Clove Oil-Nanostructured Lipid Carriers: A Platform of Herbal Anesthetics in Whiteleg Shrimp (Penaeus vannamei)
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Essential Clove Oil and Chemicals
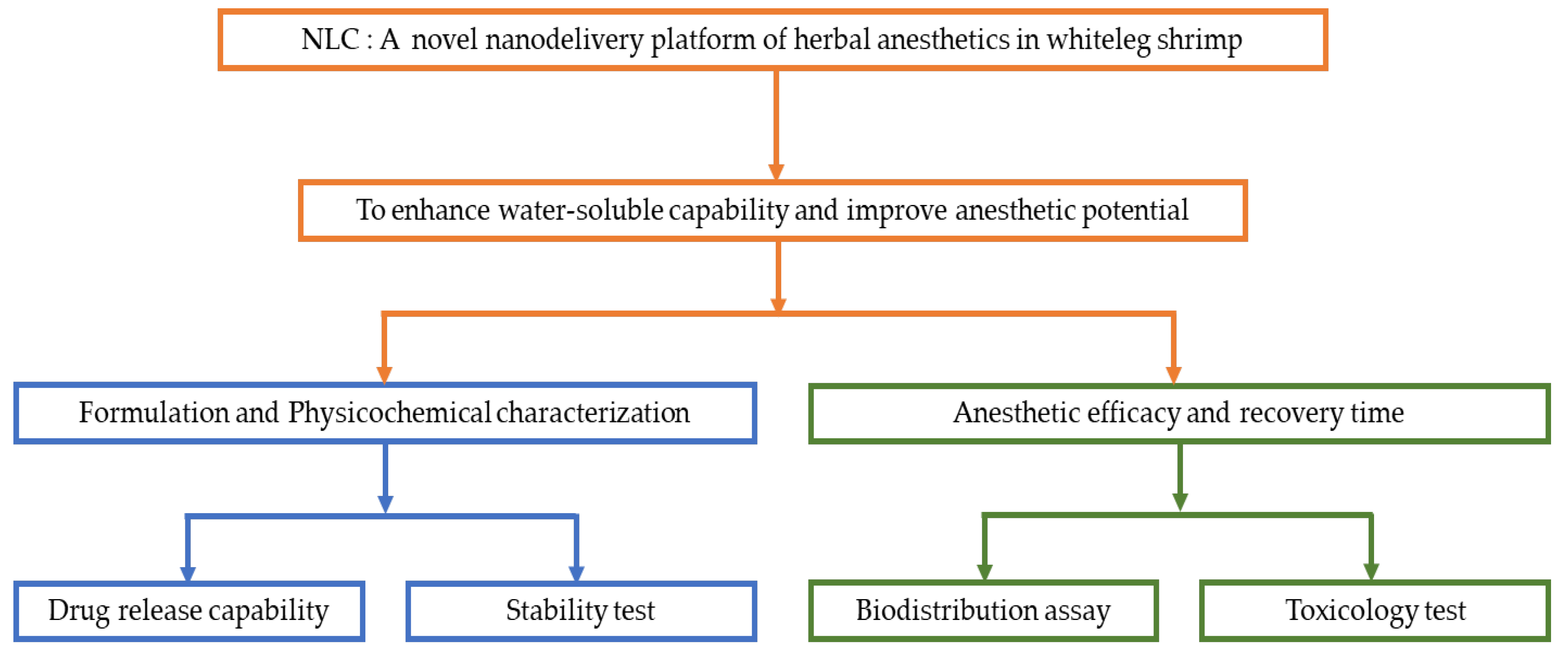
2.2. Schematic Overview of the Experimental Program
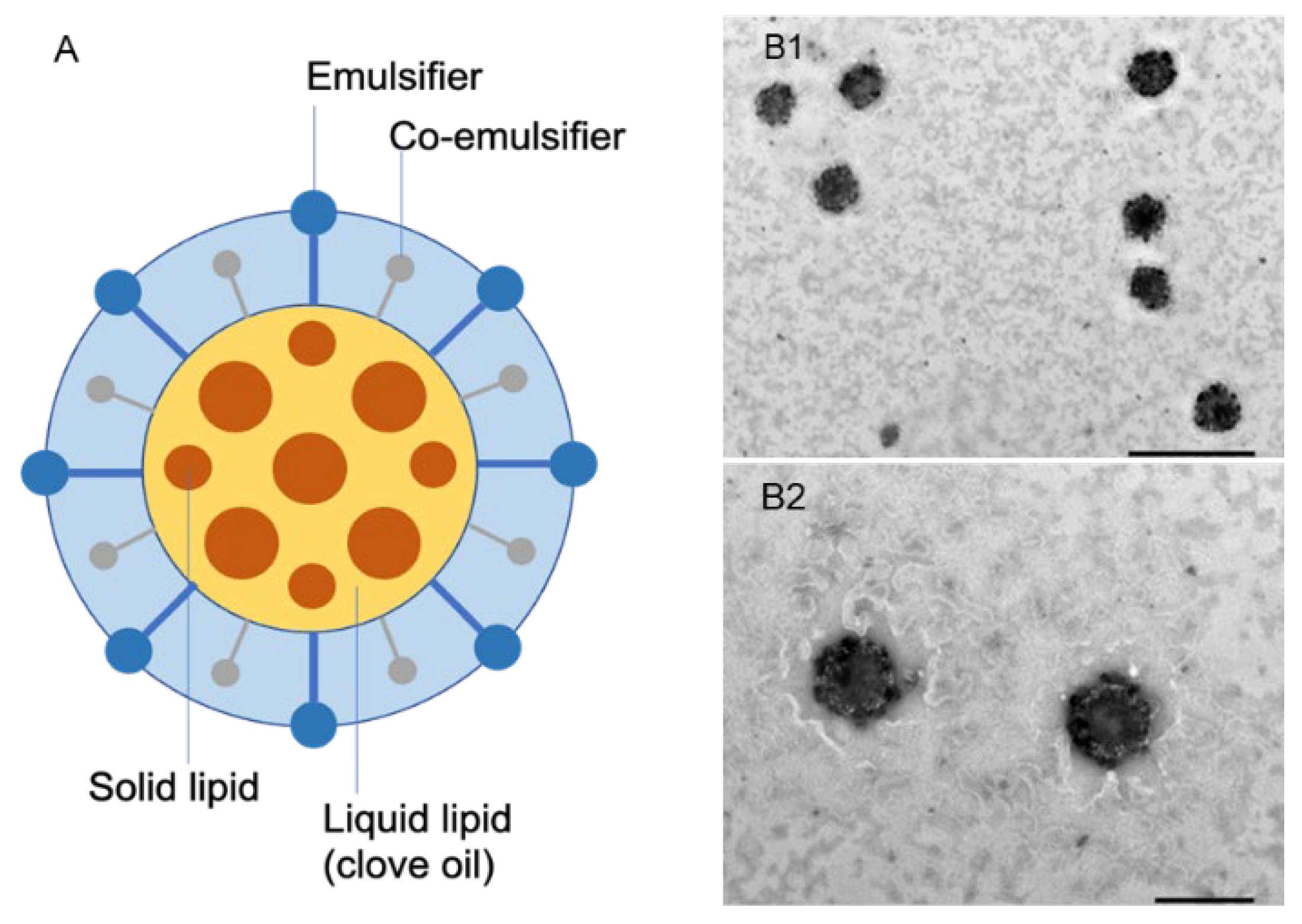
2.3. Preparation of CO-NLCs
2.4. Physicochemical Characterization of Clove Oil NLCs
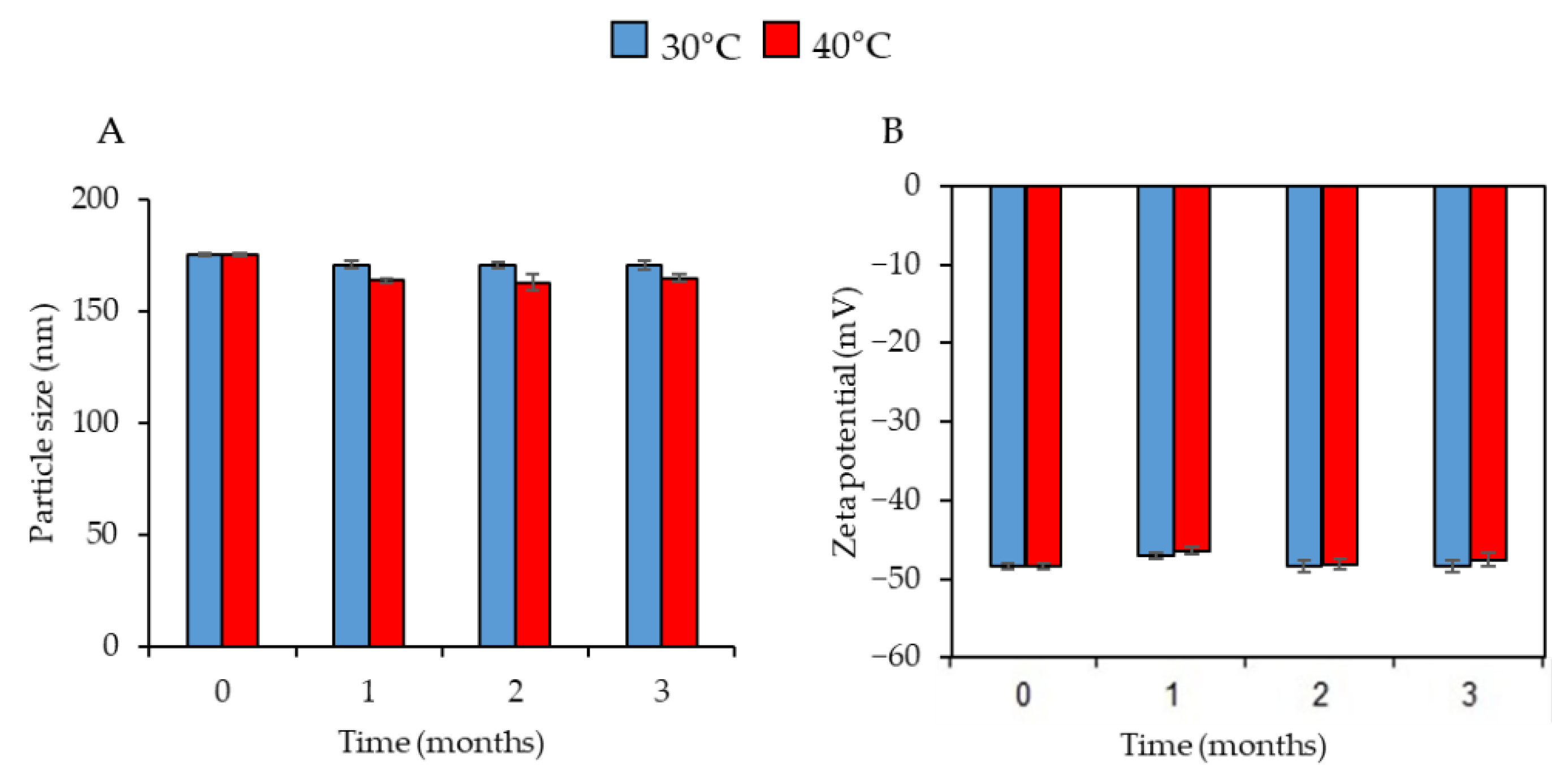
2.5. Physical Stability Study
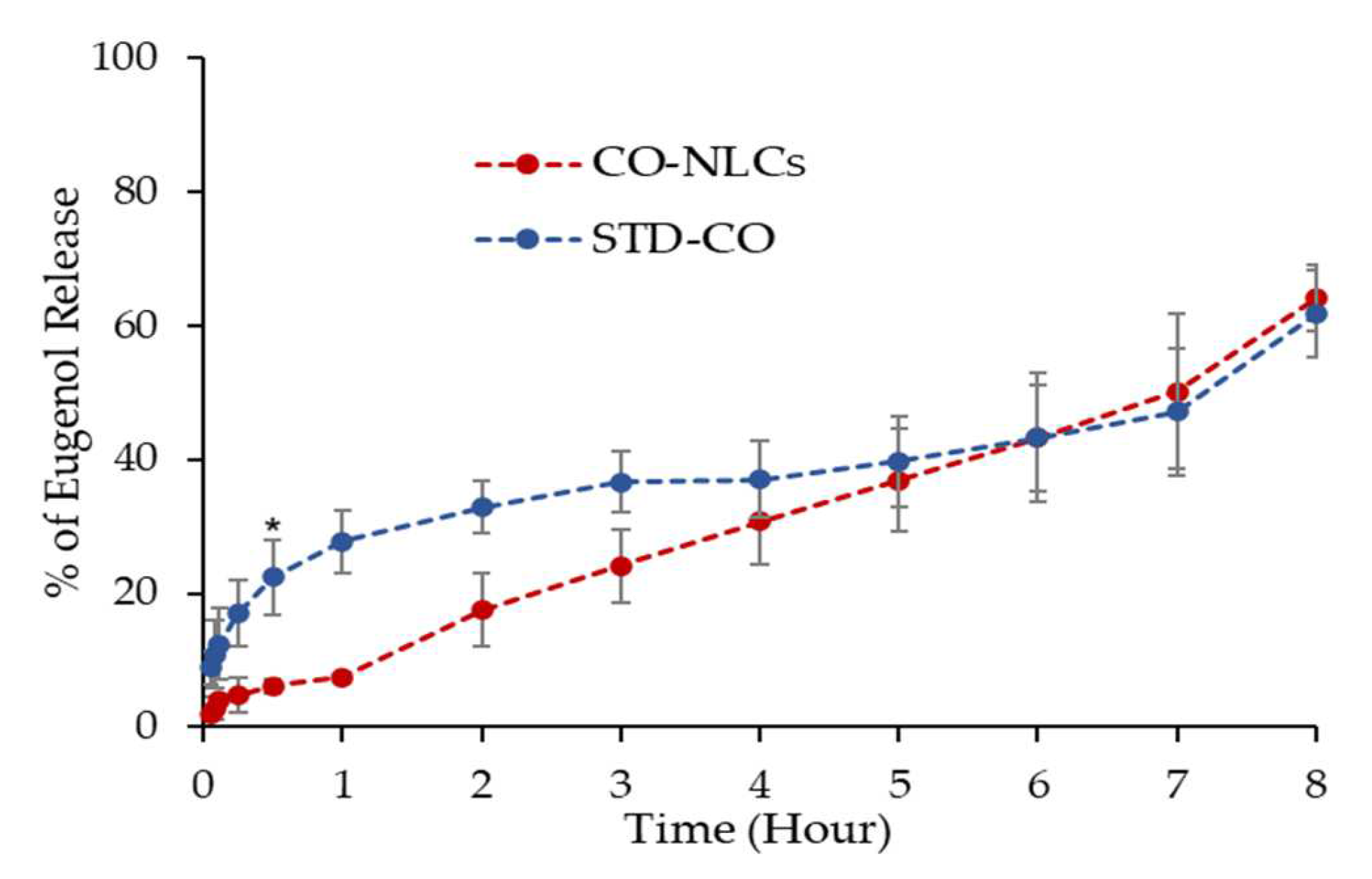
2.6. In Vitro Drug Release Study
2.7. Culture Environment
2.8. Anesthetic Concentration and Recovery Time
2.9. Acute Toxicity Study of Clove Oil NLC in Shrimp
2.10. Biodistribution Study of Clove Oil on Shrimps
2.11. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of CO-NLCs
3.2. In Vitro Drug Release
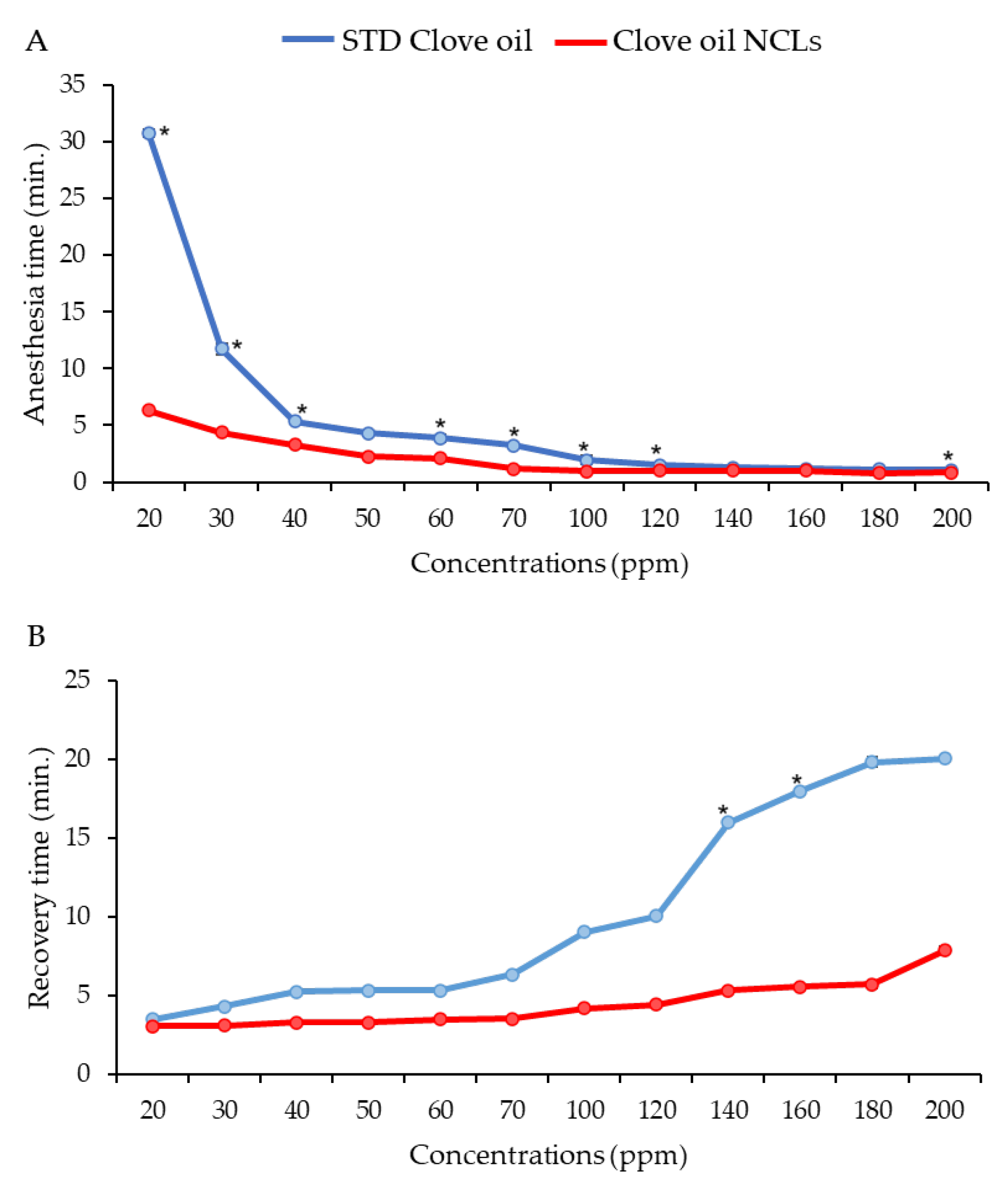
3.3. Effective Concentration for Induced Anesthesia
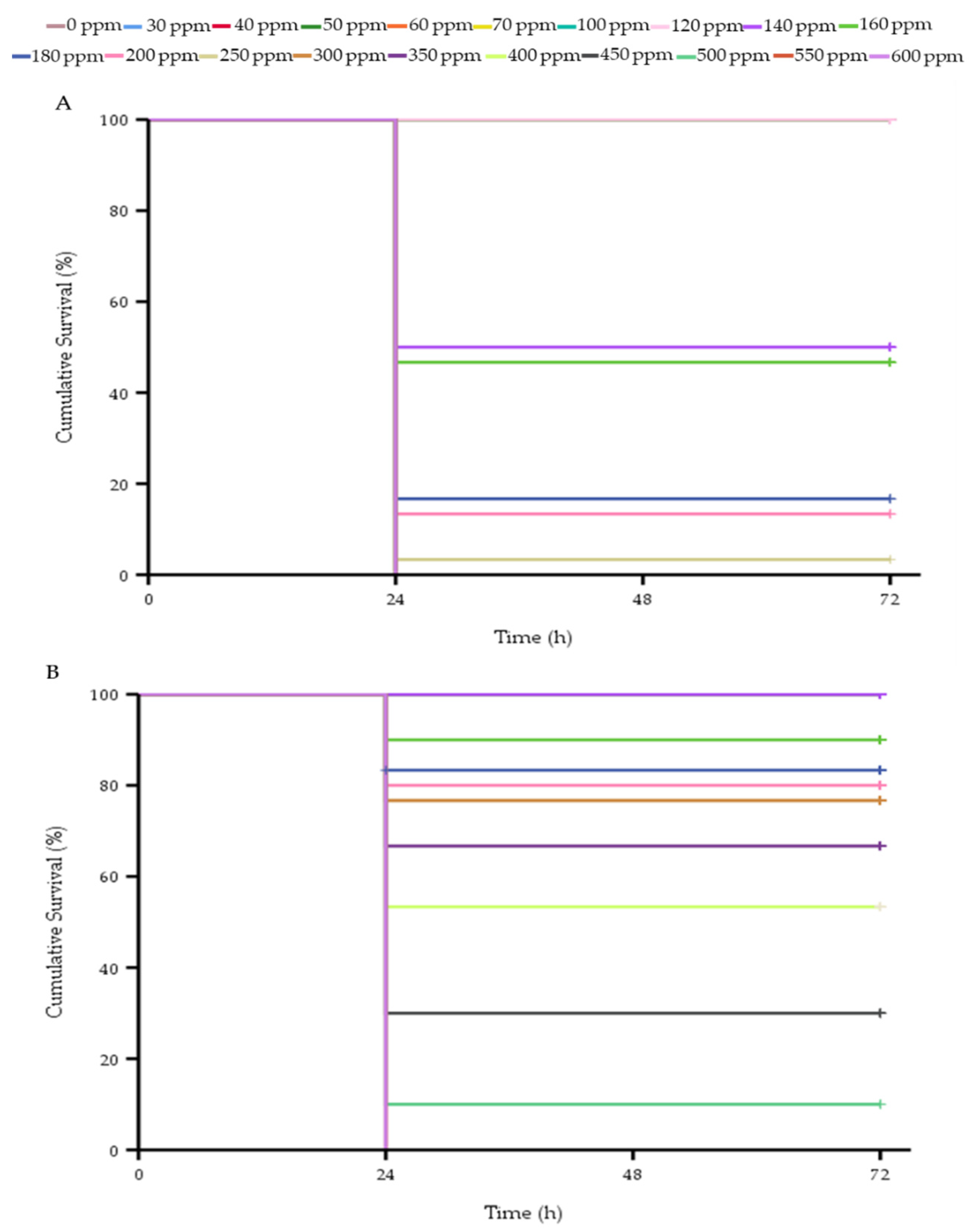
3.4. Toxicity of Clove Oil in Whiteleg Shrimp
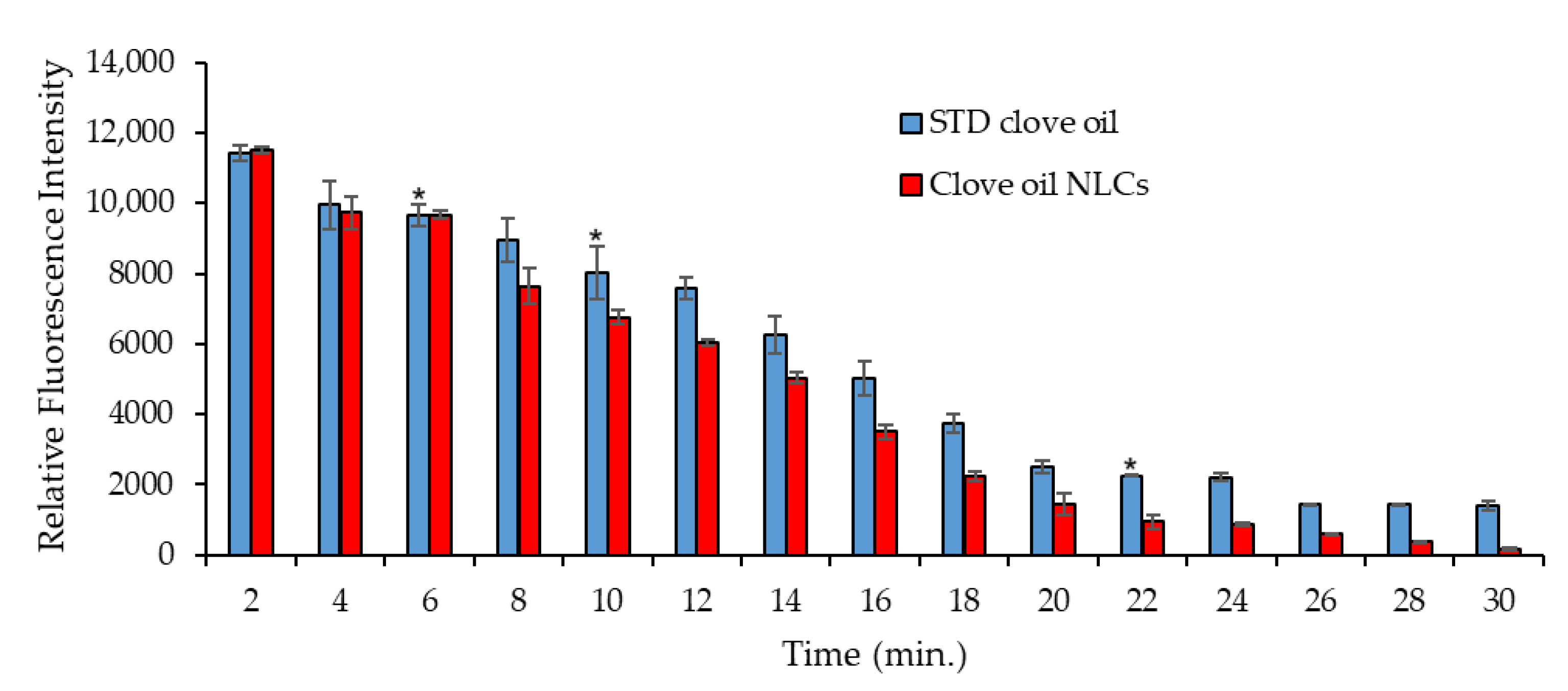
3.5. Biodistribution Study of Clove Oil in Shrimps
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Xie, S.; Wei, D.; Fang, W.; Wan, M.; Guo, T.; Liu, Y.; Yin, P.; Tian, L.; Niu, J. Optimal dietary lipid requirement of postlarval white shrimp, Litopenaeus vannamei in relation to growth performance, stress tolerance and immune response. Aquac. Nutr. 2019, 25, 1231–1240. [Google Scholar] [CrossRef]
- Mercier, L.; Palacios, E.; Campa-Córdova, Á.I.; Tovar-Ramírez, D.; Hernández-Herrera, R.; Racotta, I.S. Metabolic and immune responses in Pacific whiteleg shrimp Litopenaeus vannamei exposed to a repeated handling stress. Aquaculture 2006, 258, 633–640. [Google Scholar] [CrossRef]
- Le Moullac, G.; Soyez, C.; Saulnier, D.; Ansquer, D.; Avarre, J.C.; Levy, P. Effect of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shellfish. Immunol. 1998, 8, 621–629. [Google Scholar] [CrossRef]
- Kamble, M.T.; Rudtanatip, T.; Soowannayan, C.; Nambunruang, B.; Medhe, S.V.; Wongprasert, K. Depolymerized Fractions of Sulfated Galactans Extracted from Gracilaria fisheri and Their Antibacterial Activity against Vibrio parahaemolyticus and Vibrio harveyi. Mar. Drugs 2022, 20, 469. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.D.; Dasgupta, S.; Tidwell, J.H.; Beavers, T.; Bright, L.A.; Yasharian, D.K. Comparative Efficacy of Anesthetics for the Freshwater Prawn Macrobrachiurn rosenbergii. J. World Aquac. Soc. 2005, 36, 282–290. [Google Scholar] [CrossRef]
- Fotedar, S.; Evans, L. Health management during handling and live transport of crustaceans: A review. J. Invertebr. Pathol. 2011, 106, 143–152. [Google Scholar] [CrossRef]
- Akbari, S.; Khoshnod, M.J.; Rajaian, H.; Afsharnasab, M. The use of eugenol as an anesthetic in transportation of with Indian shrimp (Fenneropenaeus indicus) post larvae. Turk. J. Fish. Aquat. Sci. 2010, 10, 423–429. [Google Scholar] [CrossRef]
- Fanni, N.; Shaleh, F.; Santanumurti, M. The role of clove (Sygnium aromaticum) oil as anaesthetics compound for abalone (Haliotis squamata). Iraqi J. Vet. Sci. 2021, 35, 335–342. [Google Scholar] [CrossRef]
- Aydın, B.; Barbas, L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: A review. Aquaculture 2020, 520, 734999. [Google Scholar] [CrossRef]
- Mazandarani, M.; Hoseini, S.M. Anesthesia of juvenile Persian sturgeon, Acipenser persicus; Borodin 1897, by peppermint, Mentha piperita, extract–Anesthetic efficacy, stress response and behavior. Int. J. Aquat. Biol. 2017, 5, 393–400. [Google Scholar]
- Roohi, Z.; Imanpoor, M.R. The efficacy of the oils of spearmint and methyl salicylate as new anesthetics and their effect on glucose levels in common carp (Cyprinus carpio L., 1758) juveniles. Aquaculture 2015, 437, 327–332. [Google Scholar] [CrossRef]
- Bodur, T.; Afonso, J.M.; Montero, D.; Navarro, A. Assessment of effective dose of new herbal anesthetics in two marine aquaculture species: Dicentrarchus labrax and Argyrosomus regius. Aquaculture 2018, 482, 78–82. [Google Scholar] [CrossRef]
- Soltani, M.; Marmari, G.; Mehrabi, M. Acute toxicity and anesthetic effects of clove oil in Penaeus semisulcatus under various water quality conditions. Aquac. Int. 2004, 12, 457–466. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Nodeh, A.J. Changes in blood biochemistry of common carp Cyprinus carpio (Linnaeus), following exposure to different concentrations of clove solution. Comp. Clin. Pathol. 2013, 22, 9–13. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Taheri Mirghaed, A.; Yousefi, M. Application of herbal anaesthetics in aquaculture. Rev. Aquac. 2019, 11, 550–564. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus synthetic anesthetic for transport of live fish: A review. Aquac. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Fernandes, I.M.; Bastos, Y.F.; Barreto, D.S.; Lourenco, L.S.; Penha, J.M. The efficacy of clove oil as an anaesthetic and in euthanasia procedure for small-sized tropical fishes. Braz. J. Biol. 2017, 77, 444–450. [Google Scholar] [CrossRef]
- Parodi, T.V.; Cunha, M.A.; Heldwein, C.G.; de Souza, D.M.; Martins, Á.C.; Garcia, L.d.O.; Junior, W.W.; Monserrat, J.M.; Schmidt, D.; Caron, B.O. The anesthetic efficacy of eugenol and the essential oils of Lippia alba and Aloysia triphylla in post-larvae and sub-adults of Litopenaeus vannamei (Crustacea, Penaeidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Saydmohammed, M.; Pal, A.K. Anesthetic effect of eugenol and menthol on handling stress in Macrobrachium rosenbergii. Aquaculture 2009, 298, 162–167. [Google Scholar] [CrossRef]
- Li, Y.; Liang, S.; She, Q.; Han, Z.; Li, Y.; Li, X. Influence of temperature and size on menthol anaesthesia in Chinese grass shrimp Palaemonetes sinensis (Sollaud, 1911). Aquac. Res. 2018, 49, 2091–2098. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, F.; Yang, W.; Wu, Z.; Le, Y.; Yang, Q.; Yu, Y.; Jiang, S. Anaesthetic effect of eugenol at different concentrations and temperatures on black tiger shrimp (Penaeus monodon). Aquac. Res. 2020, 51, 3268–3273. [Google Scholar] [CrossRef]
- Gholipourkanani, H.; Gholinasab-Omran, I.; Ebrahimi, P.; Jafaryan, H. Anesthetic effect of clove oil loaded on lecithin based nano emulsions in gold fish, Carassius auratus. J. Fish. Aquat. Sci. 2015, 10, 553. [Google Scholar]
- Ross, L.G.; Ross, B. Anaesthetic and Sedative Techniques for Aquatic Animals, 3rd ed.; Wiley-Blackwell: New York, NY, USA, 2009. [Google Scholar]
- Yostawonkul, J.; Kitiyodom, S.; Kaewmalun, S.; Suktham, K.; Nittayasut, N.; Khongkow, M.; Namdee, K.; Ruktanonchai, U.R.; Rodkhum, C.; Pirarat, N.; et al. Bifunctional clove oil nanoparticles for anesthesia and anti-bacterial activity in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 503, 589–595. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Vieira, R.; Severino, P.; Nalone, L.A.; Souto, S.B.; Silva, A.M.; Lucarini, M.; Durazzo, A.; Santini, A.; Souto, E.B. Sucupira oil-loaded nanostructured lipid carriers (NLC): Lipid screening, factorial design, release profile, and cytotoxicity. Molecules 2020, 25, 685. [Google Scholar] [CrossRef]
- Sreedhar, R.; Kumar, V.S.; Pillai, A.K.B.; Mangalathillam, S. Omega-3 fatty acid based nanolipid formulation of atorvastatin for treating hyperlipidemia. Adv. Pharm. Bull. 2019, 9, 271. [Google Scholar] [CrossRef]
- Cansian, R.; Vanin, A.; Orlando, T.; Piazza, S.; Puton, B.; Cardoso, R.; Gonçalves, I.; Honaiser, T.; Paroul, N.; Oliveira, D. Toxicity of clove essential oil and its ester eugenyl acetate against Artemia salina. Braz. J. Biol. 2016, 77, 155–161. [Google Scholar] [CrossRef]
- Kheawfu, K.; Pikulkaew, S.; Wellendorph, P.; Jørgensen, L.v.G.; Rades, T.; Müllertz, A.; Okonogi, S. Elucidating Pathway and Anesthetic Mechanism of Action of Clove Oil Nanoformulations in Fish. Pharmaceutics 2022, 14, 919. [Google Scholar] [CrossRef]
- Schmit, O.; Mezquita, F. Experimental test on the use of MS-222 for ostracod anaesthesia: Concentration, immersion period and recovery time. J. Limnol. 2010, 69, 350. [Google Scholar] [CrossRef]
- Barrento, S.; Marques, A.; Vaz-Pires, P.; Nunes, M.L. Cancer pagurus (Linnaeus, 1758) physiological responses to simulated live transport: Influence of temperature, air exposure and AQUI-S®. J. Therm. Biol. 2011, 36, 128–137. [Google Scholar] [CrossRef]
- Tan, N.; Gao, Y.; Wang, Y.; Deng, S.; Yuan, P.; Jiang, T.; Zheng, W. The Influence of Hypothermia Hibernation Combined with CO2 Anesthesia on Life and Storage Quality of Large Yellow Croaker (Pseudosciaena crocea). Foods 2022, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Reed, C.J.; Rostron, C. Prospects of anionic nanolipoplexes in nanotherapy: Transmission electron microscopy and light scattering studies. Micron 2007, 38, 787–795. [Google Scholar] [CrossRef]
- Negussie, A.H.; Yarmolenko, P.S.; Partanen, A.; Ranjan, A.; Jacobs, G.; Woods, D.; Bryant, H.; Thomasson, D.; Dewhirst, M.W.; Wood, B.J. Formulation and characterisation of magnetic resonance imageable thermally sensitive liposomes for use with magnetic resonance-guided high intensity focused ultrasound. Int. J. Hyperth. 2011, 27, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M. Nanoliposomes: Preparation and analysis. In Liposomes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 29–50. [Google Scholar]
- Shakeel, F.; Alam, P.; Ali, A.; Alqarni, M.H.; Alshetaili, A.; Ghoneim, M.M.; Alshehri, S.; Ali, A. Investigating antiarthritic potential of nanostructured clove oil (Syzygium aromaticum) in FCA-induced arthritic rats: Pharmaceutical action and delivery strategies. Molecules 2021, 26, 7327. [Google Scholar] [CrossRef]
- Qadir, A.; Faiyazuddin, M.; Hussain, M.T.; Alshammari, T.M.; Shakeel, F. Critical steps and energetics involved in a successful development of a stable nanoemulsion. J. Mol. Liq. 2016, 214, 7–18. [Google Scholar] [CrossRef]
- Qureshi, M.J.; Mallikarjun, C.; Kian, W.G. Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets: A study in diet induced hyperlipidemic rabbits. Asian J. Pharm. Sci. 2015, 10, 40–56. [Google Scholar] [CrossRef]
- Rao, P.J.; Naidu, M.M. Nanoencapsulation of bioactive compounds for nutraceutical food. In Nanoscience in Food and Agriculture 2; Springer: Berlin/Heidelberg, Germany, 2016; pp. 129–156. [Google Scholar]
- Han, F.; Li, S.; Yin, R.; Liu, H.; Xu, L. Effect of surfactants on the formation and characterization of a new type of colloidal drug delivery system: Nanostructured lipid carriers. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 210–216. [Google Scholar] [CrossRef]
- Keck, C.M.; Kovačević, A.; Müller, R.H.; Savić, S.; Vuleta, G.; Milić, J. Formulation of solid lipid nanoparticles (SLN): The value of different alkyl polyglucoside surfactants. Int. J. Pharm. 2014, 474, 33–41. [Google Scholar] [CrossRef]
- Sladky, K.K.; Swanson, C.R.; Stoskopf, M.K.; Loomis, M.R.; Lewbart, G.A. Comparative efficacy of tricaine methanesulfonate and clove oil for use as anesthetics in red pacu (Piaractus brachypomus). Am. J. Vet. Res. 2001, 62, 337–342. [Google Scholar] [CrossRef]
- López-Cánovas, A.E.; Cabas, I.; Chaves-Pozo, E.; Ros-Chumillas, M.; Navarro-Segura, L.; López-Gómez, A.; Fernandes, J.M.; Galindo-Villegas, J.; García-Ayala, A. Nanoencapsulated Clove Oil Applied as an Anesthetic at Slaughtering Decreases Stress, Extends the Freshness, and Lengthens Shelf Life of Cultured Fish. Foods 2020, 9, 1750. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Subramanian, N.; Ray, S.; Ghosal, S.K.; Bhadra, R.; Moulik, S.P. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol. Pharm. Bull. 2004, 27, 1993–1999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elwood, R.W. Discrimination between nociceptive reflexes and more complex responses consistent with pain in crustaceans. Philos. Trans. R. Soc. B 2019, 374, 20190368. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Kwan, G.T.; Gallup, J.; Tresguerres, M. Acute fluoxetine exposure alters crab anxiety-like behaviour, but not aggressiveness. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Perrot-Minnot, M.-J.; Banchetry, L.; Cézilly, F. Anxiety-like behaviour increases safety from fish predation in an amphipod crustacea. R. Soc. Open Sci. 2017, 4, 171558. [Google Scholar] [CrossRef]
- Wycoff, S.; Weineck, K.; Conlin, S.; Suryadevara, C.; Grau, E.; Bradley, A.; Cantrell, D.; Eversole, S.; Grachen, C.; Hall, K. Effects of clove oil (eugenol) on proprioceptive neurons, heart rate, and behavior in model crustaceans. Impulse 2018, 2018, 1–21. [Google Scholar]
- Aoshima, H.; Hamamoto, K. Potentiation of GABAA receptors expressed in Xenopus oocytes by perfume and phytoncid. Biosci. Biotechnol. Biochem. 1999, 63, 743–748. [Google Scholar] [CrossRef]
- Zahl, I.H.; Samuelsen, O.; Kiessling, A. Anaesthesia of farmed fish: Implications for welfare. Fish Physiol. Biochem. 2012, 38, 201–218. [Google Scholar] [CrossRef]
- Pinto, C.G.; Fruet, G.G.; Rech, V.C.; de Vasconcellos, N.J.S.; da Silva Fernandes, L. Evaluation of the stability and ecotoxicological profile of nanostructured lipidic systems containing ginger essential oil compared to its free form. Discip. Sci. Nat. E Tecnol. 2018, 19, 389–400. [Google Scholar]
- Luis, A.I.; Campos, E.V.; De Oliveira, J.L.; Guilger-Casagrande, M.; De Lima, R.; Castanha, R.F.; De Castro, V.L.; Fraceto, L.F. Zein nanoparticles impregnated with eugenol and garlic essential oils for treating fish pathogens. ACS Omega 2020, 5, 15557–15566. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Du, H.; Yang, X.; Zhai, G. Design of chitosan-based nanoformulations for efficient intracellular release of active compounds. Nanomedicine 2014, 9, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Baati, T.; Al-Kattan, A.; Esteve, M.-A.; Njim, L.; Ryabchikov, Y.; Chaspoul, F.; Hammami, M.; Sentis, M.; Kabashin, A.V.; Braguer, D. Ultrapure laser-synthesized Si-based nanomaterials for biomedical applications: In vivo assessment of safety and biodistribution. Sci. Rep. 2016, 6, 25400. [Google Scholar] [CrossRef] [PubMed]

| Stage | Behavior of Shrimp |
|---|---|
| Induction | |
| 1 | Reaction only to strong tactile and vibration stimuli |
| 2 | Non-reactivity to stimuli |
| Recovery | |
| 1 | Start of erratic swimming without reestablishment of equilibrium |
| 2 | Attained an upright position on the bottom of the aquaria |
| Formulation | Particle Size (nm) | Zeta Potential (mV) | Polydispersity Index | Appearance |
|---|---|---|---|---|
| STD Clove oil | NA | NA | NA | Yellow transparent |
| Clove NLCs | 175 ± 0.72 | −48.37 ± 0.38 | 0.115 ± 0.230 | Milky translucent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewmalun, S.; Yata, T.; Kitiyodom, S.; Yostawonkul, J.; Namdee, K.; Kamble, M.T.; Pirarat, N. Clove Oil-Nanostructured Lipid Carriers: A Platform of Herbal Anesthetics in Whiteleg Shrimp (Penaeus vannamei). Foods 2022, 11, 3162. https://doi.org/10.3390/foods11203162
Kaewmalun S, Yata T, Kitiyodom S, Yostawonkul J, Namdee K, Kamble MT, Pirarat N. Clove Oil-Nanostructured Lipid Carriers: A Platform of Herbal Anesthetics in Whiteleg Shrimp (Penaeus vannamei). Foods. 2022; 11(20):3162. https://doi.org/10.3390/foods11203162
Chicago/Turabian StyleKaewmalun, Somrudee, Teerapong Yata, Sirikorn Kitiyodom, Jakarwan Yostawonkul, Katawut Namdee, Manoj Tukaram Kamble, and Nopadon Pirarat. 2022. "Clove Oil-Nanostructured Lipid Carriers: A Platform of Herbal Anesthetics in Whiteleg Shrimp (Penaeus vannamei)" Foods 11, no. 20: 3162. https://doi.org/10.3390/foods11203162
APA StyleKaewmalun, S., Yata, T., Kitiyodom, S., Yostawonkul, J., Namdee, K., Kamble, M. T., & Pirarat, N. (2022). Clove Oil-Nanostructured Lipid Carriers: A Platform of Herbal Anesthetics in Whiteleg Shrimp (Penaeus vannamei). Foods, 11(20), 3162. https://doi.org/10.3390/foods11203162







