The Valorization of Banana By-Products: Nutritional Composition, Bioactivities, Applications, and Future Development
Abstract
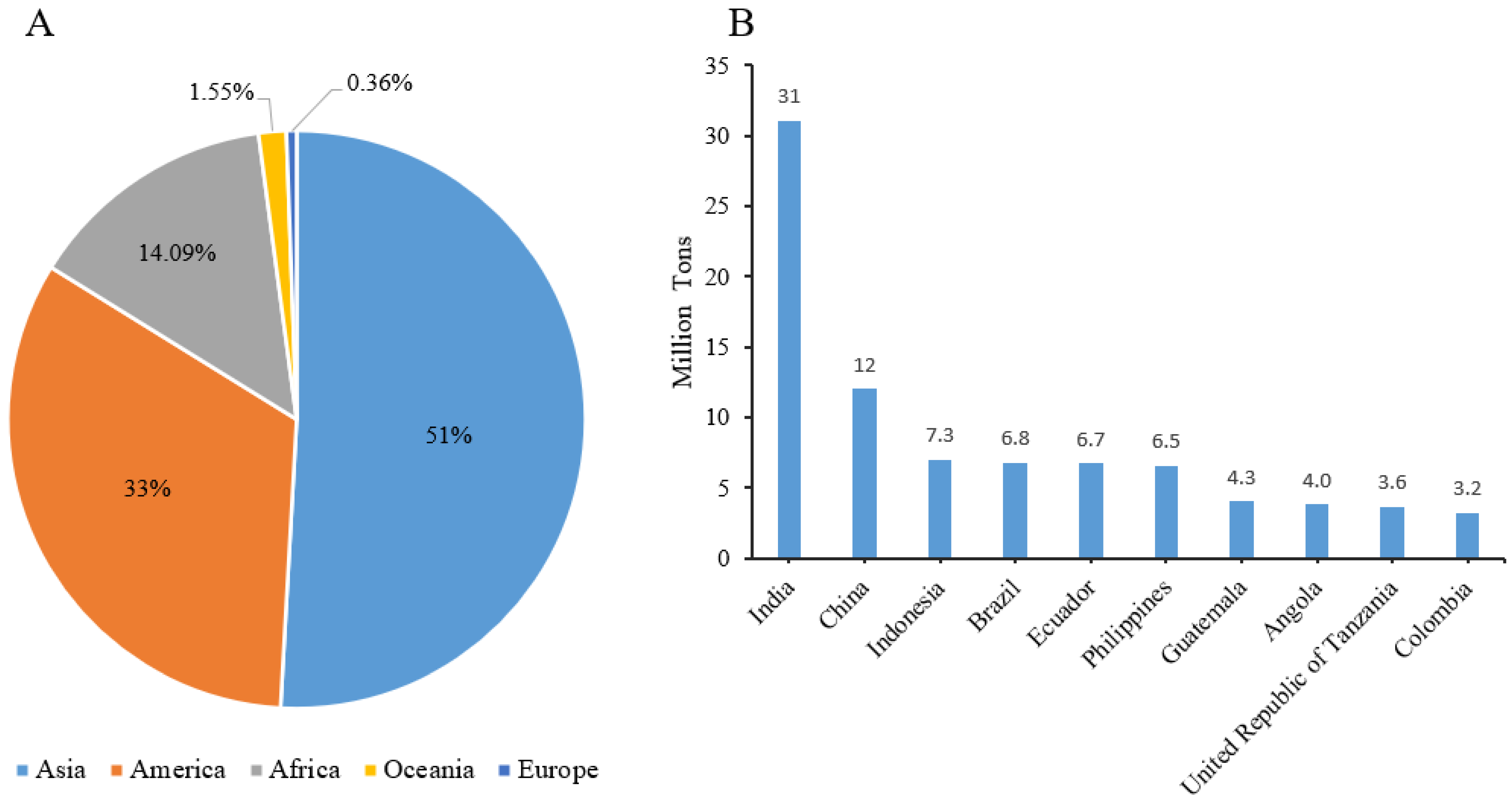
:1. Introduction

2. Banana Stem and Leaves
2.1. Fraction of Banana Stems and Leaves
2.1.1. Bio-Oil
2.1.2. Minerals
2.1.3. Dietary Fiber
2.1.4. Polyphenols and Flavonoids
2.2. Comprehensive Utilization of Banana Stems and Leaves
2.2.1. Fertilizer Utilization
2.2.2. Feed Utilization
2.2.3. Biomass Energy Utilization
2.2.4. Fiber Products
3. Banana Inflorescence
3.1. Fraction of Banana Inflorescence
3.1.1. Protein
3.1.2. Carbohydrates
3.1.3. Lipids
3.1.4. Minerals
3.1.5. Polyphenols
3.2. Biofunction
3.2.1. Antioxidant Activities
3.2.2. Antidiabetic Activities
3.2.3. Anti-Cancer Activities
3.2.4. Cardiovascular Protective Activities
3.2.5. Other Biological Activities
3.3. Comprehensive Utilization of the Banana Inflorescence
3.3.1. Pharmacological Applications
3.3.2. Food Applications
4. Banana Peel
4.1. Fraction of Banana Peel
4.1.1. Protein
4.1.2. Fatty Acids
4.1.3. Minerals
4.1.4. Dietary Fiber
4.1.5. Polyphenols
4.2. Biofunction
4.2.1. Antioxidant Activities
4.2.2. Antimicrobial Agent
4.2.3. Anti-Disease Activities
4.3. Comprehensive Utilization of Banana Peel
4.3.1. Food Processing Raw Material
| Nutrient Content | Extraction Method | Properties of Extracted Component | Application | Purpose | References |
|---|---|---|---|---|---|
| Protein | Acid extraction (glacial acetic acid) | With antioxidant and anti-fungal properties | Food or feed | Increase the content of amino acids in food or feed | [69] |
| Dietary fiber | Prior to extracting the dietary fiber, tap Water was used to rinse the wet milled BP powder. |
| Chicken sausage, fish patties, cookies | To increase the amount of dietary fiber | [89,91,92,93] |
| Fatty acids | ND | Anti-atherosclerotic, anti-thrombotic | Drugs that prevent cardiovascular disease, cancer, osteoporosis, diabetes, and other illnesses | Increasing the content of fatty acids in food | [71,72] |
| Mineral | ND |
| Used as fertilizer to supplement soil nutrients | Improve soil fertility | [94] |
| Pectinases | BP powder was added to a base medium that already contained yeast extract, K2HPO4, KH2PO4, and KNO3, following that, the samples were incubated in a solution at 37 °C while being shaken at 150 rpm. | The four lactic acid bacteria that were studied responded positively to the prebiotic effects of the banana peel powder that this enzyme hydrolyzed, suggesting that B. amyloliquefaciens TKU050 pectinase may be a good option for generating prebiotics | Release fibers by attacking non-cellulosic components in the sheath | get cellulose | [95] |
| Carbohydrate (pectin, xylose, xylooligosaccharides) | Banana peels were pre-treated with citric acid and alkaline solutions, then enzymatic hydrolysis (Cellic® CTec2) was performed on the peels | The breakdown of a banana peel demonstrated that pectin, xylose, and xylooligosaccharides all necessary carbohydrates could be removed from its cell walls with a high yield | Replenishes the role of carbohydrates in the body, but also has the effect of protecting the liver and detoxification | Using Banana Peels as a Carbohydrate Supplement | [96] |
| Polyphenols | ND | Significantly reduces the peroxide value of fish balls | Fish ball preservative | Preservation freshness to increase the storage time of fish | [78] |
4.3.2. Production of Feed
4.3.3. Fertilizer Utilization
4.3.4. Energy Utilization
4.3.5. Bioremediation
4.3.6. Food Packaging
4.3.7. Pharmacological Applications
5. Problems and Countermeasures in Utilization of Banana By-Products
5.1. Pesticide and Ripening Agent Residues
5.2. Difficulties in Collecting
5.3. Backwardness of Comprehensive Utilization Technology
5.4. Problems with the Properties of Banana By-Products Themselves
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qamar, S.; Shaikh, A. Therapeutic potentials and compositional changes of valuable compounds from banana—A review. Trends Food Sci. Technol. 2018, 79, 1–9. [Google Scholar] [CrossRef]
- Fernandes, E.R.K.; Marangoni, C.; Souza, O.; Sellin, N. Thermochemical characterization of banana leaves as a potential energy source. Energy Convers. Manag. 2013, 75, 603–608. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Essien, J.; Akpan, E.; Essien, E. Studies on mould growth and biomass production using waste banana peel. Bioresour. Technol. 2005, 96, 1451–1456. [Google Scholar] [CrossRef]
- FAOSTAT. Production Quantity of Banana-2018. Food and Agriculture Organisation of the United Nations. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 5 November 2021).
- Rosentrater, K.A.; Todey, D.; Persyn, R. Quantifying Total and Sustainable Agricultural Biomass Resources in South Dakota—A Preliminary Assessment. CIGR EJ. 2009, 11, 2–37. [Google Scholar]
- Tripathi, S.K.; Bhardwaj, N.K.; Chechi, S.; Varadhan, R. Suitability of banana stem pulp as replacement of softwood pulp for making superior grade unbleached paper from agro residue pulp. Appita J. 2019, 72, 163–178. [Google Scholar]
- Akinyemi, B.A.; Dai, C. Development of banana fibers and wood bottom ash modified cement mortars. Constr. Build. Mater. 2020, 241, 118041. [Google Scholar] [CrossRef]
- Sitthiya, K.; Devkota, L.; Sadiq, M.B.; Anal, A.K. Extraction and characterization of proteins from banana (Musa sapientum L.) flower and evaluation of antimicrobial activities. J. Food Sci. Technol. 2018, 55, 658–666. [Google Scholar] [CrossRef]
- Fidrianny, I.; Rizki, K.; Insanu, M. In vitro antioxidant activities from various extracts of banana peels using ABTS, DPPH assays and correlation with phenolic, flavonoid, carotenoid content. Int. J. Pharm. Pharm. Sci. 2014, 6, 299–303. [Google Scholar]
- Balda, S.; Sharma, A.; Capalash, N.; Sharma, P. Banana fibre: A natural and sustainable bioresource for eco-friendly applications. Clean Technol. Environ. 2021, 23, 1389–1401. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Taib, R.M.; Abdullah, N.; Aziz, N.S.M. Bio-oil derived from banana pseudo-stem via fast pyrolysis process. Biomass Bioenergy 2021, 148, 106034. [Google Scholar] [CrossRef]
- Ho, L.-H.; Tan, T.-C.; Aziz, N.A.A.; Bhat, R. In vitro starch digestibility of bread with banana (Musa acuminata X balbisiana ABB cv. Awak) pseudo-stem flour and hydrocolloids. Food Biosci. 2015, 12, 10–17. [Google Scholar] [CrossRef]
- Oleforuh-Okoleh, V.; Ogunnupebi, J.; Iroka, J. Assessment of growth performance and certain blood constituents of broiler chicks given banana leaf as a phytoadditive. Asian J. Poultry Sci. 2015, 9, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Amini Khoozani, A.; Kebede, B.; Birch, J.; Bekhit, A.E.-D.A. The effect of bread fortification with whole green banana flour on its physicochemical, nutritional and in vitro digestibility. Foods 2020, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sujithra, S.; Manikkandan, T. Extraction of anthocyanin from banana (Musa paradisiaca) flower bract and analysis of phytochemicals, antioxidant activities and anthocyanin content. J. Chem. Pharm. Sci. 2019, 12, 102–104. [Google Scholar] [CrossRef]
- Li, Z.; Guo, K.; Lin, L.; He, W.; Zhang, L.; Wei, C. Comparison of physicochemical properties of starches from flesh and peel of green banana fruit. Molecules 2018, 23, 2312. [Google Scholar] [CrossRef] [Green Version]
- Sowmya, N.; Sree, G.N.; Patil, P.; Mehta, D. Depigmenting effect of banana stem and flower on melanocytes. J. Oral Biol. Craniofac. Res. 2022, 12, 454–457. [Google Scholar]
- Gupta, G.; Baranwal, M.; Saxena, S.; Reddy, M.S. Utilization of banana waste as a resource material for biofuels and other value-added products. Biomass Convers. Biorefin. 2022, 1–20. [Google Scholar] [CrossRef]
- Harish, K.R.Y.; Srijana, M.; Madhusudhan, R.D.; Gopal, R. Coculture fermentation of banana agro-waste to ethanol by cellulolytic thermophilic Clostridium thermocellum CT2. Afr. J. Biotechnol. 2010, 9, 1926–1934. [Google Scholar] [CrossRef] [Green Version]
- Jacob, N.; Prema, P. Novel process for the simultaneous extraction and degumming of banana fibers under solid-state cultivation. Braz. J. Microbiol. 2008, 39, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petchimuthu, P.; Petchimuthu, R.; Basha, S.; Murugan, R.; Ganapathy, H.; Durairaj, U. Production of cost effective, biodegradable, disposable feminine sanitary napkins using banana fibres. Int. J. Eng. Adv. Technol. 2019, 9, 789–791. [Google Scholar]
- Ding, Z.; Han, L.; Jin, Z.; Wang, B.; Zeng, H.; Zheng, W.; He, Y.; Zang, X. Effect of Banana Stalk Organic Fertilizer on the Growth of Chinese Cabbage. Asian Agric. Res. 2016, 8, 64–68. [Google Scholar]
- de Siqueira, F.G.; Martos, E.T.; Silva, R.D.; Dias, E.S. Cultivation of Pleurotus sajor-caju on banana stalk and Bahia grass based substrates. Hortic. Bras. 2011, 29, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Amarnath, R.; Balakrishnan, V. Evaluation of the banana (Musa paradisiaca) plant by-product’s fermentation characteristics to assess their fodder potential. Int. J. Dairy Sci. 2007, 2, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Gregory, L.; Yoshihara, E.; Silva, L.K.F.; Marques, E.C.; Ribeiro, B.L.M.; Júnior, E.B.D.S.M.; Rossi, R.S.; do Amarant, A.F.T.; Hasegawa, M.Y. Anthelmintic effects of dried ground banana plant leaves (Musa spp.) fed to sheep artificially infected with Haemonchus contortus and trichostrongylus colubriformis. Afr. J. Tradit. Complem. 2017, 14, 138–144. [Google Scholar] [CrossRef]
- Marie-Magdeleine, C.; Liméa, L.; Etienne, T.; Lallo, C.H.; Archimède, H.; Alexandre, G. The effects of replacing Dichantium hay with banana (Musa paradisiaca) leaves and pseudo-stem on carcass traits of Ovin Martinik sheep. Trop. Anim. Health Prod. 2009, 41, 1531–1538. [Google Scholar] [CrossRef]
- Matekaire, T.; Mupangwa, J.; Kanyamura, E. The efficacy of banana plant (Musa paradisiaca) as a coccidiostat in rabbits. Intern. J. Appl. Res. Vet. Med. 2005, 3, 326. [Google Scholar]
- Taer, E.; Yusra, D.A.; Amri, A.; Taslim, R.; Putri, A. The synthesis of activated carbon made from banana stem fibers as the supercapacitor electrodes. Mater. Today Proc. 2021, 44, 3346–3349. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.K.; Khandal, R.; Uppaluri, R.; Ghoshal, A.K. Mechanical, thermal, and morphological properties of maleic anhydride-g-polypropylene compatibilized and chemically modified banana-fiber-reinforced polypropylene composites. J. Appl. Polym. Sci. 2010, 117, 1731–1740. [Google Scholar] [CrossRef]
- Kennedy, J. Bananas and people in the homeland of genus Musa: Not just pretty fruit. Ethnobot. Res. Appl. 2009, 7, 179–197. [Google Scholar] [CrossRef] [Green Version]
- Bastianello, S.; Testa, R.; Pezzin, A.; Silva, D. Evaluation of physical and mechanical properties of handmade recycled papers reinforced with pulp of banana tree or rice straw. Matéria 2009, 14, 1172–1178. [Google Scholar]
- Ogunsile, B.; Omotoso, M.; Onilude, M. Comparative soda pulps from the mid-rib, pseudostem and stalk of Musa paradisiaca. J. Biol. Sci. 2006, 6, 1047–1052. [Google Scholar]
- Ramu, R.; Shirahatti, P.S.; Anilakumar, K.; Nayakavadi, S.; Zameer, F.; Dhananjaya, B.; Prasad, M.N. Assessment of nutritional quality and global antioxidant response of banana (Musa sp. CV. Nanjangud Rasa Bale) pseudostem and flower. Pharmacogn. Res. 2017, 9, S74. [Google Scholar]
- Bhaskar, J.J.; Chilkunda, N.D.; Salimath, P.V. Banana (Musa sp. var. elakki bale) flower and pseudostem: Dietary fiber and associated antioxidant capacity. J. Agric. Food Chem. 2012, 60, 427–432. [Google Scholar] [CrossRef]
- Oliveira, L.; Freire, C.; Silvestre, A.; Cordeiro, N.; Torres, I.; Evtuguin, D. Lipophilic extractives from different morphological parts of banana plant “Dwarf Cavendish”. Ind. Crops Prod. 2006, 23, 201–211. [Google Scholar] [CrossRef]
- Sheng, Z.-W.; Ma, W.-H.; Jin, Z.-Q.; Bi, Y.; Sun, Z.-G.; Dou, H.-T.; Li, J.; Han, L. Investigation of dietary fiber, protein, vitamin E and other nutritional compounds of banana flower of two cultivars grown in China. Afr. J. Biotechnol. 2010, 9, 3888–3895. [Google Scholar]
- Lau, B.F.; Kong, K.W.; Leong, K.H.; Sun, J.; He, X.; Wang, Z.; Mustafa, M.R.; Ling, T.C.; Ismail, A. Banana inflorescence: Its bio-prospects as an ingredient for functional foods. Trends Food Sci. Technol. 2020, 97, 14–28. [Google Scholar] [CrossRef]
- Emaga, T.H.; Andrianaivo, R.H.; Wathelet, B.; Tchango, J.T.; Paquot, M. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007, 103, 590–600. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, N. Assessment of chemical compositions and in vitro antioxidant properties of Musa balbisiana Colla inflorescence. Int. J. Pharmaceut. Res. 2018, 10, 80–85. [Google Scholar]
- Arun, K.; Thomas, S.; Reshmitha, T.; Akhil, G.; Nisha, P. Dietary fibre and phenolic-rich extracts from Musa paradisiaca inflorescence ameliorates type 2 diabetes and associated cardiovascular risks. J. Funct. Foods 2017, 31, 198–207. [Google Scholar] [CrossRef]
- Bhaskar, J.; Kumar, A.; Salimath, P. Effect of banana (Musa sp. var. elakki bale) flower and pseudostem on antioxidant and lysosomal enzyme activities in streptozotocin-induced diabetic rats. J. Pharm. Res. 2011, 4, 1087–1091. [Google Scholar]
- Nisha, P.; Mini, S. Flavanoid rich ethyl acetate fraction of Musa paradisiaca inflorescence down-regulates the streptozotocin induced oxidative stress, hyperglycaemia and mRNA levels of selected inflammatory genes in rats. J. Funct. Foods 2013, 5, 1838–1847. [Google Scholar] [CrossRef]
- Ohtani, K. Antioxidant Function of Fruitlets in Hokkaido. J. Jpn. Soc. Food Sci. 2020, 67, 285–291. [Google Scholar] [CrossRef]
- Das, A.; Dhua, R. Development of Fresh and Minimally Processed Banana Inflorescence. Plant Arch. 2021, 21, 1561–1566. [Google Scholar] [CrossRef]
- Revadigar, V.; Al-Mansoub, M.A.; Asif, M.; Hamdan, M.R.; Majid, A.M.S.A.; Asmawi, M.Z.; Murugaiyah, V. Anti-oxidative and cytotoxic attributes of phenolic rich ethanol extract of Musa balbisiana Colla inflorescence. J. Appl. Pharm. Sci. 2017, 7, 103–110. [Google Scholar]
- Schmidt, M.M.; Prestes, R.C.; Kubota, E.H.; Scapin, G.; Mazutti, M.A. Evaluation of antioxidant activity of extracts of banana inflorescences (Musa cavendishii). CyTA-J. Food 2015, 13, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Correa, M.; Mesomo, M.C.; Pianoski, K.E.; Torres, Y.R.; Corazza, M.L. Extraction of inflorescences of Musa paradisiaca L. using supercritical CO2 and compressed propane. J. Supercrit. Fluids 2016, 113, 128–135. [Google Scholar] [CrossRef]
- Sasipriya, G.; Maria, C.L.; Siddhuraju, P. Influence of pressure cooking on antioxidant activity of wild (Ensete superbum) and commercial banana (Musa paradisiaca var. Monthan) unripe fruit and flower. J. Food. Sci. Technol. 2014, 51, 2517–2525. [Google Scholar] [CrossRef] [Green Version]
- Padam, B.; Tin, H.; Chye, F.; Abdullah, M. Antibacterial and Antioxidative Activities of the Various Solvent Extracts of Banana (Musa paradisiaca cv. Mysore) Inflorescences. J. Biol. Sci. 2012, 12, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Ranganatha, L.V.; Prasad, M.N. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S. Afr. J. Bot. 2014, 95, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, J.J.; Salimath, P.V.; Nandini, C.D. Stimulation of glucose uptake by Musa sp. (cv. elakki bale) flower and pseudostem extracts in Ehrlich ascites tumor cells. J. Sci. Food Agric. 2011, 91, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Dai, H.; Pan, S.; Ai, B.; Zheng, L.; Zheng, X.; Prinyawiwatkul, W.; Xu, Z. Phytosterols in banana (Musa spp.) flower inhibit α-glucosidase and α-amylase hydrolysations and glycation reaction. Int. J. Food Sci. Technol. 2017, 52, 171–179. [Google Scholar] [CrossRef]
- Arun, K.; Madhavan, A.; Reshmitha, T.; Thomas, S.; Nisha, P. Musa paradisiaca inflorescence induces human colon cancer cell death by modulating cascades of transcriptional events. Food Funct. 2018, 9, 511–524. [Google Scholar]
- Ramu, R.; Shirahatti, P.S.; Zameer, F.; Lakkapa, D.B.; Nagendra, M. Evaluation of banana (Musa sp. var. Nanjangud rasa bale) flower and pseudostem extracts on antimicrobial, cytotoxicity and thrombolytic activities. Int. J. Pharm. Pharm. Sci. 2015, 7, 136–140. [Google Scholar]
- Jawla, S.; Kumar, Y.; Khan, M. Antimicrobial and antihyperglycemic activities of Musa paradisiaca flowers. Asian Pac. J. Trop. Biomed. 2012, 2, S914–S918. [Google Scholar] [CrossRef]
- Divya, R.; Venkatalakshmi, P.; Vadivel, V.; Brindha, P. In vitro studies on the biological activities of flowers of banana (Musa Paradisiaca, L.). Der Pharm. Lett. 2016, 10, 238–246. [Google Scholar]
- Bhaskar, J.J.; Mahadevamma, S.; Vishwanatha, S.; Salimath, P.V. Effect of banana (Musa sp. cultivar elakki bale) flower and stem on enzyme activities of intestinal and renal disaccharidases in streptozotocin-induced diabetic rats. J. Food Biochem. 2010, 34, 564–580. [Google Scholar]
- Nadumane, V.K.; Timsina, B. Anticancer potential of banana flower extract: An in vitro study. Bangladesh J. Pharmacol. 2014, 9, 628–635. [Google Scholar]
- Su, H.-L.; Lin, Y.-H. Method for Treatment of Prostatic Hyperplasia and/or Ameliorating Urinary Disturbance with Banana Flower Extract. U.S. Patent No. 9,968,646, 15 May 2018. [Google Scholar]
- Tin, H.S.; Padam, B.S.; Vairappan, C.S.; Abdullah, M.I.; Chye, F.Y. Effect of preparation and extraction parameters of banana (Musa balbisiana cv. Saba) inflorescence on their antibacterial activities. Sains Malays. 2015, 44, 1301–1307. [Google Scholar]
- Correa, M.; Bombardelli, M.C.; Fontana, P.D.; Bovo, F.; Messias-Reason, I.J.; Maurer, J.B.B.; Corazza, M.L. Bioactivity of extracts of Musa paradisiaca L. obtained with compressed propane and supercritical CO2. J. Supercrit. Fluids 2017, 122, 63–69. [Google Scholar] [CrossRef]
- Pari, L.; Maheswari, J.U. Hypoglycaemic effect of Musa sapientum L. in alloxan-induced diabetic rats. J. Ethnopharmacol. 1999, 68, 321–325. [Google Scholar] [CrossRef]
- Novidiyanto, N.; Enardi, O.P.; Devriany, A.; Pratiwi, A.P.; Airuni, M. Acceptability and antioxidant activity level of shredded banana flower-chicken meat. Amerta Nutr. 2020, 4, 299–306. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Kubota, E.H.; Prestes, R.C.; Mello, R.O.; Rosa, C.S.; Scapin, G.; Ferreira, S. Development and evaluation of pork burger with added natural antioxidant based on extract of banana inflorescence (Musa cavendishii). CyTA-J. Food 2016, 14, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.S.; Kubota, E.H.; da Silva, C.G.; dos Santos Alves, J.; Hautrive, T.P.; Rodrigues, G.S.; Campagnol, P.C.B. Banana inflorescences: A cheap raw material with great potential to be used as a natural antioxidant in meat products. Meat Sci. 2020, 161, 107991. [Google Scholar] [CrossRef]
- Budhalakoti, N. Extraction of protein from banana by-product and its characterization. J. Food Meas. Charact. 2021, 15, 2202–2210. [Google Scholar] [CrossRef]
- Gupta, A.; Houston, B. A comprehensive review of the bioenergetics of fatty acid and glucose metabolism in the healthy and failing heart in nondiabetic condition. Heart Fail. Rev. 2017, 22, 825–842. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Comparative nutritional, functional, morphological, and diffractogram study on culinary banana (Musa ABB) peel at various stages of development. Int. J. Food Prop. 2016, 19, 2832–2853. [Google Scholar] [CrossRef] [Green Version]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef]
- Oguntoyinbo, O.; Olumurewa, J.; Omoba, O. Chemical composition, dietary fiber and antioxidant activity of fermented ripe banana peel flour. J. Food. Stab. 2020, 3, 27–42. [Google Scholar] [CrossRef]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Bindelle, J.; Agneesens, R.; Buldgen, A.; Wathelet, B.; Paquot, M. Ripening influences banana and plantain peels composition and energy content. Trop. Anim. Health Prod. 2011, 43, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Lim, T.T.; Tee, J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007, 103, 1003–1008. [Google Scholar] [CrossRef]
- Ali, M.; Imran, M.; Nadeem, M.; Khan, M.K.; Sohaib, M.; Suleria, H.A.R.; Bashir, R. Oxidative stability and Sensoric acceptability of functional fish meat product supplemented with plant−based polyphenolic optimal extracts. Lipids Health Dis. 2019, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, J.; Zafar, T. Bioactive compounds in banana fruits and their health benefits. Food Qual. Saf. 2018, 2, 183–188. [Google Scholar] [CrossRef]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant compounds from bananas (Musa Cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Changes of phytochemicals and antioxidant capacity of banana peel during the ripening process; with and without ethylene treatment. Sci. Hortic. 2019, 253, 255–262. [Google Scholar] [CrossRef]
- Mokbel, M.S.; Hashinaga, F. Antibacterial and antioxidant activities of banana (Musa, AAA cv. Cavendish) fruits peel. Am. J. Biochem. Biotechnol. 2005, 1, 125–131. [Google Scholar] [CrossRef]
- Mordi, R.; Fadiaro, A.; Owoeye, T.; Olanrewaju, I.; Uzoamaka, G.; Olorunshola, S. Identification by GC-MS of the components of oils of banana peels extract, phytochemical and antimicrobial analyses. Res. J. Phytochem. 2016, 10, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Preeti, J.; Mahmood, H.B.; Khondker, R.H.; Sitesh, C.B. Antibacterial and antioxidant activities of local seeded banana fruits. Afr. J. Pharm. Pharmacol. 2011, 5, 1398–1403. [Google Scholar]
- Dahham, S.S.; Mohamad, T.; Tabana, Y.M.; Majid, A. Antioxidant activities and anticancer screening of extracts from banana fruit (Musa sapientum). Acad. J. Cancer Res. 2015, 8, 28–34. [Google Scholar]
- Durgadevi, P.K.S.; Saravanan, A.; Uma, S. Antioxidant potential and antitumour activities of Nendran banana peels in breast cancer cell line. Indian J. Pharm. Sci. 2019, 81, 464–473. [Google Scholar]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Gopi, N.; Ekambaram, P.; Pachaiappan, R.; Velusamy, P.; Murugan, K.; Benelli, G.; Kumar, R.S. Therapeutic effects of gold nanoparticles synthesized using Musa paradisiaca peel extract against multiple antibiotic resistant Enterococcus faecalis biofilms and human lung cancer cells (A549). Microb. Pathog. 2017, 102, 173–183. [Google Scholar] [CrossRef]
- Lee, E.-H.; Yeom, H.-J.; Ha, M.-S.; Bae, D.-H. Development of banana peel jelly and its antioxidant and textural properties. Food Sci. Biotechnol. 2010, 19, 449–455. [Google Scholar] [CrossRef]
- Zaini, H.B.M.; Sintang, M.D.B.; Dan, Y.N.; Ab Wahab, N.; Hamid, M.B.A.; Pindi, W. Effect of addition of banana peel powder (Musa balbisiana) on physicochemical and sensory properties of fish patty. Br. Food J. 2019, 121, 2179–2189. [Google Scholar] [CrossRef]
- Eshak, N.S. Sensory evaluation and nutritional value of balady flat bread supplemented with banana peels as a natural source of dietary fiber. Ann. Agric. Sci. 2016, 61, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Arun, K.; Persia, F.; Aswathy, P.; Chandran, J.; Sajeev, M.; Jayamurthy, P.; Nisha, P. Plantain peel-a potential source of antioxidant dietary fibre for developing functional cookies. J. Food Sci. Technol. 2015, 52, 6355–6364. [Google Scholar] [CrossRef] [Green Version]
- Wachirasiri, P.; Julakarangka, S.; Wanlapa, S. The effects of banana peel preparations on the properties of banana peel dietary fibre concentrate. Songklanakarin J. Sci. Technol. 2009, 31, 605–611. [Google Scholar]
- Zaini, H.B.M.; Sintang, M.D.B.; Pindi, W. The roles of banana peel powders to alter technological functionality, sensory and nutritional quality of chicken sausage. Food Sci. Nutr. 2020, 8, 5497–5507. [Google Scholar] [CrossRef] [PubMed]
- Anhwange, B.A. Chemical composition of Musa sapientum (banana) peels. J. Food Technol. 2008, 6, 263–266. [Google Scholar]
- Kurhade, A.; Patil, S.; Sonawane, S.K.; Waghmare, J.S.; Arya, S.S. Effect of banana peel powder on bioactive constituents and microstructural quality of chapatti: Unleavened Indian flat bread. J. Food Meas. Charact. 2016, 10, 32–41. [Google Scholar] [CrossRef]
- Farooq, M.; Anwar, R.; Shukat, R.; Abbas, S.Q.; Ilyas, N.; Cristina, M.; Yunyang, W. Ecofriendly Utilization of by Products From Banana Peel in Food Production and Other Industrial Applications. A Review. Carpath. J. Food Sci. Technol. 2021, 13, 150–158+9. [Google Scholar]
- Hong, K.-J.; Lee, C.-H.; Kim, S.W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef]
- Nuriyasa, I.; Puspani, E.; Bidura, I. Growth, feed digestion and carcass characteristics of rabbits fed with banana peel (Acuminata balbisiana) supplementation. Pak. J. Nutr. 2019, 19, 19–24. [Google Scholar] [CrossRef]
- Kalemelawa, F.; Nishihara, E.; Endo, T.; Ahmad, Z.; Yeasmin, R.; Tenywa, M.M.; Yamamoto, S. An evaluation of aerobic and anaerobic composting of banana peels treated with different inoculums for soil nutrient replenishment. Bioresour. Technol. 2012, 126, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Pangnakorn, U. Valuable added the agricultural waste for farmers using in organic farming groups in Phitsanulok, Thailand. In Proceeding of the Prosperity and Poverty in a Globalized World-Challenges for Agricultural Research, Born, Germany, 11–13 October 2006. [Google Scholar]
- Itelima, J.; Onwuliri, F.; Onwuliri, E.; Onyimba, I.; Oforji, S. Bio-ethanol production from banana, plantain and pineapple peels by simultaneous saccharification and fermentation process. Int. J. Environ. Sci. Dev. 2013, 4, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Pisutpaisal, N.; Boonyawanich, S.; Saowaluck, H. Feasibility of biomethane production from banana peel. Energy Procedia 2014, 50, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Silva, W.O.; Nagar, B.; Soutrenon, M.; Girault, H.H. Banana split: Biomass splitting with flash light irradiation. Chem. Sci. 2022, 13, 1774–1779. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Prachpreecha, O.; Pipatpanyanugoon, K.; Sawangwong, P. A study of characterizations and efficiency of activated carbon prepared from peel and bunch of banana for methyl orange dye adsorption. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 17–26. [Google Scholar]
- Santhoskumar, A.U.; Vaishnavi, R.; Karunakaran, T. Studies on mechanical properties and biodegradation of edible food wrapper from banana peel. Asian J. Adv. Basic Sci. 2019, 7, 1–4. [Google Scholar] [CrossRef]
- Sultan, N.F.K.; Johari, W.L.W. The development of banana peel/corn starch bioplastic film: A preliminary study. Bioremediat. Sci. Technol. Res. 2017, 5, 12–17. [Google Scholar] [CrossRef]
- Pereira, A.; Maraschin, M. Banana (Musa spp.) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015, 160, 149–163. [Google Scholar] [CrossRef]
- Kumar, K.S.; Bhowmik, D.; Duraivel, S.; Umadevi, M. Traditional and medicinal uses of banana. J. Pharmacogn. Phytochem. 2012, 1, 51–63. [Google Scholar]
- Imam, M.Z.; Akter, S. Musa paradisiaca L. and Musa sapientum L.: A phytochemical and pharmacological review. J. Appl. Pharm. Sci. 2011, 1, 14–20. [Google Scholar]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [Green Version]
- Gomes, H.D.O.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. Evaluating the presence of pesticides in bananas: An integrative review. Ecotoxicol. Environ. Saf. 2020, 189, 110016. [Google Scholar] [CrossRef]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under-utilized renewable food biomass with great potential. J. Food Sci. Technol. 2014, 51, 3527–3545. [Google Scholar] [CrossRef] [Green Version]
- Tock, J.Y.; Lai, C.L.; Lee, K.T.; Tan, K.T.; Bhatia, S. Banana biomass as potential renewable energy resource: A Malaysian case study. Renew. Sustain. Energy Rev. 2010, 14, 798–805. [Google Scholar] [CrossRef]
- Adinugraha, M.P.; Marseno, D.W. Synthesis and characterization of sodium carboxymethylcellulose from cavendish banana pseudo stem (Musa cavendishii LAMBERT). Carbohydr. Polym. 2005, 62, 164–169. [Google Scholar] [CrossRef]





| Nutritional Composition | Function | Applications | References |
|---|---|---|---|
| Bio-oil | Heat generation by pyrolysis, reducing greenhouse gas emissions | Biochar, soil conditioners, ethanol, biogas | [12,21] |
| Minerals | Essential elements for human health | Making functional bread with probiotics, organic fertilizer | [16] |
| Fiber | Facilitates the digestion and absorption of many nutrients and prevents many diseases | Good nutritional quality and organoleptic acceptability when added to instant noodles, feed, clothing, paper, sanitary pads | [15,18,22,23] |
| Polyphenols and flavonoids | Strong resistance to oxidation | Gingival depigmenting agents | [19] |
| Biological Activities | Chemical Components | Extraction Method | Main Findings | Applications | References |
|---|---|---|---|---|---|
| Antioxidant activities | Total phenolics, flavonoids, tannins | 70% aqueous acetone extracts | In the β-carotene linoleic acid system, extracts showed radical scavenging (ABTS, DPPH, and superoxide), and prevention of lipid peroxidation | Reduces the chance of diseases such as cardiovascular disease and diabetes. Chicken with banana inflorescence, burger | [51] |
| Total phenolics | Methanol extracts | Extracts demonstrated lipid peroxidation inhibition in the β-carotene linoleic acid system, ferric-reducing antioxidant power (FRAP), and free radical scavenging activities (ABTS, DPPH) | [52] | ||
| Dietary fiber and associated polyphenols | Water-soluble polysaccharides and fractions | Polysaccharide fractions had DPPH radical scavenging, FRAP, and metal chelating activities | [37] | ||
| Umbelliferone and lupeol | bioassay-guided isolation of an ethanol extract | Extracts and isolated substances demonstrated reducing capability, an inhibitory impact against lipid peroxidation, and the ability to scavenge free radicals (ABTS, DPPH, and superoxide) | [53] | ||
| Anti-diabetic activities | Total phenolics; phenolic acids | extracts with water and different organic solvents | In Ehrlich ascites tumor cells, extracts had various degrees of stimulatory effects on glucose uptake | As a potential antidiabetic agent | [54] |
| Gallic acid, quercetin and epicatechin | Extracts of methanol and its fractions (petroleum ether, ethyl acetate and water fractions) | Intragastric administration of the ethyl acetate part (200 mg/kg body weight/day) for 60 days resulted in a significant decrease in blood glucose levels, lipid peroxidation products | [45] | ||
| β-sitosterol, 31-norcyclolaudenone, and (24R)-4α, 14α. 4-trimethyl-5α-cholesta-8, 25(27)-dien-3β-ol | Ethanol extract | Isolated substances prevented the production of AGEs in the BSA-fructose model and the activity of α-glucosidase and α-amylase | [55] | ||
| Anti-disease activities | GCMS profiling was used to identify steroids, fatty acids, and long chain aliphatic chemicals. | Ethanol extracts | Extracts significantly slowed the expansion of HT-29 and other cell lines | Anti-cancer drug that induces apoptosis in cancer cells | [48] |
| Polyphenols | Methanol and ethyl acetate extracts | Based on information from cellular and proteomic techniques, in HT-29 cells, methanol extract resulted in apoptotic cell death | [56] | ||
| Cardiovascular protective activities | ND | Methanol and ethyl acetate extracts | LDL oxidation and ACE activity were both reduced by methanol extract. | [48] | |
| Anti-microbial | Total phenolics | Methanol extract | Extracts showed various degrees of antibacterial activity against a number of harmful microorganisms that can be found in food | Food preservations | [57] |
| polyphenols, steroids | Extracts were made using water and a variety of organic solvents, including petroleum ether, isopropanol, chloroform, ethyl acetate, hexane, methanol, and ethanol | Extracts showed variable degrees of antibacterial activity against certain Gram-positive and Gram-negative bacteria | [58] | ||
| Anti-inflammatory | Gallic acid, quercetin and epicatechin | Extracts of methanol and its fractions (water, petroleum ether, and ethyl acetate fractions) | In the liver of streptozotocin-induced diabetic rats, the ethyl acetate fraction downregulated the mRNA expression of TNF-α, TGF-β1, NF-κB, and IL-6 and suppressed the activity of 5-LOX and COX-2 in monocytes | Anti-inflammatory drugs | [45] |
| Anti-obesity | Total phenolics | Water extracts | Pancreatic lipase activity was reduced by water extracts | Weight loss potential | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, F.; Tan, C.; Zhang, B.; Wu, W.; Shang, N. The Valorization of Banana By-Products: Nutritional Composition, Bioactivities, Applications, and Future Development. Foods 2022, 11, 3170. https://doi.org/10.3390/foods11203170
Zou F, Tan C, Zhang B, Wu W, Shang N. The Valorization of Banana By-Products: Nutritional Composition, Bioactivities, Applications, and Future Development. Foods. 2022; 11(20):3170. https://doi.org/10.3390/foods11203170
Chicago/Turabian StyleZou, Fanglei, Chunming Tan, Bo Zhang, Wei Wu, and Nan Shang. 2022. "The Valorization of Banana By-Products: Nutritional Composition, Bioactivities, Applications, and Future Development" Foods 11, no. 20: 3170. https://doi.org/10.3390/foods11203170
APA StyleZou, F., Tan, C., Zhang, B., Wu, W., & Shang, N. (2022). The Valorization of Banana By-Products: Nutritional Composition, Bioactivities, Applications, and Future Development. Foods, 11(20), 3170. https://doi.org/10.3390/foods11203170






