519–523. [Google Scholar] [CrossRef]
- Thompson, T. Folate, iron, and dietary fiber contents of the gluten-free diet. J. Am. Diet. Assoc. 2000, 100, 1389–1396. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The Gluten-Free Diet: Safety and Nutritional Quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Foschia, M.; Horstmann, S.W.; Arendt, E.K.; Zannini, E. Legumes as functional ingredients in gluten-free bakery and pasta products. Annu. Rev. Food Sci. Technol. 2017, 8, 75–96. [Google Scholar] [CrossRef]
- FAOSTAT, Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/ (accessed on 1 June 2020).
- National Nutrient Database for Standard Reference, USDA. Available online: https://fdc.nal.usda.gov/ (accessed on 1 June 2020).
- Alajaji, A.A.; El-Adawy, T.A. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Compost. Anal. 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Köksel, H.; Sivri, D.; Scanlon, M.G.; Bushuk, W. Comparison of physical properties of raw and roasted chickpeas (leblebi). Food Res. Int. 1998, 31, 659–665. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT—Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Kahraman, G.; Harsa, S.; Lucisano, M.; Cappa, C. Physicochemical and rheological properties of rice-based gluten-free blends containing differently treated chickpea flours. LWT—Food Sci. Technol. 2018, 98, 276–282. [Google Scholar] [CrossRef]
- Mohammed, I.; Ahmed, A.R.; Senge, B. Dough rheology and bread quality of wheat–chickpea flour blends. Ind. Crop. Prod. 2012, 36, 196–202. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Calasso, M.; Campanella, D.; De Angelis, M.; Gobbetti, M. Use of sourdough fermentation and mixture of wheat, chickpea, lentil and bean flours for enhancing the nutritional, texture and sensory characteristics of white bread. Int. J. Food Microbiol. 2014, 180, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Utrilla-Coello, R.G.; Osorio-Diaz, P.; Bello-Perez, L.A. Alternative use of chickpea flour in breadmaking: Chemical composition and starch digestibility of bread. Food Sci. Technol. Int. 2007, 13, 323–327. [Google Scholar] [CrossRef]
- Yamsaengsung, R.; Schoenlechner, R.; Berghofer, E. The effects of chickpea on the functional properties of white and whole wheat bread. Int. J. Food Sci. Technol. 2010, 45, 610–620. [Google Scholar] [CrossRef]
- Zafar, T.A.; Al-Hassawi, F.; Al-Khulaifi, F.; Al-Rayyes, G.; Waslien, C.; Huffman, F.G. Organoleptic and glycemic properties of chickpea-wheat composite breads. J. Food Sci. Technol. 2015, 52, 2256–2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baik, B.-K.; Han, I.H. Cooking, roasting, and fermentation of chickpeas, lentils, peas, and soybeans for fortification of leavened bread. Cereal Chem. 2012, 89, 269–275. [Google Scholar] [CrossRef]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chickpea and tiger nut flours as alternatives to emulsifier and shortening in gluten-free bread. LWT—Food Sci. Technol. 2015, 62, 225–232. [Google Scholar] [CrossRef]
- Burešová, I.; Kráčmar, S.; Dvořáková, P.; Středa, T. The relationship between rheological characteristics of gluten-free dough and the quality of biologically leavened bread. J. Cereal Sci. 2014, 60, 271–275. [Google Scholar] [CrossRef]
- Miñarro, B.; Albanell, E.; Aguilar, N.; Guamis, B.; Capellas, M. Effect of legume flours on baking characteristics of gluten-free bread. J. Cereal Sci. 2012, 56, 476–481. [Google Scholar] [CrossRef]
- Ouazib, M.; Garzon, R.; Zaidi, F.; Rosell, C.M. Germinated, toasted and cooked chickpea as ingredients for breadmaking. J. Food Sci. Technol. 2016, 53, 2664–2672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, F.G.; Aguiar, E.V.; Rosell, C.M.; Capriles, V.D. Potential of chickpea and psyllium in gluten-free breadmaking: Assessing bread’s quality, sensory acceptability, and glycemic and satiety indexes. Food Hydrocoll. 2021, 113, 106487. [Google Scholar] [CrossRef]
- Coşkuner, Y.; Karababa, E. Leblebi: A roasted chickpea product as a traditional Turkish snack food. Food Rev. Int. 2004, 20, 257–274. [Google Scholar] [CrossRef]
- Cappa, C.; Lucisano, M.; Mariotti, M. Influence of Psyllium, sugar beet fibre and water on gluten-free dough properties and bread quality. Carbohydr. Polym. 2013, 98, 1657–1666. [Google Scholar] [CrossRef]
- Hager, A.-S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K.; Czerny, M. Investigation of product quality, sensory profile and ultrastructure of breads made from a range of commercial gluten-free flours compared to their wheat counterparts. Eur. Food Res. Technol. 2012, 235, 333–344. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, Protein (Total) in Flour; AOAC: Gaithersburg, MD, USA, 1999. [Google Scholar]
- American Association of Cereal Chemists (AACC) International. Approved Methods of Analysis, Ash-Basic Method, for Corn (Method 08-01.01), 11th ed.; AACC: St. Paul, MN, USA, 1999. [Google Scholar]
- Alves, G.H.; Ferreira, C.D.; Vivian, P.G.; Monks, J.L.F.; Elias, M.C.; Vanier, N.L.; de Oliveira, M. The revisited levels of free and bound phenolics in rice: Effects of the extraction procedure. Food Chem. 2016, 208, 116–123. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Sakač, M.; Torbica, A.; Sedej, I.; Hadnađev, M. Influence of breadmaking on antioxidant capacity of gluten free breads based on rice and buckwheat flours. Food Res. Int. 2011, 44, 2806–2813. [Google Scholar] [CrossRef]
- Englyst, K.N.; Hudson, G.J.; Englyst, H.N. Starch analysis in food. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 4246–4262. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahraman, G. Development of Gluten-Free Bread Formulations Based on Chickpea Flour: Optimization of Formulation, Evaluation of Dough Properties and Bread Quality. Ph.D. Thesis, İzmir Institute of Technology, İzmir, Turkey, 2016. [Google Scholar]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of dextran-producing Weissella cibaria on baking properties and sensory profile of gluten-free and wheat breads. Int. J. Food Microbiol. 2014, 172, 83–91. [Google Scholar] [CrossRef]
- Schober, T.J. Manufacture of Gluten-Free Specialty Breads and Confectionery Products. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 130–180. [Google Scholar]
- Gray, J.A.; Bemiller, J.N. Bread staling: Molecular basis and control. Compr. Rev. Food Sci. Food Saf. 2003, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, J.; Kaur, L.; Singh Sodhi, N.; Singh Gill, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Curti, E.; Carini, E.; Tribuzio, G.; Vittadini, E. Bread staling: Effect of gluten on physico-chemical properties and molecular mobility. LWT—Food Sci. Technol. 2014, 59, 418–425. [Google Scholar] [CrossRef]
- Kim, S.K.; D’Appolonia, B.L. Bread staling studies. I. Effect of protein content on staling rate and bread crumb pasting properties. Cereal Chem. 1977, 54, 207–215. [Google Scholar]
- Every, D.; Gerrard, J.A.; Gilpin, M.J.; Ross, M.; Newberry, M.P. Staling in starch bread: The effect of gluten additions on specific loaf volume and firming rate. Starch-Stärke 1998, 50, 443–446. [Google Scholar] [CrossRef]
- Martin, M.L.; Zeleznak, K.J.; Hoseney, R.C. A mechanism of bread firming. I. Role of starch swelling. Cereal Chem. 1991, 68, 498–503. [Google Scholar]
- Cappa, C.; Lucisano, M.; Mariotti, M. Rheological properties of gels obtained from gluten-free raw materials during a short term aging. LWT—Food Sci. Technol. 2013, 53, 464–472. [Google Scholar] [CrossRef]
- Onyango, C.; Mutungi, C.; Unbehend, G.; Lindhauer, M.G. Modification of gluten-free sorghum batter and bread using maize, potato, cassava or rice starch. LWT—Food Sci. Technol. 2011, 44, 681–686. [Google Scholar] [CrossRef]
- Wójcik, M.; Różyło, R.; Schönlechner, R.; Berger, M.V. Physicochemical properties of an innovative gluten-free, low-carbohydrate and high protein-bread enriched with pea protein powder. Sci. Rep. 2021, 11, 14498. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Kaur, A.; Kumar, S.; Singh, N. Cowpea protein isolates: Functional properties and application in gluten-free rice muffins. LWT—Food Sci. Technol. 2015, 63, 927–933. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2016, 38 Pt B, 602–611. [Google Scholar] [CrossRef]
- Fares, C.; Menga, V. Effects of toasting on the carbohydrate profile and antioxidant properties of chickpea (Cicer arietinum L.) flour added to durum wheat pasta. Food Chem. 2012, 131, 1140–1148. [Google Scholar]
- Szawara-Nowak, D.; Koutsidis, G.; Wiczkowski, W.; Zieliński, H. Evaluation of the in vitro inhibitory effects of buckwheat enhanced wheat bread extracts on the formation of advanced glycation end-products (AGEs). LWT—Food Sci. Technol. 2014, 58, 327–334. [Google Scholar] [CrossRef]
- Gularte, M.A.; Gómez, M.; Rosell, C.M. Impact of legume flours on quality and in vitro digestibility of starch and protein from gluten-free cakes. Food Bioprocess Technol. 2012, 5, 3142–3150. [Google Scholar] [CrossRef] [Green Version]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, X.; Wang, P.; Jiang, H.; Li, W. Compositional, morphological, and physicochemical properties of starches from red adzuki bean, chickpea, faba bean, and baiyue bean grown in China. Food Sci. Nutr. 2019, 7, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, K.S.; Lim, S.-T. Digestibility of legume starches as influenced by their physical and structural properties. Carbohydr. Polym. 2008, 71, 245–252. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33–S50. [Google Scholar]
- Venn, B.J.; Green, T.J. Glycemic index and glycemic load: Measurement issues and their effect on diet-disease relationships. Eur. J. Clin. Nutr. 2007, 61 (Suppl. S1), S122–S131. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. Carbohydrates in Human Nutrition, FAO Food and Nutrition Paper 66. Available online: www.fao.org/docrep/w8079e/w8079e00.htm (accessed on 15 November 2016).
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to “slowly digestible starch in starch-containing foods” and “reduction of post- prandial glycaemic responses” pursuant to Article 13(5) of Regulation (EC) No 1924/20061. EFSA J. 2011, 9, 2292. [Google Scholar]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Arendt, E.K. In vitro starch digestibility and predicted glycaemic indexes of buckwheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. J. Cereal Sci. 2013, 58, 431–436. [Google Scholar] [CrossRef]
- Garsetti, M.; Vinoy, S.; Lang, V.; Holt, S.; Loyer, S.; Brand-Miller, J.C. The glycemic and insulinemic index of plain sweet biscuits: Relationships to in vitro starch digestibility. J. Am. Coll. Nutr. 2005, 24, 441–447. [Google Scholar] [CrossRef]
- de La Hera, E.; Rosell, C.M.; Gomez, M. Effect of water content and flour particle size on gluten-free bread quality and digestibility. Food Chem. 2014, 151, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Tavano, O.L.; Neves, V.A.; da Silva Júnior, S.I. In vitro versus in vivo protein digestibility techniques for calculating PDCAAS (protein digestibility-corrected amino acid score) applied to chickpea fractions. Food Res. Int. 2016, 89, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Coda, R.; Melama, L.; Rizzello, C. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.P.P.; Queiroz, J.H.; Oliveira, M.G.A.; Piovesan, N.D.; Peluzio, M.C.G.; Costa, N.M.B.; Moreira, M.A. Effects of heating on protein quality of soybean flour devoid of Kunitz inhibitor and lectin. Food Chem. 2008, 107, 649–655. [Google Scholar] [CrossRef]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duodu, K.G.; Taylor, J.R.N.; Belton, P.S.; Hamaker, B.R. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Rachman, A.; Brennan, M.A.; Morton, J.; Brennan, C.S. Effect of Egg White Protein and Soy Protein Isolate Addition on Nutritional Properties and In-Vitro Digestibility of Gluten-Free Pasta Based on Banana Flour. Foods 2020, 9, 589. [Google Scholar] [CrossRef] [PubMed]



| Sample | Bake Loss (%) | Height Max (cm) | Specific Volume (cm3/g) | Moisture (g/100 g) | Crumb Color | Crust Color | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |||||
| RF | 19.62 ± 0.47 a | 4.69 ± 0.23 ab | 2.63 ± 0.11 ab | 42.92 ± 0.96 a | 75.07 ± 0.05 a | -2.21 ± 0.19 a | 7.75 ± 0.07 d | 72.48 ± 1.08 a | -0.50 ± 0.17 d | 24.55 ± 0.35 b |
| RF + CF | 20.44 ± 0.18 a | 4.34 ± 0.03 b | 2.51 ± 0.11 b | 43.01 ± 0.48 a | 75.47 ± 0.39 a | -3.13 ± 0.21 b | 16.47 ± 0.22 b | 52.90 ± 0.39 b | 8.58 ± 0.08 b | 32.86 ± 0.53 a |
| RF + RCF | 20.43 ± 0.74 a | 5.00 ± 0.07 a | 2.89 ± 0.03 a | 42.97 ± 0.09 a | 70.56 ± 1.16 b | -2.02 ± 0.04 a | 20.59 ± 1.00 a | 56.02 ± 0.53 b | 7.14 ± 0.20 c | 33.49 ± 0.17 a |
| RF + DCF | 20.77 ± 0.57 a | 4.66 ± 0.04 ab | 2.75 ± 0.04 ab | 40.15 ± 0.05 b | 75.09 ± 0.02 a | -2.71 ± 0.22 ab | 13.42 ± 0.09 c | 48.96 ± 1.14 c | 9.80 ± 0.03 a | 31.30 ± 1.08 a |
| Sample | Crumb Porosity (%) | Cell Dimension | |||||
|---|---|---|---|---|---|---|---|
| 0.1–0.2 (mm2) | 0.2–0.5 (mm2) | 0.5–1 (mm2) | 1–5 (mm2) | 5–10 (mm2) | >10 (mm2) | ||
| RF | 45.36 ± 2.87 ab | 18.03 ± 0.44 b | 18.84 ± 3.90 a | 15.37 ± 1.52 a | 33.80 ± 2.63 a | 8.21 ± 1.34 a | 5.44 ± 1.46 a |
| RF + CF | 41.49 ± 0.11 b | 23.02 ± 0.21 a | 21.69 ± 0.91 a | 17.17 ± 1.17 a | 27.92 ± 0.67 b | 4.76 ± 0.05 b | 5.43 ± 1.15 a |
| RF + RCF | 51.41 ± 2.30 a | 23.16 ± 0.07 a | 22.23 ± 1.42 a | 16.70 ± 0.61 a | 25.81 ± 0.11 b | 5.33 ± 0.20 b | 6.77 ± 1.66 a |
| RF + DCF | 41.84 ± 1.25 b | 21.99 ± 0.41 a | 23.04 ± 1.04 a | 17.21 ± 0.42 a | 29.07 ± 0.15 ab | 5.29 ± 0.01 b | 3.40 ± 0.34 a |
| Bread | Protein (g/100 g dm) | Fat (g/100 g dm) | Ash (g/100 g dm) | TPC (mg GAE/100 g dm) | RDS (g/100 g dm) | SDS (g/100 g dm) | Total Available Starch (g/100 g dm) | %RDS (g/100 g Available Starch) | %SDS (g/100 g Available Starch) | IVPD (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| RF | 9.72 ± 0.08 b | 1.61 ± 0.38 c | 2.01 ± 0.03 b | 49.36 ± 2.47 c | 81.04 ± 0.31 a | 5.90 ± 0.62 bc | 86.94 ± 0.60 a | 93.2 ± 0.7 a | 6.8 ± 0.7 c | 83.07 ± 0.64 b |
| RF + CF | 13.02 ± 0.14 a | 4.78 ± 0.10 b | 2.78 ± 0.09 a | 65.29 ± 2.25 b | 68.81 ± 0.96 b | 6.98 ± 0.49 b | 75.79 ± 0.90 c | 90.8 ± 0.7 b | 9.2 ± 0.7 b | 84.25 ± 0.04 ab |
| RF + RCF | 13.21 ± 0.03 a | 4.58 ± 0.07 b | 2.45 ± 0.01 ab | 80.52 ± 5.13 a | 69.87 ± 0.61 b | 5.33 ± 0.60 c | 75.20 ± 0.59 c | 92.9 ± 0.8 ab | 7.1 ± 0.8 c | 85.57 ± 0.52 a |
| RF + DCF | 12.03 ± 0.71 a | 5.86 ± 0.16 a | 2.63 ± 0.21 a | 71.87 ± 2.05 ab | 67.29 ± 1.63 b | 11.14 ± 0.43 a | 78.43 ± 1.29 b | 85.8 ± 0.7 c | 14.2 ± 0.7 a | 83.58 ± 0.29 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahraman, G.; Harsa, S.; Casiraghi, M.C.; Lucisano, M.; Cappa, C. Impact of Raw, Roasted and Dehulled Chickpea Flours on Technological and Nutritional Characteristics of Gluten-Free Bread. Foods 2022, 11, 199. https://doi.org/10.3390/foods11020199
Kahraman G, Harsa S, Casiraghi MC, Lucisano M, Cappa C. Impact of Raw, Roasted and Dehulled Chickpea Flours on Technological and Nutritional Characteristics of Gluten-Free Bread. Foods. 2022; 11(2):199. https://doi.org/10.3390/foods11020199
Chicago/Turabian StyleKahraman, Gokcen, Sebnem Harsa, Maria Cristina Casiraghi, Mara Lucisano, and Carola Cappa. 2022. "Impact of Raw, Roasted and Dehulled Chickpea Flours on Technological and Nutritional Characteristics of Gluten-Free Bread" Foods 11, no. 2: 199. https://doi.org/10.3390/foods11020199
APA StyleKahraman, G., Harsa, S., Casiraghi, M. C., Lucisano, M., & Cappa, C. (2022). Impact of Raw, Roasted and Dehulled Chickpea Flours on Technological and Nutritional Characteristics of Gluten-Free Bread. Foods, 11(2), 199. https://doi.org/10.3390/foods11020199