Hot-Air Flow Rolling Dry-Blanching Pretreatment Improves the Drying Quality of Acanthopanax sessiliflorus by Increasing the Drying Rate and Inactivating Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. HMRDB Pretreatment
2.3. Drying Kinetics
2.4. PPO and POD Activity
2.5. Determination of Weight Loss
2.6. Determination of Rehydration Ratio
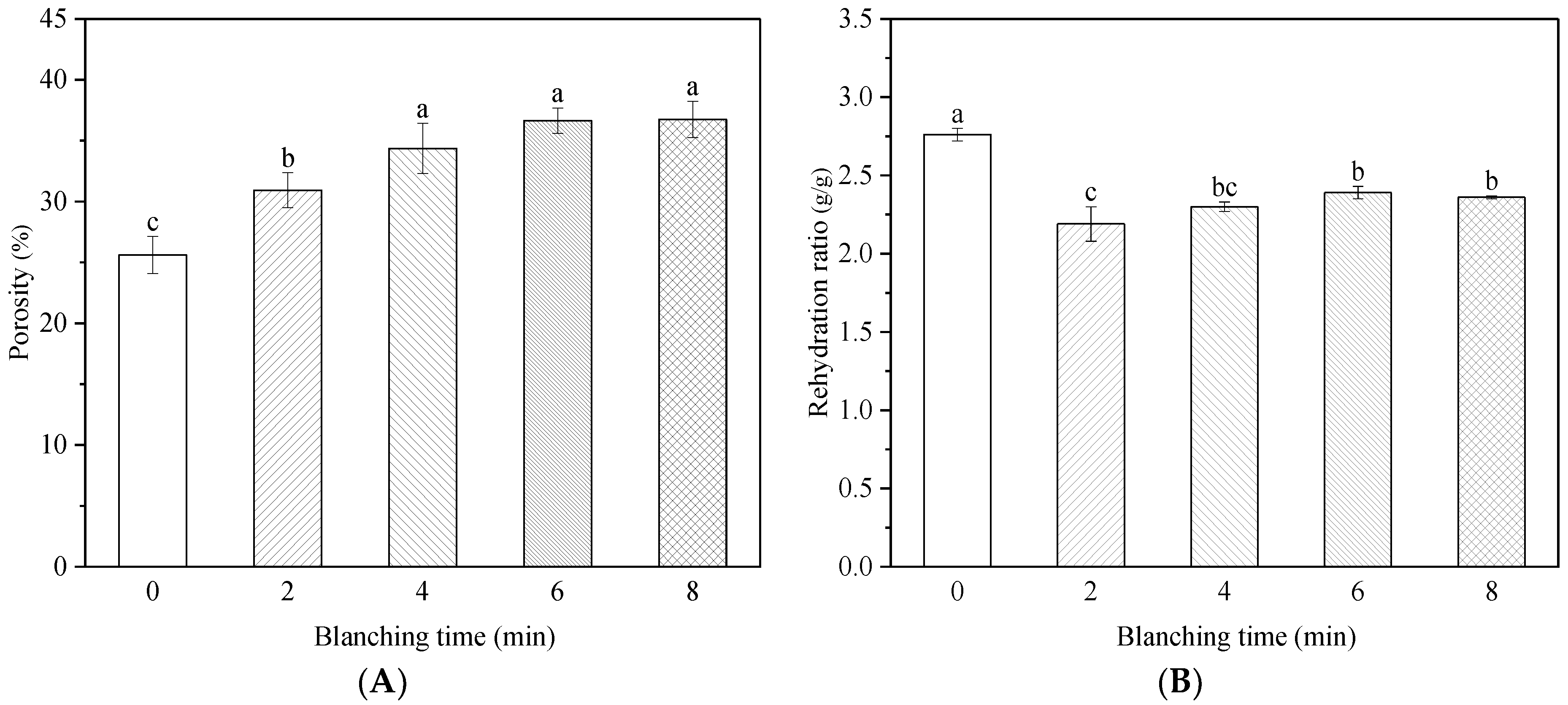
2.7. Microstructure and Porosity
2.8. Determination of Phytochemical Content and Antioxidant Activity
2.8.1. Sample Extraction
2.8.2. Total Polyphenol Content (TPC)
2.8.3. Total Flavonoid Content (TFC)
2.8.4. Total Anthocyanin Content (TAC)
2.9. Determination of Antioxidant Activity
2.10. Data Analysis
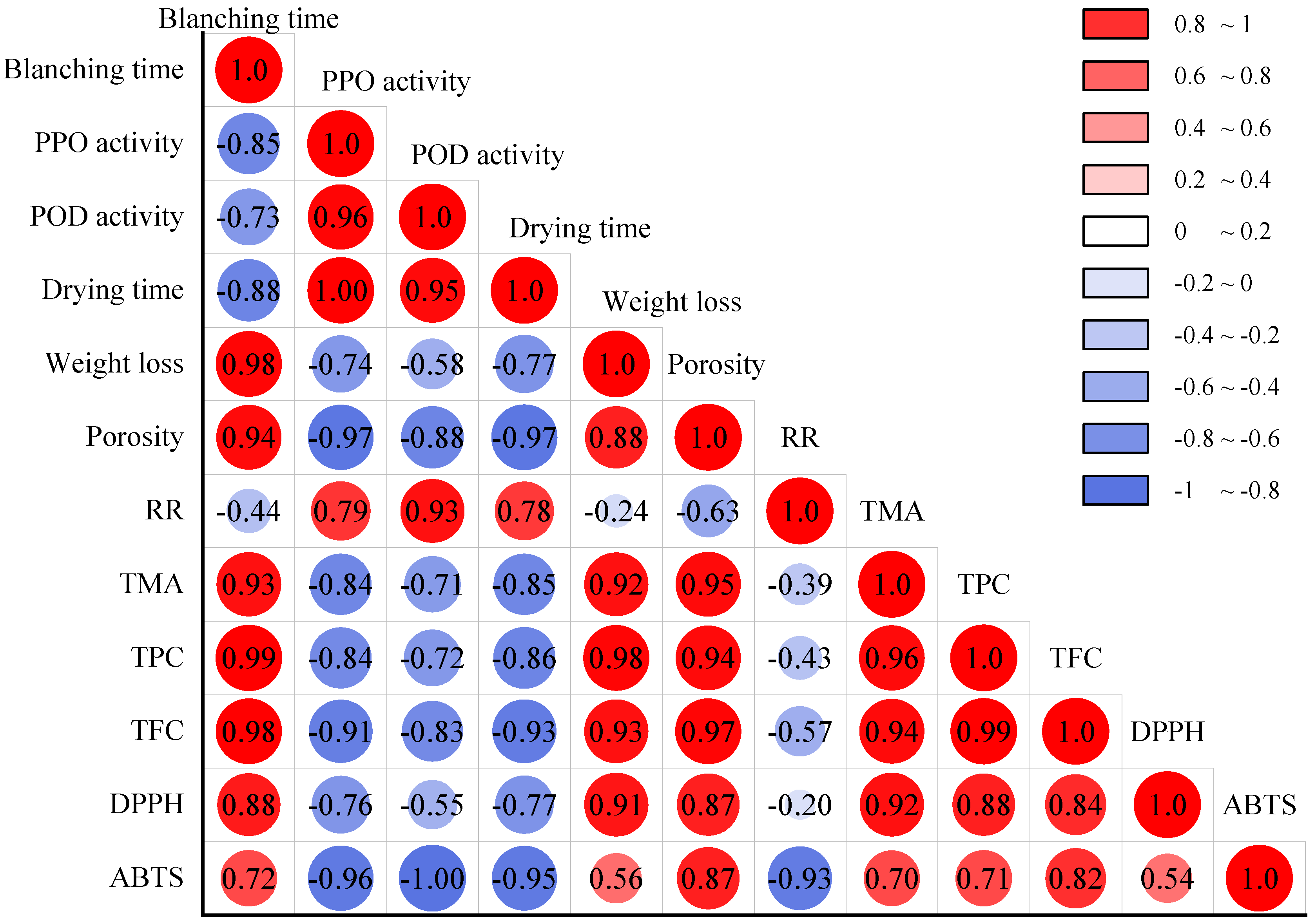
3. Results and Discussion
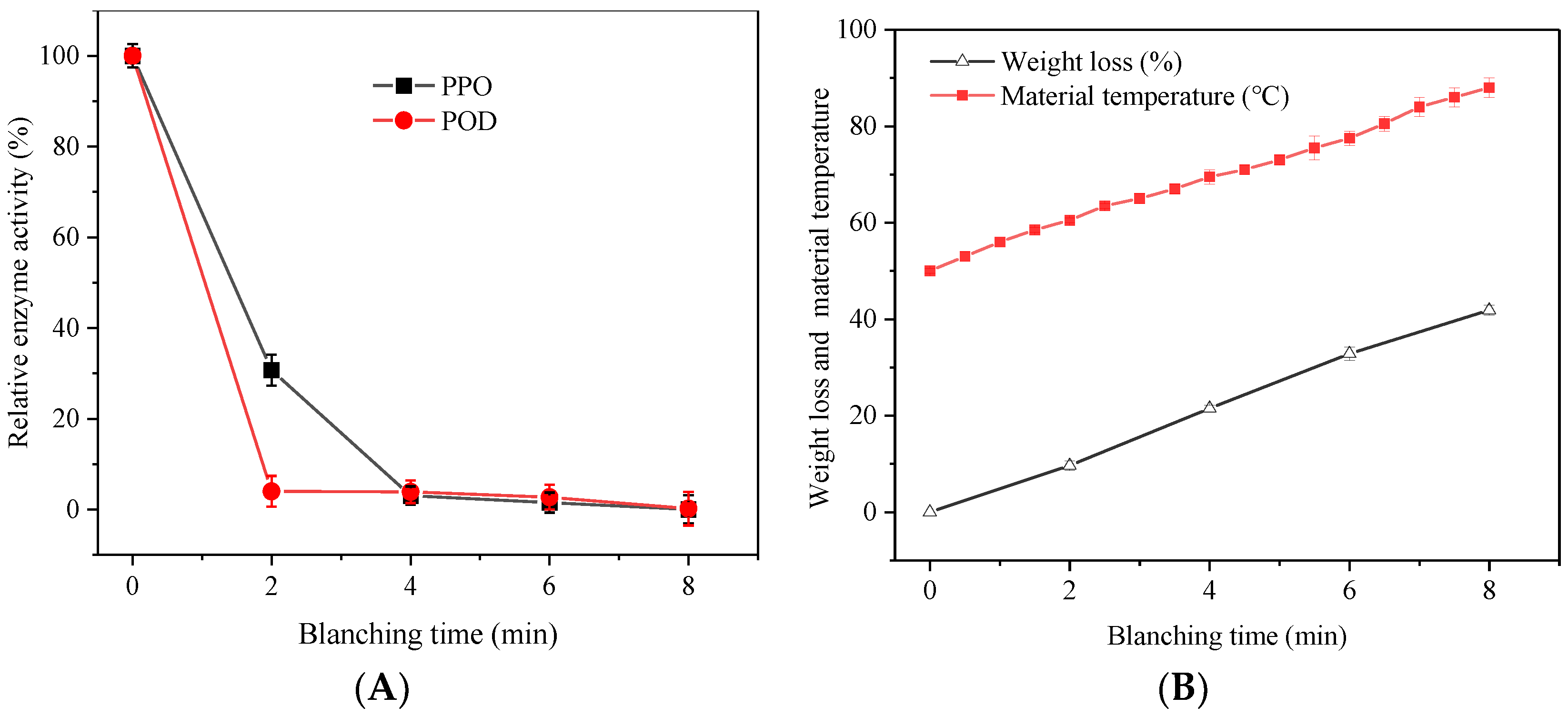
3.1. Effect of HMRDB on PPO and POD Enzyme Inactivation
3.2. Effect of HMRDB on Weight Loss
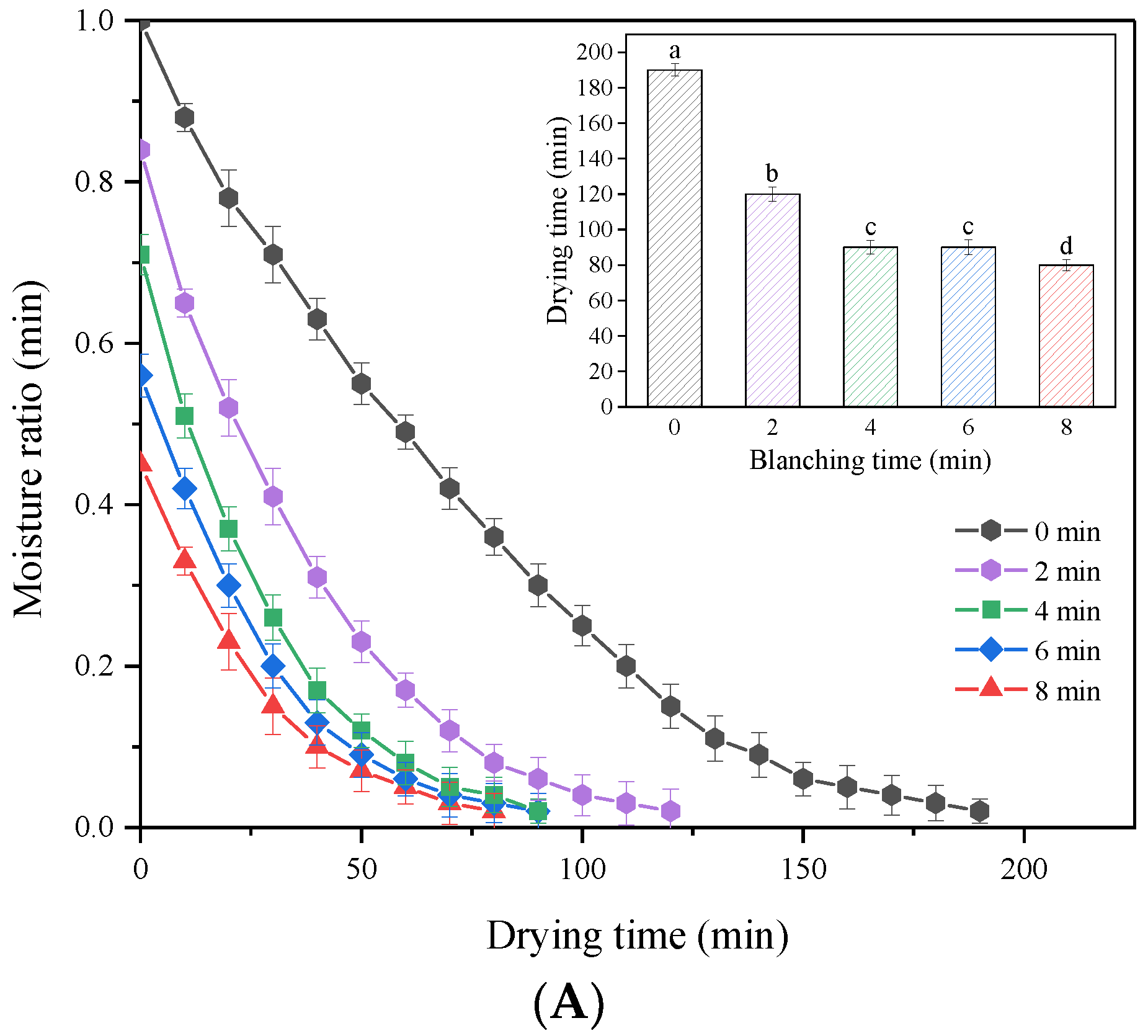
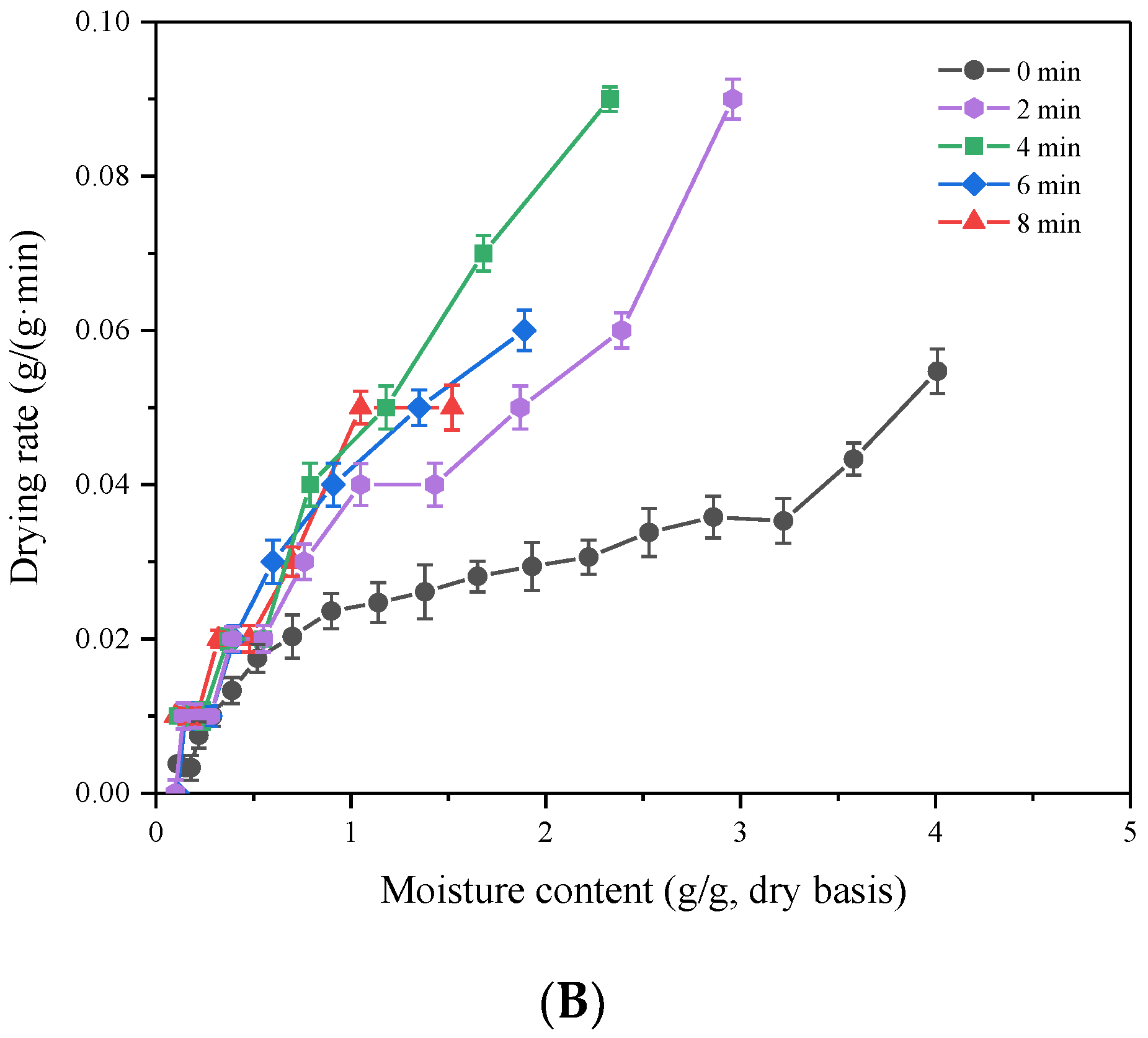
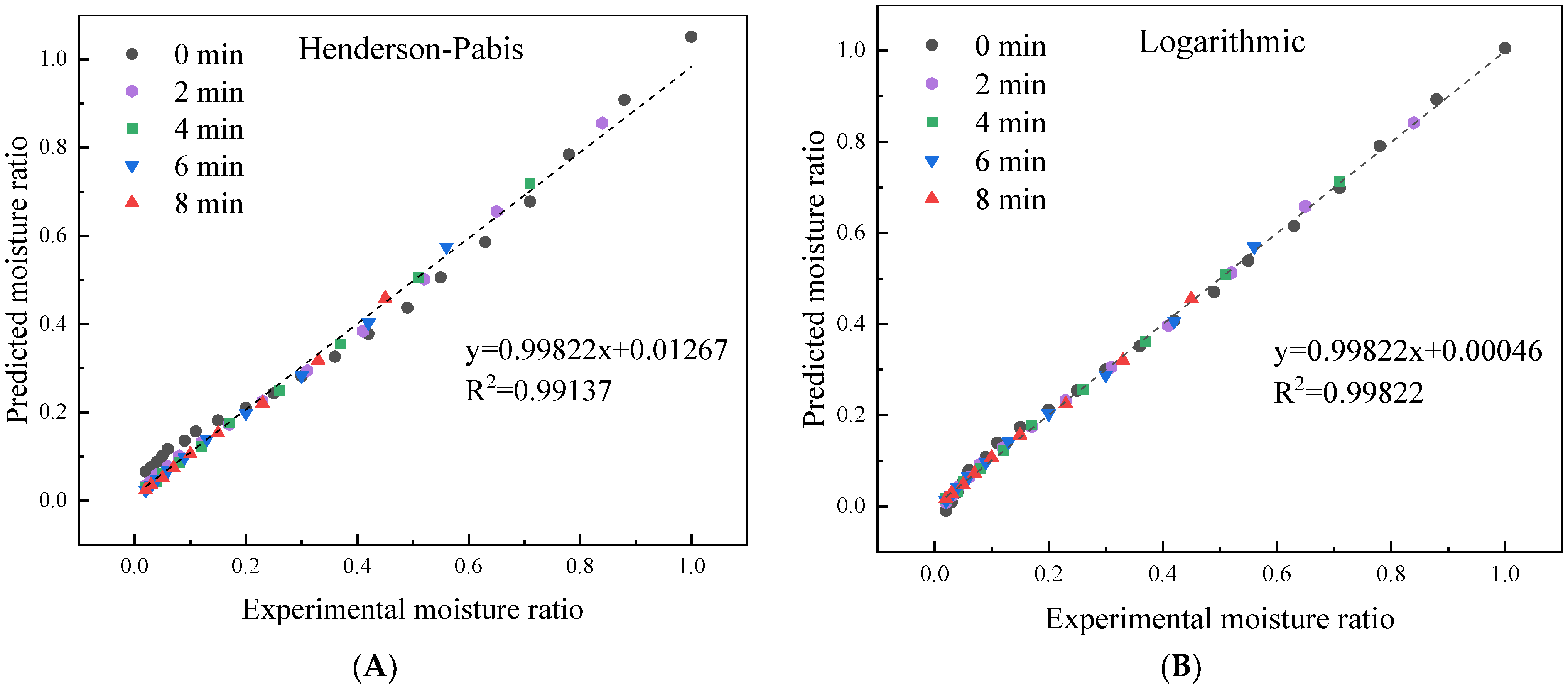
3.3. Effect of HMRDB on Drying Kinetics
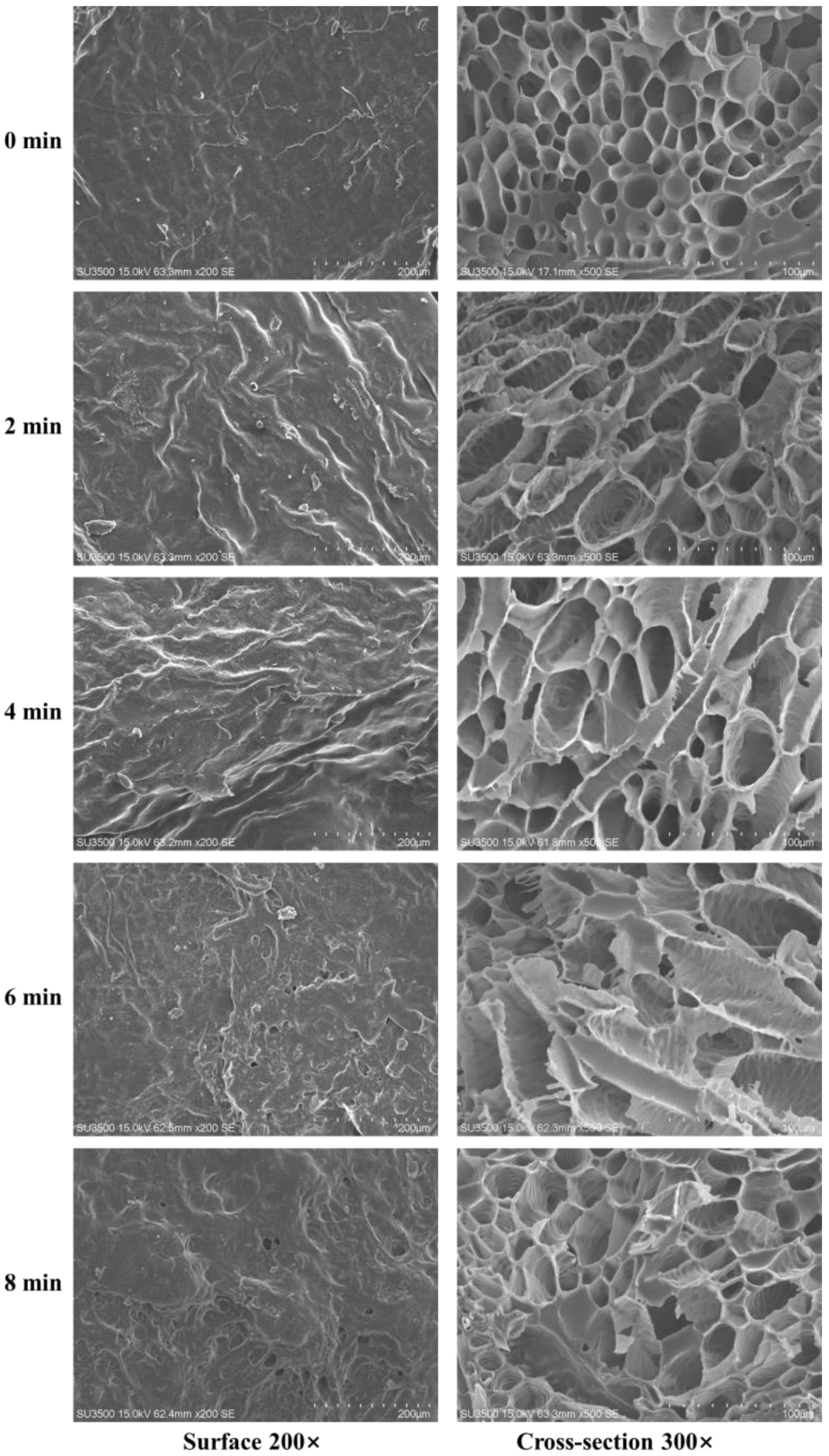
3.4. Effect of HMRDB on Microstructure
3.5. Effect of HMRDB on Rehydration Ratio
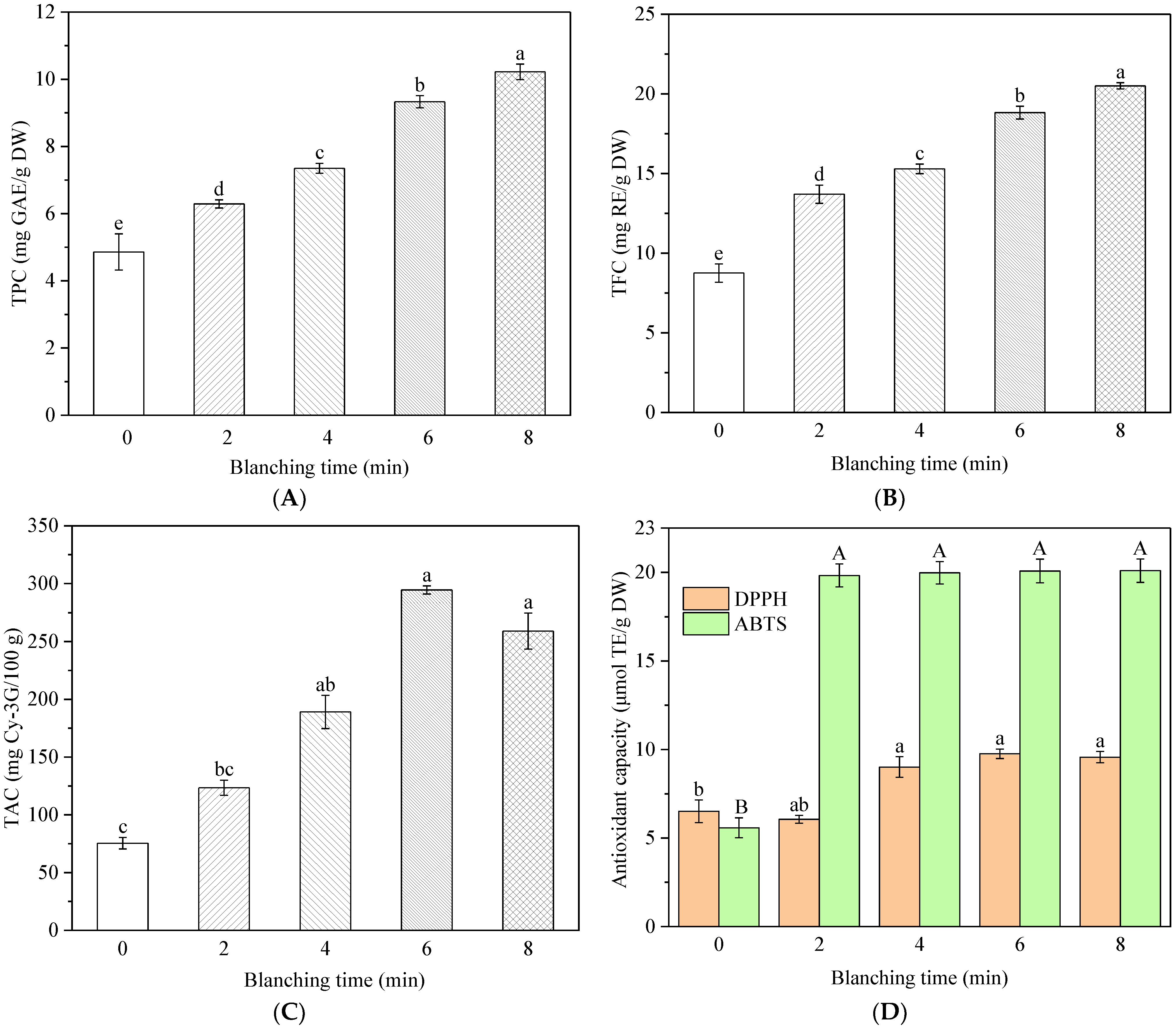
3.6. Effect of HMRDB on Phytochemical Content
3.7. Effect of HMRDB on Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xia, Y.G.; Gong, F.Q.; Guo, X.D.; Song, Y.; Li, C.X.; Liang, J.; Yang, B.Y.; Kuang, H.X. Rapid screening and characterization of triterpene saponins in Acanthopanax senticosus leaves via untargeted MSAll and SWATH techniques on a quadrupole time of flight mass spectrometry. J. Pharm. Biomed. Anal. 2019, 170, 68–82. [Google Scholar] [CrossRef]
- Guo, M.Q.; Song, F.R.; Liu, Z.Q.; Liu, S.Y. Characterization of triterpenoidic saponin mixture in crude extracts from leaves of Acanthopanax senticosus Harms by saponin structural correlation and mass spectrometry. Anal. Chim. Acta 2006, 557, 198–203. [Google Scholar] [CrossRef]
- Hong, J.H.; Cha, Y.S.; Rhee, S.J. Effects of the Cellcultured Acanthopanax senticosus Extract on Antioxidative Defense System and Membrane Fluidity in the Liver of Type 2 Diabetes Mouse. J. Clin. Biochem. Nutr. 2009, 45, 101–109. [Google Scholar] [CrossRef]
- Xia, Y.G.; Huang, Y.X.; Liang, J.; Kuang, H.X. Comparable studies of two polysaccharides from leaves of Acanthopanax senticosus: Structure and antioxidation. Int. J. Biol. Macromol. 2020, 147, 350–362. [Google Scholar] [CrossRef]
- An, N.N.; Sun, W.H.; Li, B.Z.; Wang, Y.; Shang, N.; Lv, W.Q.; Li, D.; Wang, L.J. Effect of different drying techniques on drying kinetics, nutritional components, antioxidant capacity, physical properties and microstructure of edamame. Food Chem. 2022, 373, 131412. [Google Scholar] [CrossRef]
- Thamizhiniyan, V.; Young-Woong, C.; Young-Kyoon, K. The cytotoxic nature of Acanthopanax sessiliflorus stem bark extracts in human breast cancer cells. Saudi J. Biol. Sci. 2015, 22, 752–759. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, M.; Nie, X.; Qi, Z.; Liu, X.; Jin, Q.; Zhang, X.; Yang, D. Characterization of botanical origin of selected popular purple Eleutherococcus tea grown in Yunnan province of China and quantification of Its anthocyanins using spectrophotometric method. Food Sci. Technol. 2022, 42, e91121. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Pan, Z.; Mujumdar, A.S.; Zhao, J.H.; Zheng, Z.-A.; Gao, Z.-J.; Xiao, H.-W. High-humidity hot air impingement blanching (HHAIB) enhances drying quality of apricots by inactivating the enzymes, reducing drying time and altering cellular structure. Food Control 2019, 96, 104–111. [Google Scholar] [CrossRef]
- Wang, H.; Fang, X.M.; Sutar, P.P.; Meng, J.S.; Wang, J.; Yu, X.L.; Xiao, H.W. Effects of vacuum-steam pulsed blanching on drying kinetics, colour, phytochemical contents, antioxidant capacity of carrot and the mechanism of carrot quality changes revealed by texture, microstructure and ultrastructure. Food Chem. 2021, 338, 127799. [Google Scholar] [CrossRef]
- Geng, Z.; Huang, X.; Wang, J.; Xiao, H.; Yang, X.; Zhu, L.; Qi, X.; Zhang, Q.; Hu, B. Pulsed Vacuum Drying of Pepper (Capsicum annuum L.): Effect of High-Humidity Hot Air Impingement Blanching Pretreatment on Drying Kinetics and Quality Attributes. Foods 2022, 11, 318. [Google Scholar] [CrossRef]
- Guzik, P.; Kulawik, P.; Zajac, M.; Migdal, W. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2021, 62, 7989–8008. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, C.E.; Moses, O.I.; Nwonuma, C.; Abiola, T.; Benjamin, B.O.; Folorunsho, J.O.; Olaniran, A.F.; Pan, Z. Infrared and Microwave as a dry blanching tool for Irish potato: Product quality, cell integrity, and artificial neural networks (ANNs) modeling of enzyme inactivation kinetic. Innov. Food Sci. Emerg. Technol. 2022, 78, 103010. [Google Scholar] [CrossRef]
- Taghinezhad, E.; Kaveh, M.; Szumny, A. Optimization and Prediction of the Drying and Quality of Turnip Slices by Convective-Infrared Dryer under Various Pretreatments by RSM and ANFIS Methods. Foods 2021, 10, 284. [Google Scholar] [CrossRef]
- Dibanda, R.F.; Akdowa, E.P.; Rani, P.A.; Tongwa, Q.M.; Mbofung, F.C. Effect of microwave blanching on antioxidant activity, phenolic compounds and browning behaviour of some fruit peelings. Food Chem. 2020, 302, 125308. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.R.S.; Padmini, T.; Adedeji, A.; Gariepy, Y.; Raghavan, G.S.V. A comparative study on the effect of chemical, microwave, and pulsed electric pretreatments on convective drying and quality of raisins. Dry. Technol. 2008, 26, 1238–1243. [Google Scholar] [CrossRef]
- Jiang, N.; Liu, C.; Li, D.; Zhou, Y. Effect of blanching on the dielectric properties and microwave vacuum drying behavior of Agaricus bisporus slices. Innov. Food Sci. Emerg. Technol. 2015, 30, 89–97. [Google Scholar] [CrossRef]
- Su, D.; Lv, W.; Wang, Y.; Wang, L.; Li, D. Influence of microwave hot-air flow rolling dry-blanching on microstructure, water migration and quality of pleurotus eryngii during hot-air drying. Food Control 2020, 114, 107228. [Google Scholar] [CrossRef]
- Siguemoto, E.S.; Pereira, L.J.; Gut, J.A.W. Inactivation Kinetics of Pectin Methylesterase, Polyphenol Oxidase, and Peroxidase in Cloudy Apple Juice under Microwave and Conventional Heating to Evaluate Non-Thermal Microwave Effects. Food Bioprocess Technol. 2018, 11, 1359–1369. [Google Scholar] [CrossRef]
- Hayat, K.; Zhang, X.M.; Farooq, U.; Abbas, S.; Xia, S.Q.; Jia, C.S.; Zhong, F.; Zhang, J. Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chem. 2010, 123, 423–429. [Google Scholar] [CrossRef]
- Keneni, Y.G.; Hvoslef-Eide, A.K.; Marchetti, J.M. Mathematical modelling of the drying kinetics of Jatropha curcas L. seeds. Ind. Crops Prod. 2019, 132, 12–20. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Li, Z.L.; Bi, J.F.; Zhou, L.Y.; Yi, J.Y.; Wu, X.Y. Effect of hybrid drying methods on physicochemical, nutritional and antioxidant properties of dried black mulberry. LWT Food Sci. Technol. 2017, 80, 178–184. [Google Scholar] [CrossRef]
- Xu, H.; Wu, M.; Wang, Y.; Wei, W.; Sun, D.; Li, D.; Zheng, Z.; Gao, F. Effect of Combined Infrared and Hot Air Drying Strategies on the Quality of Chrysanthemum (Chrysanthemum morifolium Ramat.) Cakes: Drying Behavior, Aroma Profiles and Phenolic Compounds. Foods 2022, 11, 2240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Xie, L.; Zielinska, M.; Pan, Z.L.; Wang, J.; Deng, L.Z.; Wang, H.; Xiao, H.W. Pulsed vacuum drying enhances drying of blueberry by altering micro-, ultrastructure and water status and distribution. LWT Food Sci. Technol. 2021, 142, 111013. [Google Scholar] [CrossRef]
- Zhao, C.C.; Ameer, K.; Eun, J.-B. Effects of various drying conditions and methods on drying kinetics and retention of bioactive compounds in sliced persimmon. LWT Food Sci. Technol. 2021, 143, 111149. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.; Espin, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Z.L. Processing and quality characteristics of apple slices under simultaneous infrared dry-blanching and dehydration with continuous heating. J. Food Eng. 2009, 90, 441–452. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, L.M.; Penas, F.J. Comparison study of conventional hot-water and microwave blanching on quality of green beans. Innov. Food Sci. Emerg. Technol. 2013, 20, 191–197. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.M.; Mujumdar, A.S.; Qian, J.Y.; Zhang, Q.; Yang, X.H.; Liu, Y.H.; Gao, Z.J.; Xiao, H.W. Effect of high-humidity hot air impingement blanching (HHAIB) on drying and quality of red pepper (Capsicum annuum L.). Food Chem. 2017, 220, 145–152. [Google Scholar] [CrossRef]
- Kingsly, R.P.; Goyal, R.K.; Manikantan, M.R.; Ilyas, S.M. Effects of pretreatments and drying air temperature on drying behaviour of peach slice. Int. J. Food Sci. Technol. 2007, 42, 65–69. [Google Scholar] [CrossRef]
- Doymaz, I.; Ismail, O. Drying and Rehydration Behaviors of Green Bell Peppers. Food Sci. Biotechnol. 2010, 19, 1449–1455. [Google Scholar] [CrossRef]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a systems approach to understanding plant-cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef]
- Imaizumi, T.; Szymanska-Chargot, M.; Pieczywek, P.M.; Chylinska, M.; Koziol, A.; Ganczarenko, D.; Tanaka, F.; Uchino, T.; Zdunek, A. Evaluation of pectin nanostructure by atomic force microscopy in blanched carrot. LWT Food Sci. Technol. 2017, 84, 658–667. [Google Scholar] [CrossRef]
- Ni, L.; Lin, D.; Barrett, D.M. Pectin methylesterase catalyzed firming effects on low temperature blanched vegetables. J. Food Eng. 2005, 70, 546–556. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.X.; Wang, H.; Wang, S.Y.; Lin, Z.A.; Zhao, L.L.; Xu, H.D. Ethanol and blanching pretreatments change the moisture transfer and physicochemical properties of apple slices via microstructure and cell-wall polysaccharides nanostructure modification. Food Chem. 2022, 381, 132274. [Google Scholar] [CrossRef] [PubMed]
- Ojediran, J.O.; Okonkwo, C.E.; Olaniran, A.F.; Iranloye, Y.M.; Adewumi, A.D.; Erinle, O.; Afolabi, Y.T.; Adeyi, O.; Adeyi, A. Hot air convective drying of hog plum fruit (Spondias mombin): Effects of physical and edible-oil-aided chemical pretreatments on drying and quality characteristics. Heliyon 2021, 7, e08312. [Google Scholar] [CrossRef]
- Fratianni, A.; Niro, S.; Messia, M.C.; Cinquanta, L.; Panfili, G.; Albanese, D.; Di Matteo, M. Kinetics of carotenoids degradation and furosine formation in dried apricots (Prunus armeniaca L.). Food Res. Int. 2017, 99, 862–867. [Google Scholar] [CrossRef]
- Heras-Ramirez, M.E.; Quintero-Ramos, A.; Camacho-Davila, A.A.; Barnard, J.; Talamas-Abbud, R.; Torres-Munoz, J.V.; Salas-Munoz, E. Effect of blanching and drying temperature on polyphenolic compound stability and antioxidant capacity of apple pomace. Food Bioprocess Tech. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- Mendez-Lagunas, L.; Rodriguez-Ramirez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef] [PubMed]

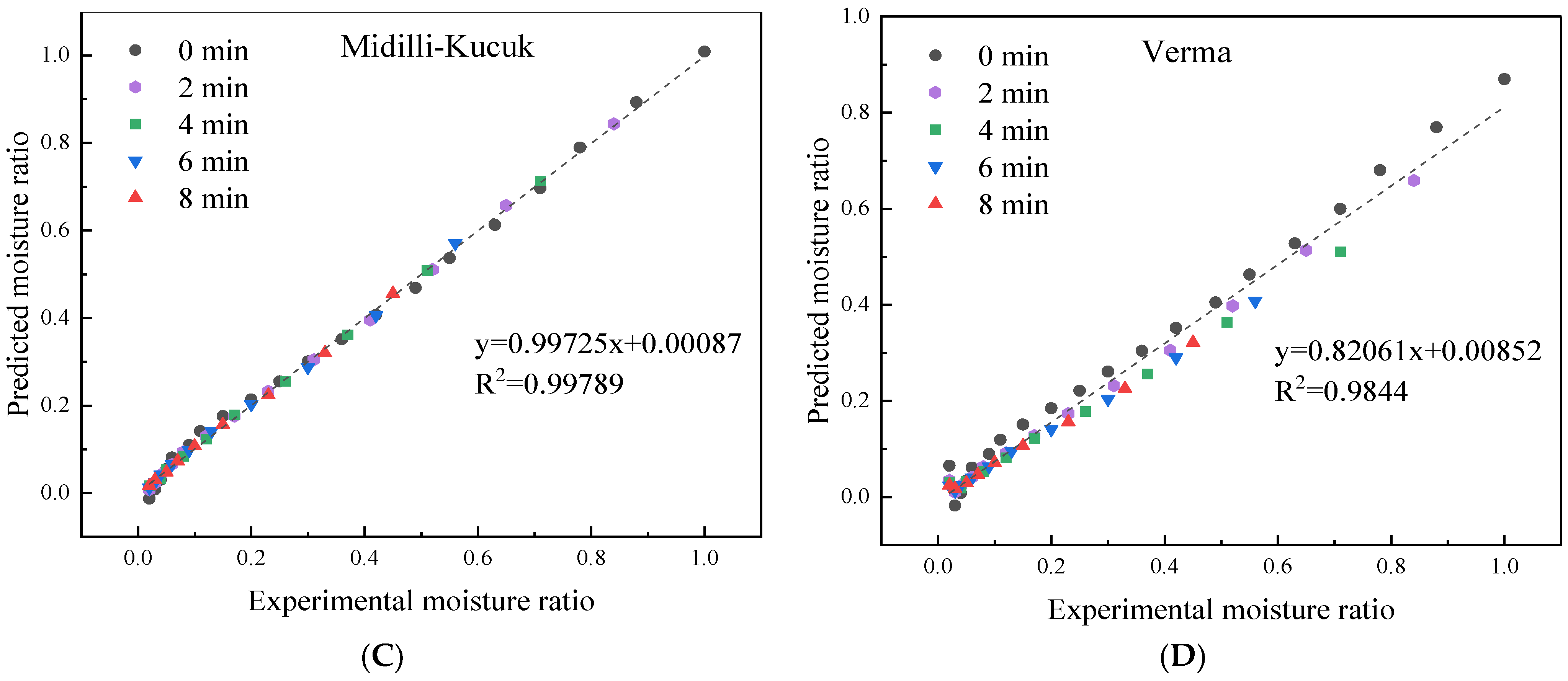
| Blanching Time (min) | 0 | 2 | 4 | 6 | 8 | |
|---|---|---|---|---|---|---|
| Henderson–Pabis y = a exp (−k t) | k | 0.0146 | 0.0267 | 0.0352 | 0.0354 | 0.0366 |
| a | 1.0512 | 0.8557 | 0.7187 | 0.5748 | 0.4589 | |
| R2 | 0.9825 | 0.9961 | 0.9985 | 0.9964 | 0.9977 | |
| χ2 | 1.79 × 10−3 | 2.98 × 10−4 | 9.26 × 10−5 | 1.38 × 10−4 | 5.93 × 10−5 | |
| RMSE | 0.0423 | 0.0173 | 0.0096 | 0.0118 | 0.0077 | |
| Logarithmic MR = a exp (−k t) + c | k | 0.0098 | 0.0232 | 0.0324 | 0.0324 | 0.0337 |
| a | 1.2002 | 0.8882 | 0.7350 | 0.5894 | 0.4703 | |
| c | −0.1949 | −0.0465 | −0.0223 | −0.0196 | −0.0149 | |
| R2 | 0.9972 | 0.9991 | 0.9995 | 0.9977 | 0.9986 | |
| χ2 | 2.99 × 10−4 | 7.90 × 10−5 | 3.66 × 10−5 | 1.02 × 10−4 | 4.23 × 10−5 | |
| RMSE | 0.0173 | 0.0089 | 0.0061 | 0.0101 | 0.0065 | |
| Midilli–Kucuk MR = a exp (−k t) + b t | k | 0.0114 | 0.0245 | 0.0335 | 0.0337 | 0.0349 |
| a | 1.0085 | 0.8434 | 0.7135 | 0.5706 | 0.4559 | |
| b | −0.0007 | −0.0003 | −0.0002 | −0.0002 | −0.0001 | |
| R2 | 0.9967 | 0.9988 | 0.9994 | 0.9975 | 0.9985 | |
| χ2 | 3.59 × 10−4 | 9.76 × 10−5 | 4.27 × 10−5 | 1.12 × 10−4 | 4.5 × 10−5 | |
| RMSE | 0.0190 | 0.0099 | 0.0065 | 0.0106 | 0.0068 | |
| Verma y = exp(−k t) + (1−a) exp (−g t) | k | 0.0117 | 0.0214 | 0.0285 | 0.0271 | 0.0277 |
| a | 1.0180 | 1.1596 | 1.2884 | 1.4314 | 1.5453 | |
| g | −0.0103 | 0.0074 | 0.0178 | 0.0195 | 0.0223 | |
| R2 | 0.9933 | 0.9991 | 0.9995 | 0.9976 | 0.9984 | |
| χ2 | 6.55 × 10−4 | 6.41 × 10−5 | 2.82 × 10−5 | 8.33 × 10−5 | 3.58 × 10−5 | |
| RMSE | 0.0256 | 0.0080 | 0.0053 | 0.0091 | 0.0060 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, N.-n.; Zhao, S.-y.; Li, D.; Wang, Y.; Wang, L.-j. Hot-Air Flow Rolling Dry-Blanching Pretreatment Improves the Drying Quality of Acanthopanax sessiliflorus by Increasing the Drying Rate and Inactivating Enzymes. Foods 2022, 11, 3186. https://doi.org/10.3390/foods11203186
An N-n, Zhao S-y, Li D, Wang Y, Wang L-j. Hot-Air Flow Rolling Dry-Blanching Pretreatment Improves the Drying Quality of Acanthopanax sessiliflorus by Increasing the Drying Rate and Inactivating Enzymes. Foods. 2022; 11(20):3186. https://doi.org/10.3390/foods11203186
Chicago/Turabian StyleAn, Nan-nan, Shi-yu Zhao, Dong Li, Yong Wang, and Li-jun Wang. 2022. "Hot-Air Flow Rolling Dry-Blanching Pretreatment Improves the Drying Quality of Acanthopanax sessiliflorus by Increasing the Drying Rate and Inactivating Enzymes" Foods 11, no. 20: 3186. https://doi.org/10.3390/foods11203186
APA StyleAn, N.-n., Zhao, S.-y., Li, D., Wang, Y., & Wang, L.-j. (2022). Hot-Air Flow Rolling Dry-Blanching Pretreatment Improves the Drying Quality of Acanthopanax sessiliflorus by Increasing the Drying Rate and Inactivating Enzymes. Foods, 11(20), 3186. https://doi.org/10.3390/foods11203186





