The Influence of Deep Eutectic Solvents Extract from Ginger on the Formation of Heterocyclic Amines and Advanced Glycation End Products in Roast Beef Patties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of DES
2.3. Ultrasonication-Assisted Extraction of Ginger with DES
2.4. Determination of Total Phenolic (TPC) and Flavonoid Content (TFC)
2.5. Antioxidant Activity Assays
2.6. Meat Preparation and Cooking
2.7. Composition, Cooking Loss, and Texture Profile Analysis
2.8. Determination of HAs
2.9. Determination of AGEs
2.10. Determination of Creatine, Creatinine, and Glucose in Roasted Beef Patties
2.11. Measurements of Protein and Lipid Oxidation of the Roasted Beef Patties
2.12. Statistical Analysis
3. Results and Discussion
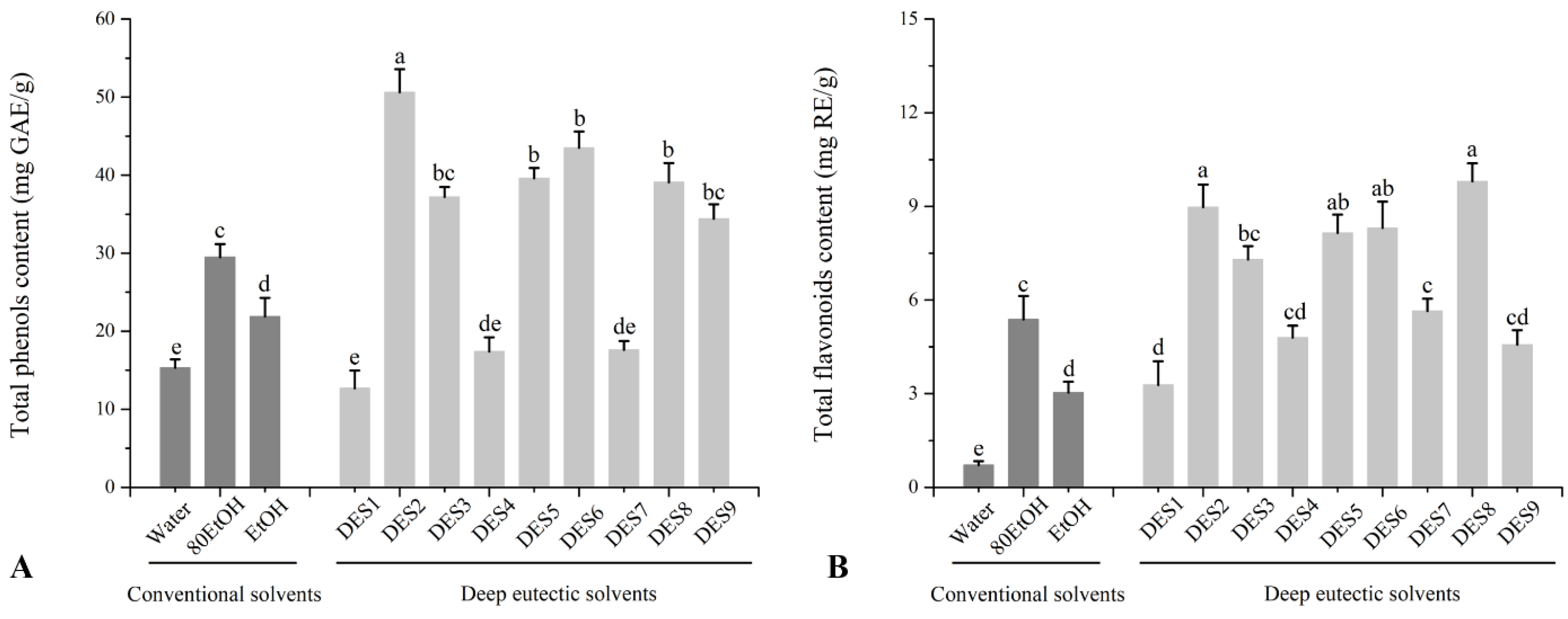
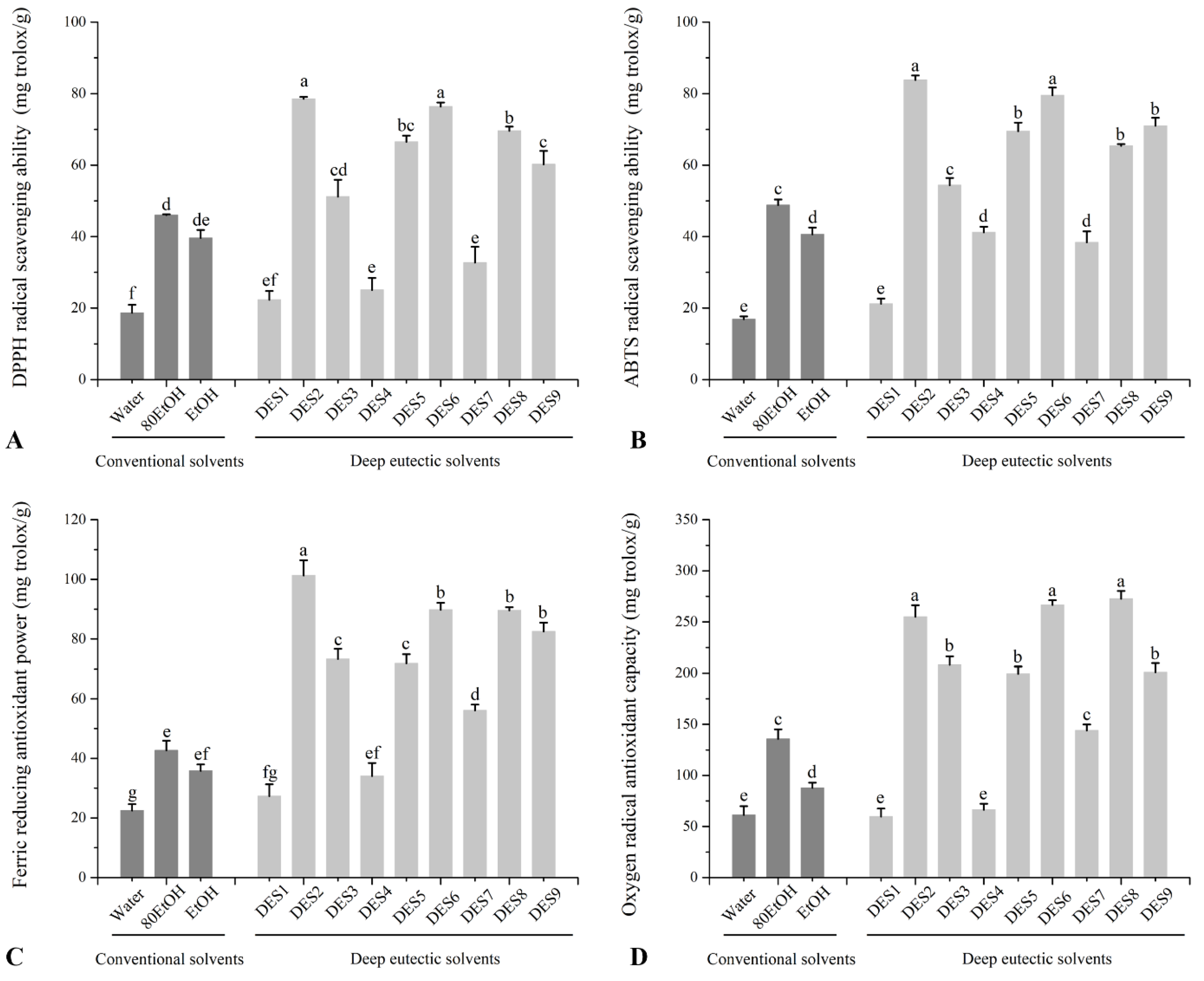
3.1. TPC, TFC, and Antioxidant Capacity of Ginger Extracts
3.2. Proximate and Texture Profile Analysis after Roasting
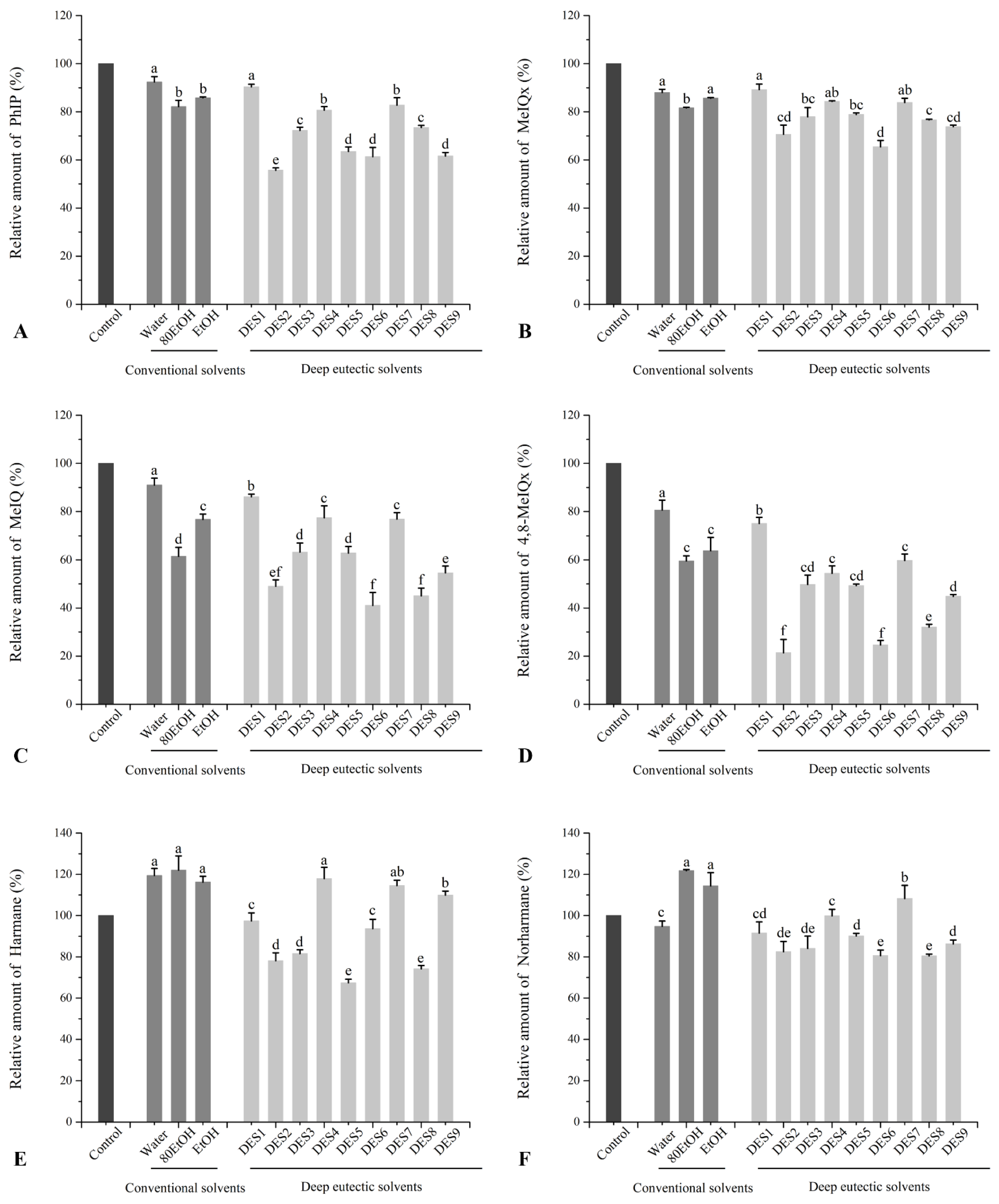
3.3. Effects of Ginger Extract on the Formation of HAs in Beef Patties
3.4. Effects of Ginger Extract on the Formation of CML and CEL in Beef Patties
3.5. Change of Glucose, Creatine, and Creatinine Contents
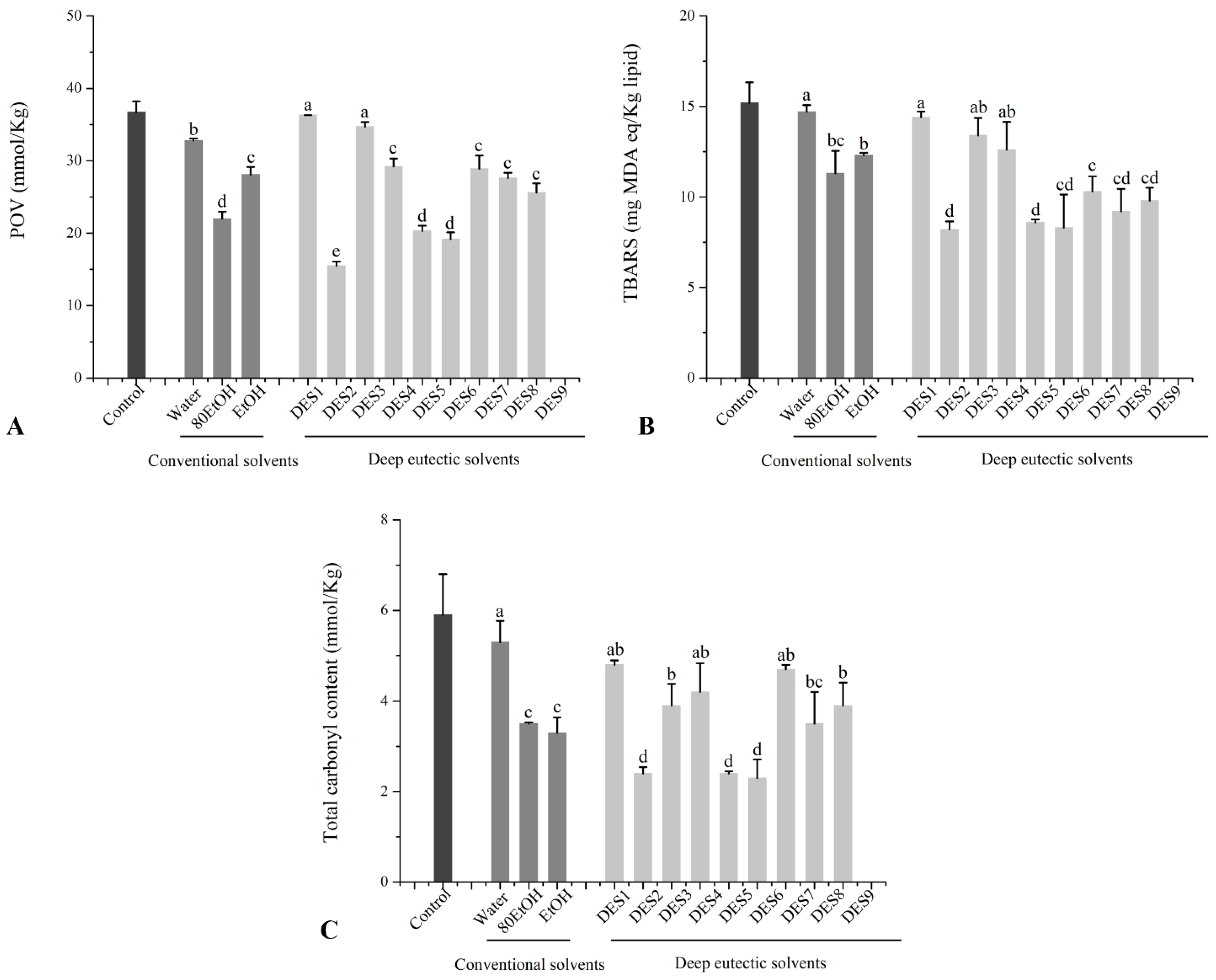
3.6. Inhibitory Effects of Ginger Extract on Protein and Lipid Peroxidation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, L.; Steele, E.M.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in consumption of ultraprocessed foods among US youths aged 2–19 years, 1999–2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Koszucka, A.; Nowak, A. Thermal processing food-related toxicants: A review. Crit. Rev. Food Sci. 2019, 59, 3579–3596. [Google Scholar] [CrossRef]
- Quan, W.; Wu, Z.; Jiao, Y.; Liu, G.; Wang, Z.; He, Z.; Tao, G.; Qin, F.; Zeng, M.; Chen, J. Exploring the relationship between potato components and Maillard reaction derivative harmful products using multivariate statistical analysis. Food Chem. 2021, 339, 127853. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. 2016, 15, 269–302. [Google Scholar] [CrossRef] [Green Version]
- Turesky, R.J. Heterocyclic aromatic amines: Potential human carcinogens. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 4, pp. 37–83. [Google Scholar]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Quan, W.; Jiao, Y.; Xue, C.; Li, Y.; Liu, G.; He, Z.; Qin, F.; Zeng, M.; Chen, J. The effect of exogenous free N ε-(carboxymethyl) lysine on diabetic-model Goto-Kakizaki rats: Metabolomics analysis in serum and urine. J. Agri. Food Chem. 2021, 69, 783–793. [Google Scholar] [CrossRef]
- Quan, W.; Li, Y.; Jiao, Y.; Xue, C.; Liu, G.; Wang, Z.; He, Z.; Qin, F.; Zeng, M.; Chen, J. Simultaneous generation of acrylamide, β-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system. Food Chem. 2020, 332, 127387. [Google Scholar] [CrossRef]
- Rannou, C.; Laroque, D.; Renault, E.; Prost, C.; Sérot, T. Mitigation strategies of acrylamide, furans, heterocyclic amines and browning during the Maillard reaction in foods. Food Res. Inter. 2016, 90, 154–176. [Google Scholar] [CrossRef]
- Xue, C.; Quan, W.; Li, Y.; He, Z.; Qin, F.; Wang, Z.; Chen, J.; Zeng, M. Mitigative capacity of Kaempferia galanga L. and kaempferol on heterocyclic amines and advanced glycation end products in roasted beef patties and related mechanistic analysis by density functional theory. Food Chem. 2022, 385, 132660. [Google Scholar] [CrossRef]
- Jiao, Y.; Quan, W.; He, Z.; Gao, D.; Qin, F.; Zeng, M.; Chen, J. Effects of Catechins on N ε-(Carboxymethyl) lysine and N ε-(Carboxyethyl) lysine Formation in Green Tea and Model Systems. J. Agri. Food Chem. 2019, 67, 1254–1260. [Google Scholar] [CrossRef]
- Xue, C.; He, Z.; Qin, F.; Chen, J.; Zeng, M. Effects of amides from pungent spices on the free and protein-bound heterocyclic amine profiles of roast beef patties by UPLC–MS/MS and multivariate statistical analysis. Food Res. Inter. 2020, 135, 109299. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Li, Y.; Pan, Z.; Chen, Z.; Lu, J.; Yuan, J.; Zhu, Z.; Zhang, J. Ultrasonication-assisted synthesis of alcohol-based deep eutectic solvents for extraction of active compounds from ginger. Ultrason. Sonochem. 2020, 63, 104915. [Google Scholar] [CrossRef]
- Xue, C.; Deng, P.; Quan, W.; Li, Y.; He, Z.; Qin, F.; Wang, Z.; Chen, J.; Zeng, M. Ginger and curcumin can inhibit heterocyclic amines and advanced glycation end products in roast beef patties by quenching free radicals as revealed by electron paramagnetic resonance. Food Control 2022, 138, 109038. [Google Scholar] [CrossRef]
- Gumus, D.; Kizil, M. Comparison of the reducing effects of blueberry and propolis extracts on heterocyclic aromatic amines formation in pan fried beef. Meat Sci. 2022, 186, 108746. [Google Scholar] [CrossRef]
- Khan, M.R.; Busquets, R.; Azam, M. Blueberry, raspberry, and strawberry extracts reduce the formation of carcinogenic heterocyclic amines in fried camel, beef and chicken meats. Food Control 2021, 123, 107852. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Ali Redha, A. Review on extraction of phenolic compounds from natural sources using green deep eutectic solvents. J. Agri. Food Chem. 2021, 69, 878–912. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Długosz, O.; Ochnik, M.; Sochocka, M.; Franz, D.; Orzechowska, B.; Anna, C.-K.; Agata, D.; Banach, M. Antimicrobial and antiviral activity of selenium sulphide nanoparticles synthesised in extracts from spices in natural deep eutectic solvents (NDES). Sustain. Mater. Technol. 2022, 32, e00433. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, W.; Soladoye, O.P. Towards innovative food processing of flavonoid compounds: Insights into stability and bioactivity. LWT 2021, 150, 111968. [Google Scholar] [CrossRef]
- Quan, W.; Qie, X.; Chen, Y.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effect of milk addition and processing on the antioxidant capacity and phenolic bioaccessibility of coffee by using an in vitro gastrointestinal digestion model. Food Chem. 2020, 308, 125598. [Google Scholar] [CrossRef]
- Qie, X.; Chen, Y.; Quan, W.; Wang, Z.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Analysis of β-lactoglobulin–epigallocatechin gallate interactions: The antioxidant capacity and effects of polyphenols under different heating conditions in polyphenolic–protein interactions. Food Funct. 2020, 11, 3867–3878. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs/Edited by William Horwitz; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Li, Y.; He, J.; Quan, W.; He, Z.; Qin, F.; Tao, G.; Wang, Z.; Zeng, M.; Chen, J. Effects of polyphosphates and sodium chloride on heterocyclic amines in roasted beef patties as revealed by UPLC-MS/MS. Food Chem. 2020, 326, 127016. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, Z.; Chen, Q.; Sun, F.; Xu, M.; Kong, B. Influence of different ratios of sucrose and green tea leaves on heterocyclic aromatic amine formation and quality characteristics of smoked chicken drumsticks. Food Control 2022, 133, 108613. [Google Scholar] [CrossRef]
- Li, Y.; Xue, C.; Quan, W.; Qin, F.; Wang, Z.; He, Z.; Zeng, M.; Chen, J. Assessment the influence of salt and polyphosphate on protein oxidation and Nε-(carboxymethyl) lysine and Nε-(carboxyethyl) lysine formation in roasted beef patties. Meat Sci. 2021, 177, 108489. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Li, Z.-H.; Liu, L.-L.; Wang, H.; Yang, S.-H.; Zhang, J.-S.; Zhang, Y. Green extraction using deep eutectic solvents and antioxidant activities of flavonoids from two fruits of Rubia species. LWT 2021, 148, 111708. [Google Scholar] [CrossRef]
- Percevault, L.; Limanton, E.; Nicolas, P.; Paquin, L.; Lagrost, C. Electrochemical determination and antioxidant capacity modulation of polyphenols in deep eutectic solvents. ACS Sustain. Chem. Eng. 2020, 9, 776–784. [Google Scholar] [CrossRef]
- Li, M.; Lin, S.; Wang, R.; Gao, D.; Bao, Z.; Chen, D.; Tang, Y.; Sun, N.; Zhang, S. Inhibitory effect and mechanism of various fruit extracts on the formation of heterocyclic aromatic amines and flavor changes in roast large yellow croaker (Pseudosciaena crocea). Food Control 2022, 131, 108410. [Google Scholar] [CrossRef]
- Cao, H.; Chen, B.H.; Inbaraj, B.S.; Chen, L.; Alvarez-Rivera, G.; Cifuentes, A.; Zhang, N.; Yang, D.J.; Simal-Gandara, J.; Wang, M. Preventive potential and mechanism of dietary polyphenols on the formation of heterocyclic aromatic amines. Food Front. 2020, 1, 134–151. [Google Scholar] [CrossRef]

| Name of DES | Component 1 (HBA) | Component 2 (HBD) | Molar Ratio |
|---|---|---|---|
| DES1 | Choline chloride | Glycerol | 1:2 |
| DES2 | Choline chloride | Lactic acid | 1:2 |
| DES3 | Choline chloride | Xylitol | 2:1 |
| DES4 | Betaine | Glycerol | 1:2 |
| DES5 | Betaine | Lactic acid | 1:2 |
| DES6 | Betaine | Xylitol | 2:1 |
| DES7 | L-carnitine | Glycerol | 1:2 |
| DES8 | L-carnitine | Lactic acid | 1:2 |
| DES9 | L-carnitine | Xylitol | 2:1 |
| Precursor Ion (m/z) | Product Ion (m/z) | Cone Voltage (V) | Collision Voltage (eV) | Dwell Time (s) | |
|---|---|---|---|---|---|
| HAs | |||||
| DMIP | 163 | 148 | 30 | 25 | 0.10 |
| Phe-p-1 | 171 | 127 | 30 | 35 | 0.10 |
| 1,5,6-TMIP | 177 | 162 | 30 | 25 | 0.15 |
| Harmane | 183 | 115 | 30 | 30 | 0.15 |
| Norharmane | 169 | 115 | 30 | 35 | 0.10 |
| AαC | 183 | 140 | 30 | 35 | 0.15 |
| MeAαC | 198 | 181 | 30 | 30 | 0.15 |
| Glu-p-1 | 199 | 145 | 30 | 35 | 0.10 |
| IQ | 199 | 130 | 30 | 35 | 0.15 |
| IQ [4,5-b] | 199 | 115 | 30 | 35 | 0.10 |
| IQx | 200 | 185 | 30 | 35 | 0.10 |
| MeIQ | 213 | 198 | 30 | 30 | 0.10 |
| MeIQx | 214 | 131 | 30 | 35 | 0.10 |
| PhIP | 225 | 210 | 30 | 35 | 0.15 |
| 4,8-DiMeIQx | 228 | 212 | 30 | 30 | 0.15 |
| 7,8-DiMeIQx | 228 | 213 | 30 | 35 | 0.15 |
| 4,7,8-DiMeIQx | 242 | 227 | 30 | 30 | 0.10 |
| AGEs | |||||
| CML | 205 | 84 | 20 | 18 | 0.15 |
| d4-CML | 209 | 88 | 20 | 18 | 0.15 |
| CEL | 219 | 84 | 22 | 20 | 0.15 |
| d4-CEL | 223 | 88 | 22 | 20 | 0.15 |
| Group | pH | Protein (g/100g) | Cooking Loss (%) | Ash (%) |
|---|---|---|---|---|
| Control | 5.58 ± 0.13 a | 42.9 ± 1.74 ab | 50.9 ± 1.22 a | 4.46 ± 0.08 a |
| Water | 5.77 ± 0.09 a | 43.4 ± 0.57 a | 48.3 ± 2.96 a | 4.31 ± 0.01 a |
| 80EtOH | 5.74 ± 0.07 a | 45.1 ± 2.56 a | 52.3 ± 1.05 a | 4.16 ± 0.09 a |
| EtOH | 5.62 ± 0.04 a | 44.3 ± 2.53 a | 49.9 ± 2.90 a | 4.53 ± 0.03 a |
| DES1 | 5.69 ± 0.05 a | 46.2 ± 2.98 a | 50.2 ± 1.17 a | 4.27 ± 0.05 a |
| DES2 | 5.01 ± 0.19 b | 44.0 ± 1.94 a | 52.3 ± 1.08 a | 4.33 ± 0.12 a |
| DES3 | 5.73 ± 0.16 a | 45.2 ± 2.38 a | 49.9 ± 2.46 a | 4.86 ± 0.04 a |
| DES4 | 5.81 ± 0.03 a | 46.7 ± 1.08 a | 52.9 ± 1.38 a | 4.88 ± 0.20 a |
| DES5 | 4.61 ± 0.12 b | 46.2 ± 2.19 a | 48.0 ± 1.80 a | 4.85 ± 0.07 a |
| DES6 | 5.88 ± 0.05 a | 46.9 ± 0.67 a | 52.5 ± 1.21 a | 4.45 ± 0.13 a |
| DES7 | 5.33 ± 0.02 a | 43.1 ± 1.04 ab | 48.7 ± 2.10 a | 4.55 ± 0.04 a |
| DES8 | 4.67 ± 0.09 b | 47.7 ± 1.96 a | 48.1 ± 2.89 a | 4.79 ± 0.05 a |
| DES9 | 5.78 ± 0.17 a | 45.9 ± 1.26 a | 52.7 ± 1.36 a | 4.86 ± 0.07 a |
| Group | Hardness (N) | Springiness (mm) | Gumminess (N) | Cohesiveness (N) | Chewiness (mJ) |
|---|---|---|---|---|---|
| Control | 8298 ± 51 ab | 0.39 ± 0.02 a | 4954 ± 57 a | 0.65 ± 0.15 ab | 3728 ± 52 a |
| Water | 8276 ± 43 b | 0.49 ± 0.08 a | 4832 ± 83 a | 0.91 ± 0.14 a | 3807 ± 77 a |
| 80EtOH | 8294 ± 59 ab | 0.52 ± 0.07 a | 4905 ± 30 a | 0.96 ± 0.16 a | 3722 ± 73 a |
| EtOH | 8328 ± 96 a | 0.32 ± 0.03 a | 4859 ± 79 a | 0.83 ± 0.19 a | 3698 ± 49 ab |
| DES1 | 8394 ± 59 a | 0.44 ± 0.07 a | 4858 ± 96 a | 0.73 ± 0.05 a | 3672 ± 65 ab |
| DES2 | 8393 ± 103 a | 0.36 ± 0.02 a | 4901 ± 44 a | 0.69 ± 0.11 a | 3634 ± 62 ab |
| DES3 | 8478 ± 101 a | 0.46 ± 0.06 a | 4961 ± 48 a | 0.57 ± 0.17 ab | 3729 ± 65 a |
| DES4 | 8306 ± 83 ab | 0.4 ± 0.07 a | 4834 ± 110 a | 0.74 ± 0.04 a | 3799 ± 29 a |
| DES5 | 8344 ± 50 a | 0.5 ± 0.02 a | 4924 ± 34 a | 0.78 ± 0.02 a | 3710 ± 88 a |
| DES6 | 8437 ± 99 a | 0.59 ± 0.10 a | 4927 ± 103 a | 0.75 ± 0.12 a | 3842 ± 34 a |
| DES7 | 8257 ± 67 ab | 0.52 ± 0.01 a | 4841 ± 101 a | 0.71 ± 0.01 a | 3654 ± 72 ab |
| DES8 | 8455 ± 60 a | 0.57 ± 0.03 a | 4801 ± 98 a | 0.66 ± 0.16 a | 3628 ± 64 ab |
| DES9 | 8240 ± 96 ab | 0.51 ± 0.06 a | 4832 ± 41 a | 0.60 ± 0.03 b | 3733 ± 21 a |
| Group | Creatine (mg/g) | Creatinine (μmol/L) | Glucose (mg/g) |
|---|---|---|---|
| Control | 0.95 ± 0.04 f | 1.22 ± 0.08 cd | 0.11 ± 0.01 d |
| Water | 1.21 ± 0.03 e | 1.55 ± 0.19 c | 0.13 ± 0.01 d |
| 80EtOH | 2.35 ± 0.07 c | 1.85 ± 0.16 bc | 0.21 ± 0.03 bc |
| EtOH | 2.48 ± 0.26 c | 2.45 ± 0.21 b | 0.12 ± 0.01 d |
| DES1 | 1.33 ± 0.29 ef | 1.19 ± 0.05 cd | 0.17 ± 0.00 b |
| DES2 | 5.30 ± 0.20 a | 3.26 ± 0.02 a | 0.40 ± 0.08 a |
| DES3 | 2.72 ± 0.02 c | 2.19 ± 0.16 b | 0.26 ± 0.07 a |
| DES4 | 1.48 ± 0.28 d | 2.52 ± 0.11 b | 0.22 ± 0.03 b |
| DES5 | 4.80 ± 0.27 a | 3.34 ± 0.21 a | 0.36 ± 0.06 a |
| DES6 | 5.97 ± 0.08 a | 2.54 ± 0.12 b | 0.31 ± 0.09 a |
| DES7 | 1.89 ± 0.11 d | 2.30 ± 0.05 b | 0.27 ± 0.00 b |
| DES8 | 4.22 ± 0.04 b | 3.39 ± 0.02 a | 0.28 ± 0.07 a |
| DES9 | 4.44 ± 0.13 b | 2.47 ± 0.03 b | 0.29 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Jiao, Y.; Luo, J.; He, Z.; Zeng, M.; Shen, Q.; Chen, J.; Quan, W. The Influence of Deep Eutectic Solvents Extract from Ginger on the Formation of Heterocyclic Amines and Advanced Glycation End Products in Roast Beef Patties. Foods 2022, 11, 3161. https://doi.org/10.3390/foods11203161
Xu Y, Jiao Y, Luo J, He Z, Zeng M, Shen Q, Chen J, Quan W. The Influence of Deep Eutectic Solvents Extract from Ginger on the Formation of Heterocyclic Amines and Advanced Glycation End Products in Roast Beef Patties. Foods. 2022; 11(20):3161. https://doi.org/10.3390/foods11203161
Chicago/Turabian StyleXu, Yang, Ye Jiao, Jie Luo, Zhiyong He, Maomao Zeng, Qingwu Shen, Jie Chen, and Wei Quan. 2022. "The Influence of Deep Eutectic Solvents Extract from Ginger on the Formation of Heterocyclic Amines and Advanced Glycation End Products in Roast Beef Patties" Foods 11, no. 20: 3161. https://doi.org/10.3390/foods11203161
APA StyleXu, Y., Jiao, Y., Luo, J., He, Z., Zeng, M., Shen, Q., Chen, J., & Quan, W. (2022). The Influence of Deep Eutectic Solvents Extract from Ginger on the Formation of Heterocyclic Amines and Advanced Glycation End Products in Roast Beef Patties. Foods, 11(20), 3161. https://doi.org/10.3390/foods11203161






